Low parathyroid hormone level predicts infection-related mortality in incident dialysis patients: a prospective cohort study
Article information
Abstract
Background/Aims
Parathyroid hormone (PTH) is an important factor influencing immunologic dysfunction, but the effect of PTH level on infection-related outcomes remains unclear in incident dialysis.
Methods
We evaluated a multicenter prospective cohort study of 1,771 incident dialysis patients (1,260 hemodialysis and 511 peritoneal dialysis) in Korea. Patients were divided into three groups based on serum intact PTH (iPTH) level. The primary outcomes were all-cause and infection-related mortality and multivariate Cox regression analysis was performed to evaluate the role of iPTH in all-cause and infection-related mortality.
Results
During the follow-up period of 27.3 months, 175 patients (9.9%) died, and infection-related death represented 20% of all-cause mortality. Both all-cause mortality and infection-related mortality rates (p < 0.001 and p = 0.003, by logrank) were markedly higher in patients with serum iPTH < 150 pg/mL than in the other groups. Multivariate Cox regression analysis revealed that patients with serum iPTH < 150 pg/mL remained at higher risk for infection-related mortality than patients in the target range of 150 ≤ iPTH < 300 pg/mL, after adjusting for confounding variables (hazard ratio [HR], 2.52; 95% confidence interval, 1.06 to 5.99; p = 0.04). The HR of infection-related mortality in patients with serum iPTH < 150 pg/mL was significantly higher in patients with low serum phosphorus, low Ca × P product, low serum alkaline phosphatase and those older than 65 years.
Conclusions
Low serum iPTH level is an independent predictor of infection-related mortality in incident dialysis patients.
INTRODUCTION
A link between intact parathyroid hormone (iPTH) and mortality has been investigated as a biomarker of chronic kidney disease–mineral bone disorder (CKD-MBD) for decades. Most epidemiologic studies reported that higher serum iPTH level is associated with increased all-cause mortality in dialysis patients [1-3]. However, the impact of iPTH level on mortality is controversial in dialysis patients [4-6].
Infectious disease is the second most common cause of mortality in CKD patients [7]. Renal dysfunction is accompanied by disturbances of the immune system and subsequent susceptibility to infections [8]. PTH receptors are located in circulating human lymphocytes and are known as immunoregulatory factors [9]. Thus, PTH may be an important factor influencing immunologic dysfunction, but the roles of PTH in the immune system in dialysis patients remain unclear.
An improved understanding of the role of PTH in infection could lead to identification of preventive and therapeutic targets of CKD-MBD for infection control in dialysis patients. However, there are insufficient data to identify the relationships between infection-related outcomes and serum iPTH level in dialysis patients. Therefore, we performed this study to determine whether serum iPTH level is associated with infection-related outcome in incident dialysis patients through a multicenter prospective observational cohort study in Korea.
METHODS
Study design
All patients in this study participated in the Clinical Research Center for End Stage Renal Disease (CRC registry for ESRD). The CRC registry for ESRD is an observational prospective cohort performed in patients with ESRD from 31 medical centers in Korea (Supplementary Material 1). This cohort study started in April 2009 and ended in July 2015. A total of 2,208 incident dialysis patients were enrolled during this period. Incident dialysis patients were defined as newly diagnosed ESRD patients within 3 months of initiating hemodialysis (HD) or peritoneal dialysis (PD). We excluded patients for whom information about the level of iPTH at the time of enrollment was not available (n = 437). A total of 1,260 patients (71.1%) with HD and 511 patients (28.9%) with PD were included in the final analysis. Although Korean working group recommendations for management of CKD-MBD were recently established, most dialysis patients are managed with therapeutic interventions for CKD-MBD parameters according to Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines in Korea [10]. Therefore, we categorized enrolled patients into three groups based on serum iPTH level at baseline as follows [11]: serum iPTH < 150 pg/mL (low), 150 ≤ serum iPTH < 300 pg/mL (target range), and serum iPTH ≥ 300 pg/mL (high). Fig. 1 shows the design and flowchart of participants in this study. Our research protocol was approved by the Institutional Review Board at each center (KC16ONME0595) and performed in accordance with the Declaration of Helsinki. All participants provided informed consent.
