Efficacy and safety of alirocumab in Korean patients with hypercholesterolemia and high cardiovascular risk: subanalysis of the ODYSSEY-KT study
Article information
Abstract
Background/Aims
Efficacy and safety data of alirocumab, a fully human monoclonal antibody to proprotein convertase subtilisin/kexin type 9 (PCSK9), is not yet well established in the Korean population. We assessed them in ODYSSEY-KT through the pre-specified Korean subanalysis.
Methods
In the ODYSSEY-KT study, South Korean and Taiwanese patients with hypercholesterolemia and high cardiovascular risks were randomized (1:1) to alirocumab or placebo. Alirocumab was self-administered subcutaneously at 75 mg every 2 weeks with a maximally tolerated statin dose with or without other lipid-modifying therapies. Alirocumab dose was increased to 150 mg every 2 weeks at week 12 if low density lipoprotein cholesterol (LDL-C) ≥ 70 mg/dL at week 8. Primary endpoint was percent change in LDL-C from baseline to week 24. Results from Korean cohort (n = 83: 40 for alirocumab and 43 for placebo, respectively) analyses are reported here.
Results
In alirocumab group, the least square of mean change percent in LDL-C levels was –65.7% (placebo: 11.1%; p < 0.0001) and 92.0% of them achieved LDL-C < 70 mg/dL (placebo: 12.7%; p < 0.0001) at week 24. Alirocumab also showed significantly greater improvements in high density lipoprotein cholesterol (HDL-C), non-HDL-C, total cholesterol, lipoprotein(a), and apolipoprotein B than placebo (p < 0.05). Two consecutive calculated LDL-C values < 25 mg/dL were observed in 37.5% of alirocumab-treated patients. Overall, 45.0% alirocumab-treated and 51.2% placebo-treated patients experienced treatment-emergent adverse events (TEAEs) without discontinuation of treatment due to TEAEs.
Conclusions
Alirocumab has demonstrated to be effective in improvement of LDL-C and related lipid profiles in Korean cohort. Alirocumab was generally well tolerated with no significant safety signals.
INTRODUCTION
Dyslipidemia is a major risk factor for cardiovascular disease (CVD), the second-leading cause of mortality in Korea [1-3] and is also associated with increased prevalence of metabolic syndrome. In recent years, the prevalence of dyslipidemia has been increasing steadily in Korea [4,5]. The association between higher low density lipoprotein cholesterol (LDL-C) level and CVD is well established and guidelines recommend < 70 or < 100 mg/dL as target levels in patients with very high or high CV risk, respectively [6,7].
Guidelines for the management of dyslipidemia in Korea were established in 2015 [8]. These guidelines recommend lifestyle modification and pharmacological management of dyslipidemia for prevention of CVD. The CEPHEUS Pan-Asian survey showed suboptimal achievement of target LDL-C and total cholesterol (TC) with existing treatments in Korea [9].
Among several lipid lowering therapies (LLTs), proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors modulate cholesterol homeostasis via its ability to upregulate LDL receptor expression on hepatocyte cell membrane and by decreasing plasma LDL-C levels [10]. Recent consensus statements from both the American College of Cardiology and European Society of Cardiology/European Atherosclerosis Society recommend the use of PCSK9 inhibitors or ezetimibe in patients with very high or high cardiovascular (CV) risk who have high baseline LDL-C levels (70 to 189 mg/dL) uncontrolled by maximally tolerated doses of statins [11,12]. Alirocumab is a fully human monoclonal antibody that inhibits PCSK9 and has been clinically evaluated for its ability to reduce LDL-C levels. Several ODYSSEY phase 3 studies have demonstrated that alirocumab can reduce LDL-C levels substantially when used as monotherapy or when combined with background statin therapy with or without other LLTs [13-21].
However, the safety and efficacy of alirocumab has not been established in the Korean population. The ODYSSEY KT study was performed to assess the safety and efficacy of alirocumab among Korean and Taiwanese patients with uncontrolled serum cholesterol levels and coronary heart disease (CHD) or those at risk for CHD [22]. Here we present a pre-specified subanalysis for Korean patients.
