Feasibility of primary percutaneous coronary intervention via the distal radial approach in patients with ST-elevation myocardial infarction
Article information
Abstract
Background/Aims
Recently, distal radial approach (DRA), called as snuffbox approach, has gained the interest of interventional cardiologists, but there is a lack of data about the feasibility of DRA as an alternative route for primary percutaneous coronary intervention (PCI).
Methods
A total of 138 patients presenting with ST-elevation myocardial infarction (STEMI) in whom primary PCI via the DRA was attempted at three hospitals from October 2017 to September 2019 were analyzed.
Results
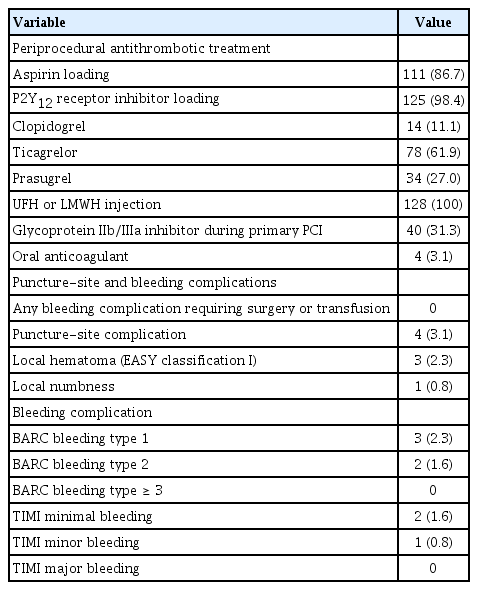
The success rate of snuffbox puncture in the setting of STEMI was 92.8% (128/138). Successful primary PCI via the DRA was achieved in all 128 patients. The snuffbox puncture time, defined as the time interval from local anesthesia induction to successful sheath cannulation, was 2.7 ± 1.6 minutes, and snuffbox puncture was performed within 5 minutes in 95.3% of patients. Moreover, the percentage of the puncture time in the door-to-balloon time was 3.3%. The left DRA was selected in 103 patients (80.5%), and primary PCI via the DRA was performed using a 6-Fr guiding catheter in 125 patients (97.7%). There was no major bleeding; however, there were four cases (3.1%) of access-site complications, including three cases of local hematoma (≤ 5 cm diameter) and one case of local numbness, which improved 3 months later.
Conclusions
In the setting of STEMI, the DRA could be a feasible alternative access route for primary PCI.
INTRODUCTION
The radial approach for coronary angiography (CAG) and percutaneous coronary intervention (PCI) is preferred owing to several advantages, including reduced vascular complications, patient comfort, and early ambulation, when compared with the femoral approach [1-3]. Moreover, a recent largescale randomized trial demonstrated that the radial approach in patients with acute coronary syndrome was associated with a significantly lower incidence of cardiovascular mortality and major bleeding at 1 year compared with the femoral approach [4]. With these advantages, the radial approach is recommended as the standard approach for PCI procedures in most clinical settings [5]. The radial approach is also recommended over the femoral approach when an experienced radial operator performs primary PCI in patients with ST-elevation myocardial infarction (STEMI) [6].
Recently, the distal radial approach (DRA), called as the snuffbox approach, has gained the interest of interventional cardiologists. Data supporting the feasibility of the DRA for CAG and PCI have been published, demonstrating potential benefits over the conventional radial approach in terms of patient and operator comfort, shorter hemostasis duration, less bleeding, and access-site complications [7-9]. The DRA could be considered an alternative access route in selected patients, such as those with a high bleeding risk or renal impairment in whom the radial artery needs to be preserved for arteriovenous fistula creation. However, there is a lack of data about the DRA as an alternative route for primary PCI in patients with STEMI, who often need treatment with potent antithrombotic agents such as ticagrelor or prasugrel or the use of glycoprotein IIb/IIIa inhibitors. As a result, the feasibility of the DRA for primary PCI is still unclear. Therefore, this study aimed to investigate the feasibility of a DRA in patients with STEMI.