Clinical data acquisition
Demographic and clinical data were collected at the time of study enrollment. Baseline demographic and clinical data were collected from clinical charts and medical histories. Age, sex, body mass index (BMI), causes of ESRD, incidence of diabetes mellitus (DM), hypertension, coronary artery diseases (CAD), malignancy and human immunodeficiency virus (HIV) infection, type of vascular access in HD, type of PD, systolic and diastolic blood pressures (BP), and laboratory investigations were recorded. Blood samples were collected at the time of pre-dialysis in HD and at visit of outpatient clinic in PD from enrolled participants who fasted overnight. Laboratory data were available for hemoglobin, white blood cell (WBC) count, blood urea nitrogen, creatinine, albumin, sodium, potassium, corrected calcium, phosphorus, alkaline phosphatase (ALP), iPTH, total cholesterol, and high-sensitivity C-reactive protein (hs-CRP) from blood samples. The modified Charlson comorbidity index was recorded at the time of dialysis. Each patient’s nutrient status was assessed by subjective global assessment (SGA). Serum iPTH level was measured using a radioimmunoassay kit. Serum calcium level was corrected for albumin concentration using the following formula: corrected calcium = measured calcium + (4 − measured albumin) × 0.8.
Clinical outcomes
The primary outcome measures were all-cause and infection-related mortality. All participants were followed until death or the research was terminated, with data censored when a patient underwent renal transplantation or was lost to follow-up because of patient refusal of further participation or transfer to a nonparticipating hospital. Deaths that occurred during the study were reported to study investigators, who identified causes of death according to the research classification system at each clinical center.
Statistical analysis
Continuous variables were presented as the mean ± standard deviation, and categorical variables were presented as number with percentage. Differences among groups for categorical variables were analyzed by Pearson’s chisquare test. Continuous data were compared using oneway analysis of variance to detect differences among the three groups. Absolute mortality rate was presented per 100 person-years of follow up. Survival curves for serum iPTH level were estimated by Kaplan-Meier method, and significance of the survival curve was assessed by log rank test. Hazard ratios (HRs) with 95% confidence intervals (CIs) for all-cause and infection-related mortality were calculated by Cox proportional hazard regression analysis. The Cox proportional hazard regression models were adjusted for known CKD-MBD parameters based on prior studies and clinical insight and significant (p < 0.1) predictors for all-cause and infection-related mortality in univariate Cox regression analysis including age, sex, DM, CAD, malignancy, HIV infection, BMI, systolic BP, diastolic BP, modified Charlson comorbidity index, hemoglobin, creatinine, albumin, potassium, corrected calcium, phosphorus, alkaline phosphatase, hs-CRP, and SGA. All statistical analyses were performed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA). A p < 0.05 was considered to indicate statistical significance.
RESULTS
Patient characteristics
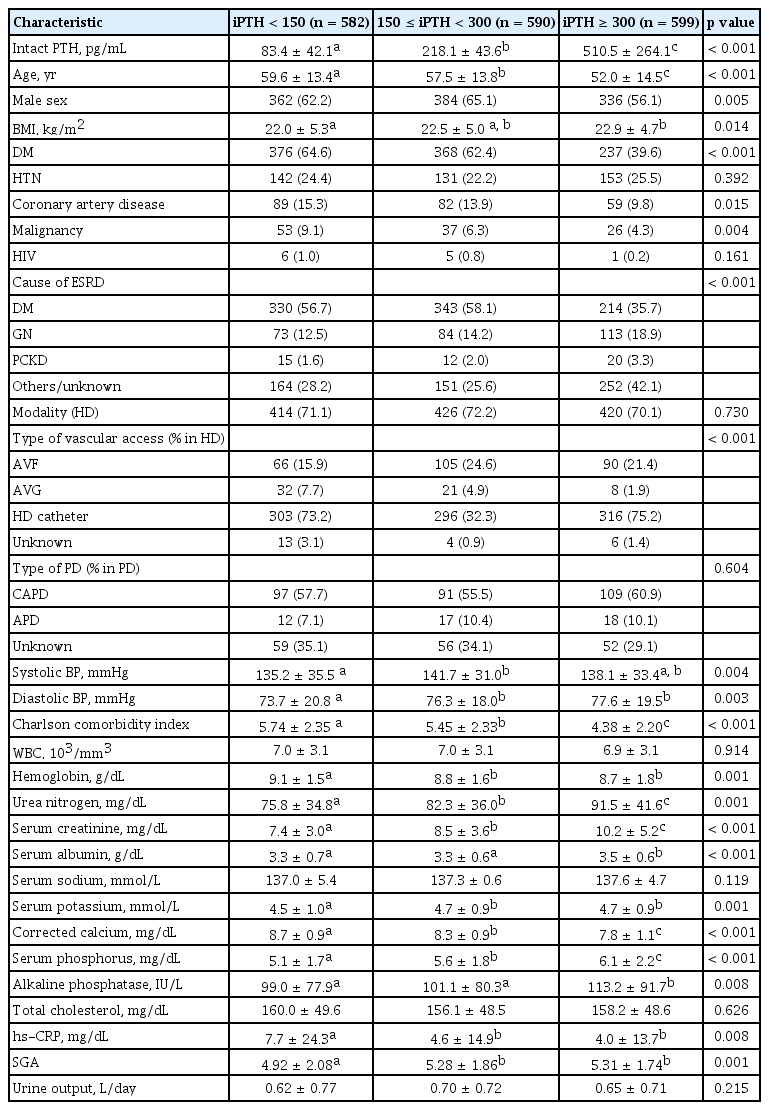