METHODS
Study design
The ODYSSEY KT study (NCT02289963) was a randomized, double-blind, placebo-controlled, parallel-group, and multicenter study [22]. The Korean cohort study was conducted in 16 study centers across Korea between January 2015 and April 2016. The protocol was approved by the relevant independent Institutional Review Boards (Keimyung University Dongsan Medical Center IRB 2014-09-009). The study was performed according to the principles of the Declaration of Helsinki and applicable amendments and the International Conference on Harmonisation guidelines for Good Clinical Practice. All participating patients provided written informed consent.
Patients
Eligible patients (aged ≥ 18 years) had established CHD or CHD risk equivalents and hypercholesterolemia that was inadequately controlled with a maximally tolerated statin dose (with or without other LLTs) at a stable dose ≥ 4 weeks prior to screening. CHD risk equivalents were defined as history of CVD, moderate chronic kidney disease, or diabetes with multiple risk factors. Inadequately controlled hypercholesterolemia was defined as LDL-C ≥ 70 mg/dL in patients with documented CVD history or ≥ 100 mg/dL in patients without CVD history. The following were considered maximally tolerated statin doses: atorvastatin, 40 to 80 mg/day; rosuvastatin, 20 mg/day; and simvastatin, 40 mg/day. Patients receiving daily dose of atorvastatin, rosuvastatin, or simvastatin dose and considered appropriate by the investigator were also eligible to participate. Exclusion criteria for this study are described in Supplementary Table 1.
Treatment regimen
The total study duration was 35 weeks, consisting of a 3-week screening period, 24 weeks of double-blind treatment, and an 8-week follow-up period. Eligible patients were randomized 1:1 to receive either alirocumab 75 mg every 2 weeks or placebo every 2 weeks using an auto-injector device through the subcutaneous route. Study visits were on weeks 0, 4, 8, 12, 16, and 24. Follow visit was done 8 weeks after the end of treatment visit. Patients on alirocumab who did not achieve LDL-C < 70 mg/dL at week 8 were uptitrated to alirocumab 150 mg every 2 weeks from week 12 onwards. Blinding was maintained in these patients.
Endpoints
The primary efficacy endpoint was the percent change in calculated LDL-C values between baseline and week 24 in the intent-to-treat (ITT) population. Other key endpoints included the percent change from baseline to week 24 in non-high density lipoprotein cholesterol (non-HDL-C), TC, HDL-C, fasting triglycerides (TGs), lipoprotein(a) (Lp(a)), apolipoprotein A1 and apolipoprotein B (Apo B) and the proportion of patients reaching a calculated LDL-C < 70 mg/dL at week 24. Analyses of lipid samples were performed by a central laboratory using standard procedures and individual site laboratory tests for lipid parameters were not allowed during the study period. LDL-C levels were calculated using the Friedewald formula. LDL-C levels were reflexively measured via β-quantification when TG levels were > 400 mg/dL. In addition, LDL-C levels were systematically measured (via the β-quantification method) at weeks 0 and 24 for efficacy analysis purposes.
Safety was assessed throughout the study by monitoring treatment-emergent adverse events (TEAEs), serious adverse events (SAEs), laboratory data, and vital signs. TEAEs were defined as adverse events (AEs) that occurred during the time from first administration of blinded study drug to 10 weeks after last administration of blinded study drug.
Statistical analysis
The sample size calculation has been described previously [22]. Briefly, a total sample size of 40 patients (20 patients in each group) was required to achieve 95% power to detect a mean percent change difference in LDL-C of 30% with a 0.05 two-sided significance level and assuming a common standard deviation (SD) of 25% with all patients having an evaluable primary endpoint. At last, total sample size (n = 83) was bigger than the estimated minimum size to satisfy the requirement because we also intended to identify the safety data as well as efficacy. Calculations were done using nQuery Advisor version 7.0 (Statsols, Cork, Ireland).