METHODS
Study population
A total of three experienced radial operators, defined as operators who perform at least 50% of all PCI procedures in patients with acute coronary syndrome via the radial approach, participated in this study [2]. The operators attempted the DRA in patients with STEMI who had a well-palpable pulse in the anatomical snuffbox area. We retrospectively collected data from patients with STEMI who were planned to undergo primary PCI via the DRA at three hospitals between October 2017 and September 2019. STEMI was defined as ST-segment elevation in at least two contiguous leads, with ischemic symptoms and elevated cardiac markers (creatine kinase-MB or troponin I/T). Primary PCI was defined as PCI in patients presenting within 12 hours of symptom onset or > 12 hours from symptom onset with clinical and/or electrocardiographic evidence of ongoing ischemia [6].
The study protocol was approved by the Institutional Review Board of each hospital (approval number: CNUH2019-185, CR319045, CBNUH-2019-06-010), which waived the requirement for informed consent owing to the retrospective observational study design.
Preparation for the DRA
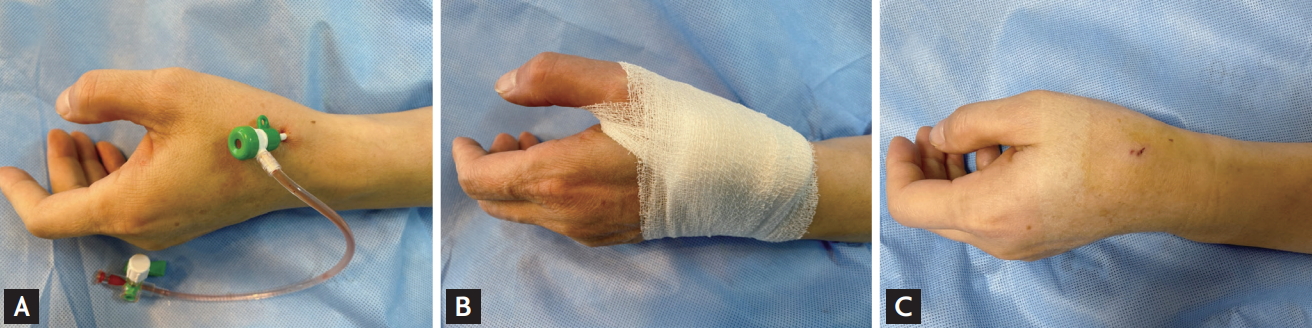
Local anesthesia on the anatomical snuffbox was achieved through lidocaine hydrochloride injection by using a 26-gauge needle. Thereafter, puncture was performed by using a 20-gauge 2-piece needle with the through-andthrough or anterior wall puncture technique, or a 21-gauge open needle with the anterior wall puncture technique. After a successful puncture, a 0.025-inch straight wire or a 0.018-inch hair wire was inserted, followed by insertion of the 5- or 6-Fr radial sheath (Prelude Radial, Merit Medical, South Jordan, UT, USA; or Radiofocus Introducer II, Terumo Corp., Tokyo, Japan) (Fig. 1A). The selection of the puncture device was left at the physician’s discretion. Hemostasis was obtained using a 4 × 4-inch sterile gauze dressing wrapped with cohesive elastic bandage for 3 hours, according to the method of each hospital (Fig. 1B). Access-site hematoma classified according to the Early Discharge After Transradial Stenting of Coronary Artery (EASY) trial classification and local hematoma defined as EASY classification I (≤ 5 cm diameter of hematoma) (Fig. 1C) [10].
Medical treatment and primary PCI procedure
Primary PCI was performed according to the current updated STEMI treatment guidelines. Patients who underwent primary PCI received a loading dose of aspirin (300 mg) and a P2Y12 receptor inhibitor (180 mg ticagrelor or 60 mg prasugrel or 300 to 600 mg clopidogrel) before the procedure if they were not previously taking these medications. The choice of medication including antiplatelet agents, the use of intravascular imaging guidance or glycoprotein IIb/IIIa inhibitors, and the need for stent implantation were determined according to the clinical decision of physicians. Unfractionated heparin (50 to 70 U/kg) was administered during the procedure to maintain the activated clotting time at 250 to 300 seconds.