The clinical and laboratory characteristics of participants are described in Table 1. The mean age of enrolled patients was 56.3 ± 14.3 years, and 61.1% were men. Patients with low serum iPTH level (serum iPTH < 150 pg/mL) were significantly older, had lower BMI, and were more likely to be male and to have DM, CAD, and malignancy than patients with high serum iPTH level (serum iPTH ≥ 300 pg/mL). Among causes of ESRD, diabetic renal disease was more common in patients with low serum iPTH level than in those with high serum iPTH level. The prevalence of hypertension and HIV infection, WBC, serum sodium, and total cholesterol levels were not significantly different among the three groups. Patients with low serum iPTH level showed significantly lower serum creatinine, serum albumin, serum potassium, serum phosphorus, ALP, and nutritional status compared to patients with high serum iPTH level. Patients with low serum iPTH level had higher Charlson comorbidity index, hemoglobin, corrected calcium, and hs-CRP than patients with high serum iPTH level. The CKD-MBD parameters were assessed to identify the difference of distribution between HD and PD (Table 2). Serum phosphorus, calcium × phosphorus (Ca × P) product, and serum iPTH level did not significantly differ between HD and PD patients, and the proportion of patients with corrected calcium level < 8.4 mg/dL in HD increased more than in PD (p = 0.036) (Table 2).
Relationship between serum iPTH level and allcause or infection-related mortality in incident dialysis patients
The median duration of follow-up was 27.3 months (interquartile range, 8 to 24). During the follow-up period, 723 patients were dropped out from the study for reasons other than death (40.8% of all patients). One hundred seventy-five deaths were recorded during the study period, and the absolute mortality rate of this study was 4.29 deaths per 100 person-years. The causes of death were cardiovascular disease including sudden cardiac death, myocardial infarction, congestive heart failure, and stroke (55/175, 31.4%), followed by infection (35/175, 20%), and other diseases caused by malignancy, liver failure, chronic obstructive lung disease, and others (85/175, 48.6%). All-cause and infection-related mortality were further increased in patients with low serum iPTH level compared to the other groups (p < 0.001 and p = 0.002). However, cardiovascular mortality was not significantly different among the three groups (p = 0.580) (Table 3). Kaplan-Meier curve of survival according to serum iPTH level at baseline is shown in Fig. 2. Patients with low serum iPTH level had poorer survival for both allcause and infection-related death than those with normal or high serum iPTH level (p < 0.001 and p = 0.003, respectively, by log rank). On closer inspection, all-cause mortality was considerably increased in patients with low serum iPTH level compared to those with high serum iPTH level (p < 0.001), but there was no significant difference between patients with low serum iPTH level and those with serum iPTH level within the target range (p = 0.086, by log rank). In contrast, infection-related mortality was substantially increased in patients with low serum iPTH level compared to both those with high or within the target range of serum iPTH level (p = 0.003 or p = 0.013, respectively, by log rank test) (Fig. 2). The causes of death by infection were respiratory infection including pneumonia (19/35, 54.3%), sepsis of unknown origin (11/35, 31.4%), gastrointestinal infection (2/35, 5.8%), and other causes (3/35, 8.7%).