The primary efficacy endpoint was analyzed using a mixed effect model with repeated measures (MMRM) with all post-baseline data available and missing data accounted for. Fixed categorical effects of treatment group (alirocumab vs. placebo), time (weeks 4, 8, 12, 16, and 24), randomization strata, treatment-by-time point interaction, strata-by-time point interaction, and continuous fixed covariates of baseline LDL-C value and baseline value-by-time point interaction were included in the MMRM. The 95% confidence intervals (CIs) of the differences were estimated from the model.
Other key endpoints were analyzed in a sequential order using a hierarchical inferential approach when the primary endpoint was significant at 5% α level. Continuous secondary endpoints with an anticipated normal distribution were analyzed using MMRM. Continuous secondary endpoints with a normal distribution (lipids other than Lp(a) and TGs) were analyzed as for the primary endpoint while those without normal distribution (Lp(a) and TGs) and binary secondary endpoints were analyzed using multiple imputation approach followed by robust regression. Target achievement for LDL-C was analyzed by multiple imputations followed by logistic regression. The ITT population included all randomized patients with LDL-C values available at baseline and at least one other post-randomization time point till the end of study. The modified intent-to-treat (mITT) included the randomized patients who had received at least one dose or part of the study with an evaluable primary endpoint during the treatment period and within one of the analysis windows. All randomized patients who received at least one dose or part of a dose of the alirocumab comprised the safety set. Safety data were analyzed by descriptive statistics and were focused on the TEAE period. All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Patient characteristics