Statistical analysis
All categorical variables were reported as numbers with percentages. All continuous variables were expressed as means with standard deviations or median with interquartile ranges, when appropriate. Statistical analysis was performed using SPSS version 22.0 for Windows (IBM Co., Armonk, NY, USA).
RESULTS
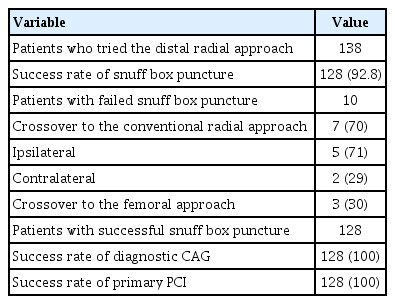
The DRA was attempted in 138 patients with STEMI during the study period. The success rate of snuffbox puncture was 92.8% (128/138). However, the success rate of primary PCI was 100% in all 128 patients in whom successful snuffbox puncture was achieved (Table 1).
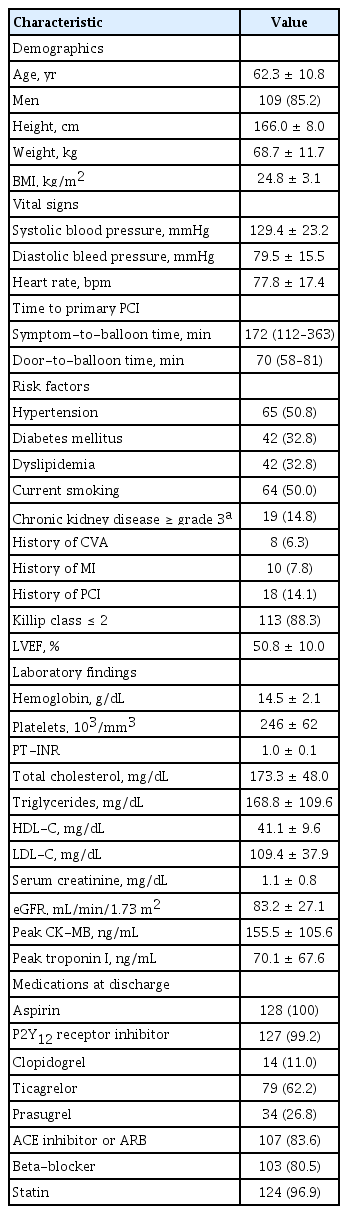
Table 2 summarizes the baseline characteristics of 128 patients who underwent successful primary PCI via the DRA. The average age of the patients was 62.3 ± 10.8 years, and 85.2% were men. The median symptom-to-balloon and door-to-balloon (D2B) times were 172 and 70 min, respectively. A total of 19 patients (14.8%) had ≥ grade 3 chronic kidney disease, defined as an estimated glomerular filtration rate of < 60 mL/min/1.73 m2. There were 113 patients (88.3%) with Killip classification ≤ 2, and the mean left ventricular ejection fraction was 50.8 ± 10.0%.