Effects of serum iPTH level on all-cause and infection-related mortality
Table 4 shows univariable and multivariable Cox hazard regression analyses for all-cause and infection-related mortality according to serum iPTH level at baseline. In univariate Cox regression analysis, the HR for all-cause mortality of patients with low serum iPTH level was 1.34 (95% CI, 0.96 to 1.87; p = 0.09), and that of patients with high serum iPTH level was 0.53 (95% CI, 0.35 to 0.81; p = 0.003) when patients with target serum iPTH level (150 to 300 pg/mL) were used as the reference category. The HR for infection-related mortality in a crude model of patients with low serum iPTH level was 2.61 (95% CI, 1.16 to 5.89; p = 0.02), and that of patients with high serum iPTH level was 0.73 (95% CI, 0.25 to 2.10; p = 0.55) when patients with target serum iPTH level (150 to 300 pg/mL) were used as the reference category.
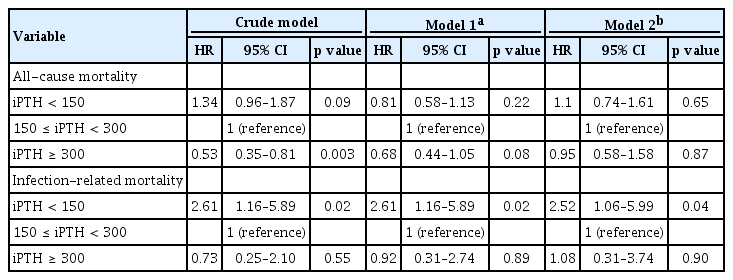
Univariable and multivariable Cox hazard regression analyses for all-cause and infection-related mortality according to the levels of serum iPTH levels
In the multivariable analysis, high or low serum iPTH level was not associated with increased all-cause mortality in model 1 (serum iPTH < 150 pg/mL [HR, 0.81; 95% CI, 0.58 to 1.13; p = 0.22], serum iPTH > 300 pg/mL [HR, 0.68; 95% CI, 0.44 to 1.05; p = 0.08]) or model 2 (serum iPTH < 150 pg/mL [HR, 1.1; 95% CI, 0.74 to 1.61; p = 0.65], serum iPTH > 300 pg/mL [HR, 0.95; 95% CI, 0.58 to 1.58; p = 0.87]) after adjustment for age, sex, DM, CAD, malignancy, HIV infection, BMI, systolic BP, diastolic BP, modified Charlson comorbidity index, hemoglobin, creatinine, albumin, potassium, corrected calcium, phosphorus, alkaline phosphatase, hs-CRP, and SGA. However, multivariate Cox regression analysis showed that patients with serum iPTH < 150 pg/mL remained at higher risk for infection-related mortality than those within the target range of serum iPTH after adjusting for confounding variables (model 1 [serum iPTH < 150 pg/mL: HR, 2.61; 95% CI, 1.16 to 5.89; p = 0.02; serum iPTH > 300 pg/mL: HR, 0.92; 95% CI, 0.31 to 2.74; p = 0.89] and model 2 [serum iPTH < 150 pg/mL: HR, 2.52; 95% CI, 1.06 to 5.99; p = 0.04; serum iPTH > 300 pg/mL: HR, 1.08; 95% CI, 0.31 to 3.74; p = 0.90]) after adjustment for age, sex, DM, CAD, malignancy, HIV infection, BMI, systolic BP, diastolic BP, modified Charlson comorbidity index, hemoglobin, creatinine, albumin, potassium, corrected calcium, phosphorus, alkaline phosphatase, hs-CRP, and SGA.
Subgroup analysis of infection-related mortality by risk factors according to serum iPTH level
The results of subgroup analysis exploring associations between serum iPTH level and infection-related mortality in various subgroups of patients are displayed in Fig. 3. In subgroup analysis, there were no significant interactions between low serum iPTH level and DM, serum albumin, hs-CRP, SGA, and comorbidity index in infection-related mortality. However, there was a tendency for significant interactions to exist between low serum iPTH level and age (< 65 or ≥ 65 year), serum phosphorus (P < 3.5 or P ≥ 3.5 mg/dL), Ca × P product (Ca × P < 55 or Ca × P ≥ 55), and serum ALP (ALP < 100 IU or ALP ≥ 100). The HR of infection-related mortality in the low serum iPTH group was significantly higher in patients with lower serum phosphorus, Ca × P product, and serum ALP and was substantially higher in patients older than 65 years compared with those 65 years and younger.