A total of 83 patients were enrolled in the Korean subgroup of the ODYSSEY KT study. Both the ITT and safety populations consisted of 83 patients (40 patients in the alirocumab group and 43 patients in the placebo group) while the mITT population comprised 82 patients (40 patients in the alirocumab group and 42 patients in the placebo group) (Fig. 1). Both treatment groups were matched for age, gender, weight, body mass index, medical history, and statin type by intensity. More patients in alirocumab group were taking ezetimibe than those in placebo group (n = 7, 17.5%; and n = 4, 9.3%, respectively), but it did not reach the significance level of 0.05 in the post hoc chi-square test (Table 1). Additionally, no significant differences were found in lipid parameters between alirocumab and placebo groups at baseline (p > 0.05; post hoc t tests) (Table 2).
Efficacy analyses
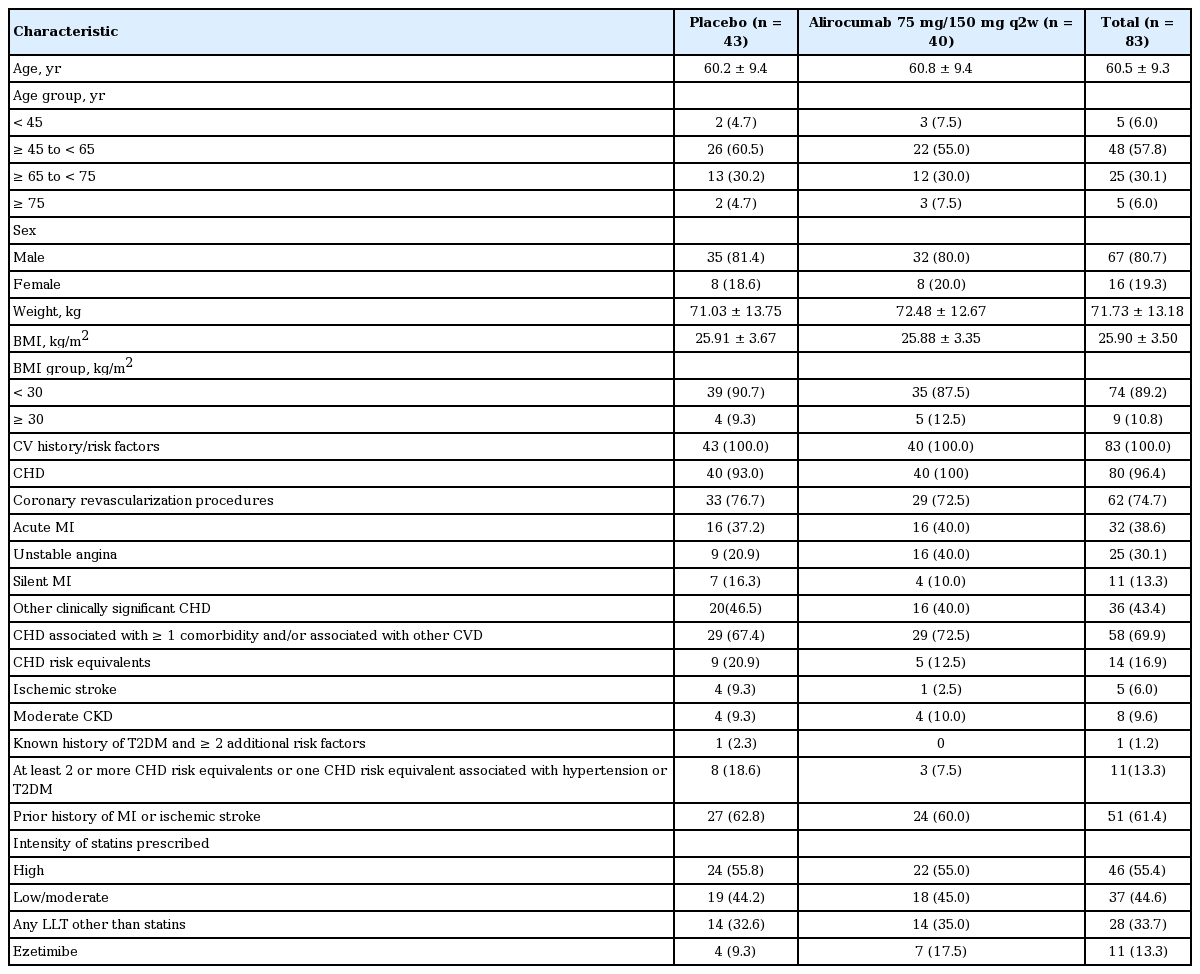
There was a reduction in LDL-C levels at week 4 and it was maintained till week 24. The calculated least squares mean (LSM) ± standard error (SE) of LDL-C levels in the ITT population receiving alirocumab decreased from 90.7 ± 3.6 mg/dL at baseline to 33.8 ± 4.5 mg/dL at week 24 (Fig. 2). In comparison, LSM ± SE of LDL-C levels increased from 95.1 ± 4.1 at baseline to 102.9 ± 4.4 at week 24 in patients receiving placebo. The percent change in LSM ± SE of LDL-C from baseline to week 24 was –65.7% ± 4.4% in the alirocumab group in comparison with 11.1% ± 4.2%) in the placebo group. The difference in LSM ± SE for alirocumab versus placebo was –76.7% ± 6.1% (95% CI, –88.9 to –64.6; p < 0.0001) (Table 3 and Fig. 3). Similarly, the percent change in LDL-C from baseline for the mITT population given as LSM ± SE was –67.3% ± 4.4% in the alirocumab group in comparison with 11.1% ± 4.2% in the placebo group and the difference in LSM ± SE for alirocumab versus placebo was –78.5% ± 6.1% (95% CI, –90.6 to –66.3; p < 0.0001) (Fig. 4).

Calculated low density lipoprotein cholesterol levels (least-squares mean ± standard error) in intent-to-treat population during treatment phase. q2w, every 2 weeks.