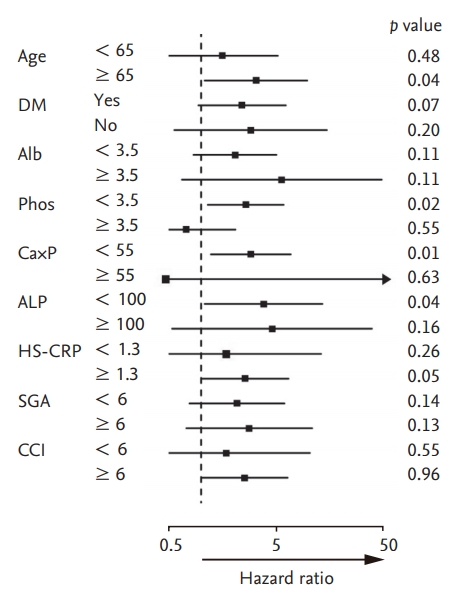
Hazard ratio (95% confidence interval) for infection-related mortality associated with lower serum intact parathyroid hormone (iPTH) levels (< 150 pg/mL) in subgroups of dialysis patients. DM, diabetes mellitus; Alb, albumin; Phos, phsophorus; Ca×P, calcium × phosphorus product; ALP, alkaline phosphatase; hs-CRP, high sensitive C-reactive protein; SGA, subjective global assessment; CCI, Charlson comorbidity index.
DISCUSSION
The results of our study clearly demonstrated that low iPTH level was associated with increased risk of allcause and infection-related mortality in incident dialysis patients. Infection-related mortality was especially increased in patients with low serum iPTH level even after adjustment of confounding factors. These findings suggest that low serum iPTH level is an independent risk factor for infection-related mortality in incident dialysis patients.
The relationship between serum iPTH level and allcause mortality in dialysis patients is still controversial. Most epidemiologic studies have suggested that the association between iPTH and all-cause mortality in dialysis patients is U-shaped [12-15]. Some other researches indicate that there is no correlation between serum iPTH level and mortality risk [1,4,16]. In the present study, low serum iPTH level (serum iPTH < 150 pg/mL) was associated with highest mortality rates, and high serum iPTH level (serum iPTH ≥ 300 pg/mL) was associated with lowest mortality rates in incident dialysis patients. This discrepancy between the current study and previous studies may be due to differences in study design, follow-up period, and timing of iPTH measurement.
Our observations are of importance as this is the first prospective observational study about infection-related outcomes of incident dialysis patients according to serum iPTH level in the Korean population. To date, no epidemiologic study about the relationship between serum iPTH level and infection-related mortality has been published. Although the follow-up period was short, enrolled participants formed a relatively homogenous group within 3 months of starting dialysis. Thus, it seems that our study group is more suitable to evaluate the impact of low iPTH level on infection in dialysis patients. Another interesting finding of this study is that patients with low serum iPTH level had poorer survival in infection-related death than those with serum iPTH level in the target range, whereas survival rates for all-cause death were not different between patients with low serum iPTH level and those with target range of serum iPTH level, as shown in Table 4, Fig. 1. This finding means that serum iPTH level at the time of starting dialysis is more meaningful in infection-related mortality than all-cause mortality. Further long-term study is needed to evaluate the impact of serum iPTH level on infection- related mortality in dialysis patients.
The results of our study suggest that low serum iPTH level increases the susceptibility of dialysis patients to infection, but the pathophysiologic mechanisms are not well established. For this, we speculate possible mechanisms. First, PTH had a stimulatory function on T lymphocytes, and low iPTH might be associated with decrease in cellular immunity. Previous studies demonstrated that iPTH stimulated proliferation of T lymphocytes and increased interleukin-2 (IL-2) production in human peripheral blood mononuclear cells (PBMCs) and rat PBMCs in a 5/6 nephrectomy model, and PTH inactivation by treatment of acetic acid or parathyroidectomy abolished T lymphocyte stimulation [17,18]. Furthermore, human CD4/CD8 ratio was significantly lower in prevalent HD patients with serum iPTH level below 65 pg/mL than in those with PTH level greater than 300 pg/mL, suggesting an immunosuppressive status associated with low serum iPTH [19]. Second, low serum iPTH level is related to malnutrition-inflammation-complex syndrome (MICS) [20]. Low serum iPTH level reflects a state of malnutrition, which may increase infection risk [21], and inflammation suppresses PTH secretion, and low PTH induces inflammation [22,23]. One long-term observational study reported that PTH is directly correlated with serum albumin, serum creatinine, and serum phosphorus and was correlated inversely with age and serum calcium [6]. In our study, low serum iPTH level combined with serum phosphorus level lower than 3.5 g/dL or with Ca × P product lower than 55 was related to significantly increased infection-related mortality in subgroup analysis. Among the biochemical parameters of CKD-MBD, hypophosphatemia reflects poor nutritional status and has been reported as an independent predictor for all-cause mortality in dialysis patients [13,15,24]. Therefore, low serum iPTH level combined with low serum phosphorus is a strong indicator of malnutrition and may have a deleterious effect on infection in dialysis patients. This suggests that malnutrition has a strongly detrimental effect due to increased risk of infection in incident dialysis patients.