Least squares mean differences (vs. placebo) in LDL-C and secondary lipid parameters in patients of alirocumab group in intent-to-treat population
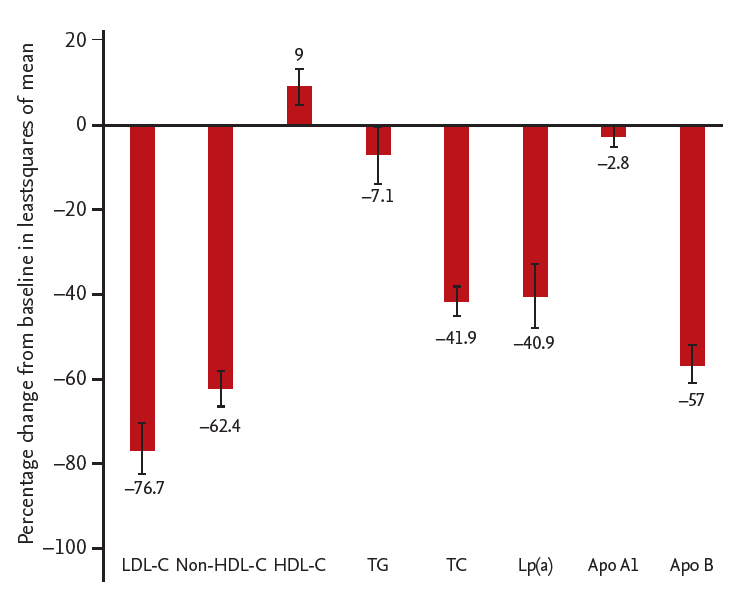
Least squares mean difference (vs. placebo) in low density lipoprotein cholesterol (LDL-C) and secondary lipid parameters in patients in the alirocumab group in intentto-treat population. LDL-C levels given were calculated values. Triglyceride levels were ascertained under fasting conditions. HDL-C, high density lipoprotein cholesterol; TG, triglyceride; TC, total cholesterol; Lp(a), lipoprotein(a); Apo A1, apolipoprotein A1; Apo B, apolipoprotein B.
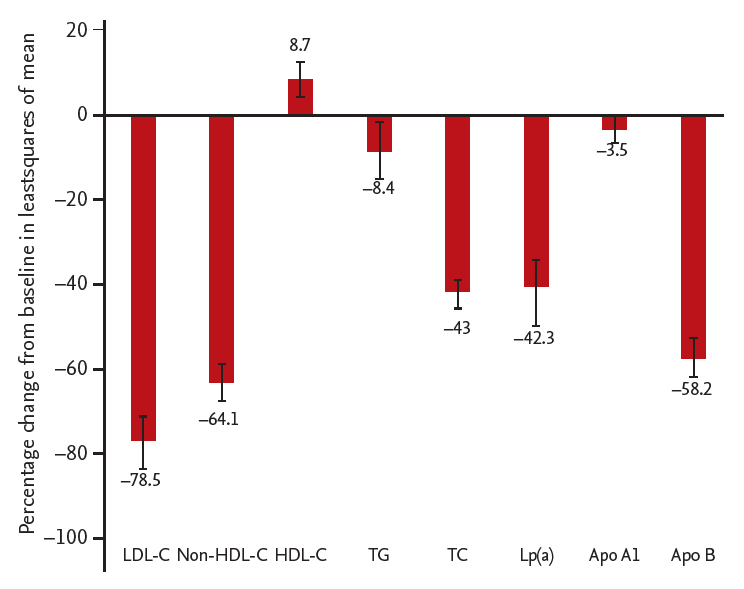
Least squares mean difference (vs. placebo) in low density lipoprotein cholesterol (LDL-C) and secondary lipid parameters in patients in the alirocumab group in modified intent-to-treat population. LDL-C levels given were calculated values. Triglyceride levels were ascertained under fasting conditions. HDL-C, high density lipoprotein cholesterol; TG, triglyceride; TC, total cholesterol; Lp(a), lipoprotein(a); Apo A1, apolipoprotein A1; Apo B, apolipoprotein B.
Significant differences in LSM ± SE for alirocumab versus placebo were observed at week 24 in the following lipid parameters: non-HDL-C (–62.4% ± 4.5%; 95% CI, –71.4 to –53.4; p < 0.0001), HDL-C (9.0% ± 4.2%; 95% CI, 0.7 to 17.4; p = 0.034), TC (–41.9% ± 3.4%; 95% CI, –48.6 to –35.3; p < 0.0001), Lp(a) (–40.9% ± 7.7%; 95% CI, –56.1 to –25.7; p < 0.0001), and ApoB (–57.0% ± 4.5%; 95% CI, –66.0 to –48.1; p < 0.0001) (Table 3 and Fig. 3). The same trend was found in mITT comparisons (Supplementary Table 2).
The proportion of patients achieving LDL-C < 70 mg/dL at week 24 was 92.0% in the alirocumab group and 12.7% in the placebo group (combined estimate for odds ratio, 157.4; 95% CI, 19.0 to 1,306.7; p < 0.0001). Three alirocumab-treated patients (7.5%) had LDL-C values ≥ 70 mg/dL at week 8 and were uptitrated to alirocumab 150 mg every 2 weeks at week 12. During the treatment period, 15 alirocumab-treated patients (37.5%) had two consecutive calculated LDL-C values < 25 mg/dL, among whom four patients (10.0%) displayed two consecutive LDL-C values < 15 mg/dL.
Safety analysis
A total of 18 patients (45.0%) in the alirocumab group and 22 patients (51.2%) in the placebo group experienced a TEAE. The most commonly reported TEAEs in the alirocumab group by system organ class (SOC) and preferred term (PT) classification were nervous system disorders (n = 5, 12.5%), majority of which were headaches (n = 4, 10.0%); followed by infections and infestations (n = 4, 10.0%), majority of which were nasopharyngitis (n = 3, 7.5%); and injury, poisoning, and procedural complications (n = 4, 10.0%), majority of which were due to fall (n = 2, 5.0%) (Table 4). The most commonly reported TEAEs in placebo group by SOC and PT classification were infections and infestations (n = 6, 14.0%), followed by gastrointestinal disorders (n = 5, 11.6%) and general disorders and administration site conditions (n = 5, 11.6%) (Table 4). Among alirocumab-treated patients who had two consecutive calculated LDL-C < 25 mg/dL (n = 15), one case of hepatobiliary disorders (hepatitis acute) and one case of injury, poisoning and procedural complications (fall and radius fracture for the same case) were identified.
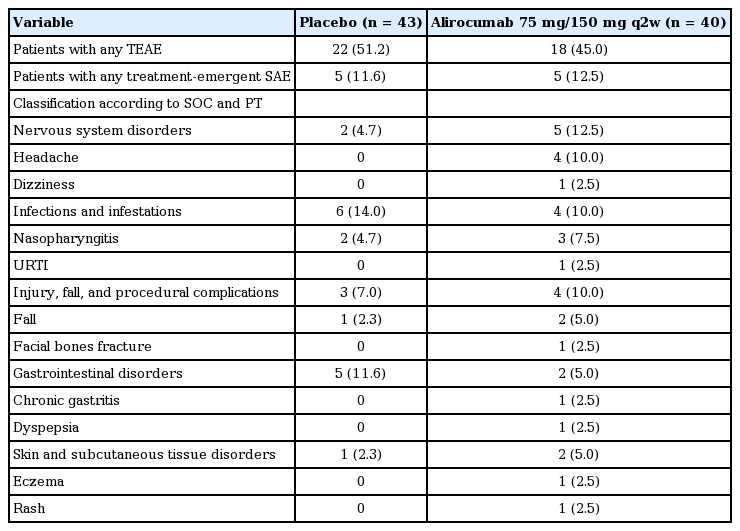
Adverse events and laboratory evaluations in safety population (with incidence of ≥ 5.0% in alirocumab group)
Eight treatment-emergent SAEs were experienced by five patients (12.5%) from the alirocumab group. These SAEs were unstable angina, brachiocephalic artery stenosis, traumatic fracture, radius fracture (one patient each) and two ligament sprains and two muscle strains (one patient). Eight treatment-emergent SAEs were experienced by five patients (11.6%) from the placebo group. These SAEs were road traffic accident and arterial injury (one patient); acute myocardial infarction on two occasions (one patient); unstable angina and peripheral arterial disease (one patient); pulmonary tuberculosis (one patient) and coronary artery stenosis (one patient). None of the reported SAEs were deemed related to study drug by both the investigator and the sponsor. No treatment-related discontinuation or death was reported due to TEAEs in either group.