We also demonstrated that low serum iPTH level combined with serum ALP level lower than 100 IU/L was related to significantly increased infection-related mortality in subgroup analysis. Most epidemiologic studies showed that serum ALP level has a linear and incremental association with all-cause mortality in pre-dialysis and dialysis patients [25-27]. However, the relationship between serum ALP level and infection-related mortality has not been elucidated. Low serum ALP could also be indicative of low-turnover bone disease. Adynamic bone disease is characterized by markedly low bone turnover, reducing osteoanabolic stimulation caused by oversuppression of PTH. In clinical practice, the combination of low iPTH and low bone ALP levels may be suggestive of adynamic bone disease in lieu of histomorphometric analysis of bone biopsies [28]. One study suggested that adynamic bone disease is a surrogate marker of MICS and increased mortality associated with adynamic bone disease is caused by MICS [29]. Hence, clinicians should attend more carefully to controlling low serum iPTH level in high-risk patients, such as patients with hypophosphatemia, low Ca × P product, and low serum ALP level.
Immune dysfunction is a characteristic of advanced CKD, and infection is a frequent cause of mortality in ESRD. Mortality rates due to sepsis in dialysis patients are several hundred times higher than those observed in the general population [7]. Traditionally, uremic toxins, comorbidity conditions, anemia, and malnutrition are responsible for innate and adaptive immune dysfunction in CKD [30,31]. However, recent studies have also highlighted implications for biochemical parameters of CKD-MBD regarding infection that extend beyond mineral homeostasis and bone metabolism. An observational study, the Dialysis Outcomes and Practice Patterns Study (DOPPS), found that elevated serum ALP level was associated with higher risk of infection-related death in HD patients, independent of calcium, phosphorus, and iPTH levels [25]. Two recent multicenter prospective studies in Korea also showed that elevated serum ALP level and low serum phosphorus level were independently associated with higher risk of infection-related mortality in dialysis patients [32,33]. To the best of our knowledge, the present study is the first multicenter prospective study showing significant associations between serum iPTH level and mortality risk of infection, rather than cardiovascular disease, in incident dialysis patients.
There are some limitations of the present study. First, we did not analyze the impacts of therapeutic interventions for CKD-MBD on infection-related mortality. Second, we did not assess other biochemical markers related to CKD-MBD, such as fibroblast growth factor-23 or vitamin D metabolites. Third, we cannot exclude residual confounding given the observational study design. Fourth, we did not perform bone biopsy and so cannot determine whether low-turnover bone disease itself increased the infection-related mortality in the low serum iPTH level. Last, the number of patients who were dropped out from the study for reasons other than death was relatively high. This may be due to the high percentage of patients with ‘transfer to a nonparticipating hospital (n = 282, 39.0%)’ and ‘kidney transplantation (n = 125, 17.3%).’ In Korea, creation of vascular access and initiation of HD are performed at university hospitals or general hospitals, but maintenance HD is usually performed at private clinics. In addition, enrolled patients were incident dialysis patients, and a considerable number of patients received kidney transplantation during the follow up period. Despite these limitations, our study clearly demonstrates the clinical significance of low serum iPTH for infection-related mortality in incident dialysis patients.
In conclusion, this prospective cohort study found that low serum iPTH level was associated with increased risk of subsequent infection in incident dialysis patients. This association suggests that serum iPTH level is an independent risk factor for infection.
KEY MESSAGE
1. Low serum intact parathyroid hormone (iPTH) level is an independent risk factor for infection-related mortality in incident dialysis patients.
2. Infection-related mortality in the subgroup analysis of incident dialysis patients with low serum iPTH level was significantly high in patients with lower serum phosphorus, Ca × P product, and serum alkaline phosphatase and was substantially higher in patients older than 65 years compared with those 65 years and younger.
3. The pathophysiologic mechanisms between low serum iPTH level and infection susceptibility in dialysis patients can be suggested that low iPTH might be associated with decrease in cellular immunity and related to malnutrition-inflammation-complex syndrome.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HC15C1129). This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT, Republic of Korea (grant number: 2018R1C1B5045006).