DISCUSSION
The ODYSSEY KT study was the first to evaluate the safety and efficacy of alirocumab 75 mg (with a provision to uptitrate the dose to 150 mg) every 2 weeks for 24 weeks in Korean patients with inadequately controlled hypercholesterolemia and at high risk of CVD. The main findings are as follows. (1) The percent change of LDL-C from baseline in the ITT population was –65.7% in the alirocumab group and the difference of LDL-C versus placebo was –76.7% (p < 0.0001). (2) Alirocumab treatment additionally improved lipid parameters including HDL-C, non-HDL-C, TC, Lp(a), and Apo B versus placebo in the Korean cohort (p < 0.05). (3) Alirocumab did not induce higher rates of TEAEs and SAEs than placebo. None of the eight treatment-emergent SAEs in the alirocumab group, were deemed related to alirocumab.
In recent years, improvements in laboratory lipid measurements through PCSK9 inhibition have revealed to be associated with better clinical prognosis. Evolocumab reduced the risk of CV events (composite of CV death, myocardial infarction, stroke, hospitalization for unstable angina, or coronary revascularization) by 15% at 3 years of follow-up in FOURIER clinical trial [23]. Most recently in ODYSSEY outcomes trial, alirocumab proved 15% reduction in CV events (composite of CHD death, non-fatal myocardial infarction, fatal or non-fatal ischemic stroke, or unstable angina requiring hospitalization) and appeared associated with 15% lower rate of all-cause death at 4 years of follow-up among patients with “recent acute coronary syndrome” defined as an index hospitalization for acute myocardial infarction or unstable angina 1 to 12 months before the enrollment [24]. In summary, it can be implicated that the improvements in LDL-C and related lipid parameters observed in current study might lead to clinical benefits in CV morbidity and mortality among Korean population. It requires further investigation.
There were several findings in the current Korean cohort that were unique relative to the entire ODYSSEY KT population [22]. The use of high-intensity statins was lower in the Korean cohort in comparison to the entire KT population (55.4% vs. 72.4%). In contrast, the use of LLTs other than statins were higher in the Korean cohort that in the study population (33.7% vs. 23.1%). Additionally, baseline Lp(a) and fasting TG levels were higher in the Korean cohort. Compared to the entire ODYSSEY KT study population, nominally larger percent change in LDL-C from baseline in the alirocumab group appeared at week 12 (–62.3% vs. –57.9%) and was maintained week 24 (–65.7% vs. –57.1%) in the Korean cohort. Similarly, among alirocumab group, the proportion of patients achieving LDL-C values < 70 mg/dL (92.0% vs. 85.8%) and the proportion of patients with two consecutive LDL-C values < 15 mg/dL (10.0% vs. 9.3%) or < 25 mg/dL (37.5% vs. 27.8%) were nominally higher in the Korean cohort. In short, LDL-C reduction through alirocumab treatment in Korean cohort appeared at least consistent with the results from whole ODYSSEY KT cohort. With regards to safety data, a smaller proportion of overall patients in the Korean cohort displayed TEAEs in comparison to the ODYSSEY KT study population (48.2% vs. 60.3%). Similarly, the incidence of TEAEs in the alirocumab-treated patients was lower in the Korean cohort in comparison to those in the ODYSSEY KT population (45.0% vs. 58.8%). In addition, neither death nor permanent discontinuation due to TEAE were observed in the Korean cohort, whereas one death (influenza A infection) and three permanent discontinuations due to TEAEs (one in placebo group and two in alirocumab group) were reported in the Taiwanese study population.
The results show that, with similar design and inclusion criteria, Korean patients exhibited consistent LDL-C reduction with previous ODYSSEY trials. In the ODYSSEY COMBO I and II studies, treatment with addon alirocumab to background statin therapy reduced LDL-C levels by at least 48.2% [14,17]. In the ODYSSEY OPTIONS I and II studies, add-on alirocumab to atorvastatin 20 or 40 mg resulted in a reduction of LDL-C levels of 44.1% and 54%, respectively [13], while add-on alirocumab to rosuvastatin 10 or 20 mg resulted in a reduction of LDL-C levels of 50.6% and 36.3%, respectively [15]. In patients with heterozygous familial hypercholesterolemia (heFH), alirocumab treatment reduced LDL-C levels by at least 51.4% [16]. In patients with statin intolerance, alirocumab treatment reduced LDL-C levels by 45% [18]. Another study reported that 150 mg of alirocumab every 2 weeks induced –62.0% of change in LDL-C level at week 24 from baseline [19]. Considering that only three out of 40 patients were uptitrated to 150 mg in Korean cohort, it might suggest that 75 mg every 2 weeks is enough for the majority of Korean dyslipidemic patients. In studies on Japanese patients (with or without heFH), alirocumab treatment reduced LDL-C levels by at least 40% [20,21]. Similar to the results observed in this Korean population, a pooled analysis of eight ODYSSEY phase 3 trials (n = 4,629) indicated that alirocumab provided consistent reductions in LDL-C regardless of the statin dose and type [25]. In the ODYSSEY LONG TERM trial (n = 2,341), TEAEs were observed in 81% of alirocumab-treated patients [19], which is more than that observed in the Korean cohort (45%). The most common TEAEs in the ODYSSEY LONG TERM trial of alirocumab-treated patients were general allergic reactions (10.1%) and local injection-site reactions (5.9%) [19], while the most common TEAEs in the Korean cohort were headache (10.0%) and nasopharyngitis (7.5%) (Table 4).
While the patients in the ODYSSEY KT study were receiving background statin therapy, it is important to note that high-dose statins are known to upregulate PCSK9 expression [26] and could therefore interfere with PCSK9 inhibitors.
Although several reports suggested that low-to-moderate dose statins are sufficient in Korean population [27,28], it still appears to be inadequate in achieving the goal of LDL-C < 70 mg/dL in patients with very high CV risk [6-8]. Further investigations are needed to assess the necessity of more aggressive treatment policy including combination of alirocumab for Korean dyslipidemic patients.
There are several limitations to this study. The relatively small number of patients (n = 40) in the alirocumab group may not be representative of the relevant Korean population. There was a 4:1 ratio of males to females in the study, and very few patients were < 45 or > 75 years. As this study recruited patients already on statins or other LLTs, it is not known how the Korean population would respond to alirocumab as monotherapy. The small number of patients and the limited trial period may be insufficient to capture all relevant AEs.
In conclusion, this study demonstrated that alirocumab showed significantly greater efficacy in reduction of LDL-C levels, as well as improvements in HDL-C, non-HDL-C, TC, Lp(a), and Apo B than placebo in Korean patients. In addition, alirocumab was generally well tolerated. These results indicate that Korean dyslipidemic patients could achieve higher efficacy in LDL-C reduction with alirocumab augmentation than with conventional LLTs only.
KEY MESSAGE
1. Alirocumab add on treatment can make an additional low density lipoprotein cholesterol level reduction up to 65% to 75% compared with statin only therapy.
2. Alirocumab treatment additionally improved lipid parameters including high density lipoprotein cholesterol (HDL-C), non-HDL-C, total cholesterol, lipoprotein(a), and apolipoprotein B versus placebo in the Korean patients.
3. Although alirocumab showed stronger effect on lipid modification than placebo, alirocumab did not induce higher rates of treatment-emergent adverse events and serious adverse events than statin only therapy.
Notes
This study was funded by Sanofi and Regeneron Pharmaceuticals, Inc.
Acknowledgements
This study was supported by Sanofi and Regeneron in data collection and analysis, assistance with statistical analysis, and critical review of the article. Medical writing and editorial support in the preparation of this publication was provided by Satyendra Shenoy from Describe Scientific Writing & Communications and Derek Ho who were paid for by Sanofi and Anahita Gouri and Rohan Mitra from Sanofi. The authors, individually and collectively, are responsible for all content and editorial decisions. Chang-Wook Nam, Dong-Soo Kim, Chong-Jin Kim received payment from neither Sanofi nor Regeneron Pharmaceuticals, Inc. directly or indirectly (through a third party) related to the development/presentation of this publication.