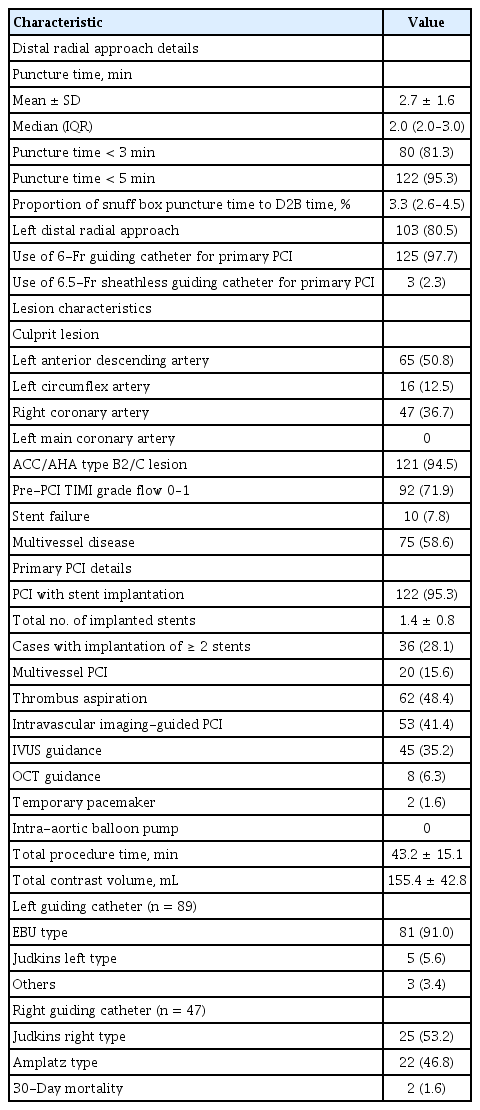
Procedural characteristics including the details of the DRA and 30-day mortality data are presented in Table 3. With respect to the details of the DRA, the mean and median times of snuffbox puncture, defined as the time interval from local anesthesia induction to successful sheath cannulation, were 2.7 ± 1.6 and 2.0 minutes (quartile 1 to 3: 2.0 to 3.0), respectively. Snuffbox puncture was done within 3 and 5 minutes in 81.3% (104/128) and 95.3% (122/128) of patients, respectively (Fig. 2). With respect to the safety of the DRA for primary PCI, the percentage of the puncture time in the D2B time was evaluated and the percentage was 3.3% (quartile 1 to 3: 2.6 to 4.5) (Fig. 3). The left DRA was selected in 103 patients (80.5%), and primary PCI via the DRA was performed using a 6-Fr guiding catheter in 125 patients (97.7%). In three patients (2.3%), 6.5-Fr sheathless guiding catheter was used. With respect to lesion characteristics, 50.8% (65/128) of culprit lesions were located in the left anterior descending artery and the proportion of type B2/C was 94.5% (121/128). Implantation of > 2 stents and multivessel PCI were performed in 36 (28.1%) and 20 patients (15.6%), respectively. Thrombus aspiration was needed in 62 patients (48.4%) and intravascular imaging-guided PCI was conducted in 53 patients (41.4%) (intravascular ultrasound in 45 and optical coherence tomography guidance in eight).
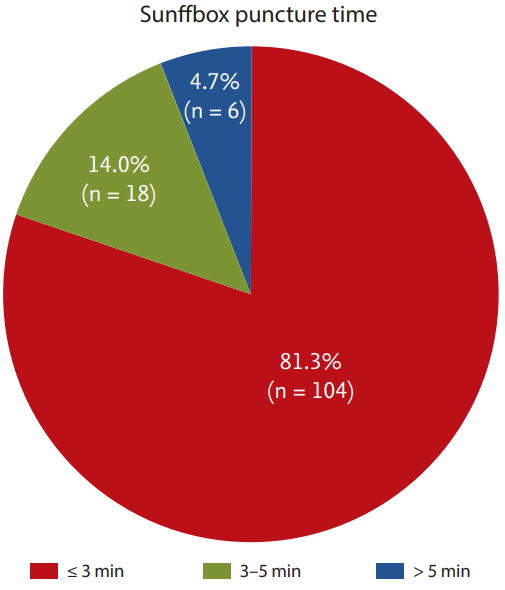
Distribution of the snuffbox puncture time in 128 patients who underwent a successful primary percutaneous coronary intervention via the distal radial approach (Snuffbox puncture time was defined as time interval from local anesthesia induction to successful sheath cannulation).

Percentage of the snuff box puncture time in the door-to-balloon time (Horizontal lines represent median with interquartile range).
During primary PCI, temporary pacemaker, requiring inguinal preparation, was inserted in two (1.6%), but there was no case of inserted intra-aortic balloon pump via the femoral approach. In terms of the guiding catheter for the procedure, the extra-backup-type catheter was used in 91 of 89 cases (91.0%) of left coronary artery lesion. Among 47 cases of right coronary artery lesion, Judkins right and Amplatz type catheters were used in 25 (53.2%) and 22 cases (46.8%), respectively. Concerning the clinical outcome, the overall incidence of death at 30 days was 2.0% (2/100), including two cases of in-hospital cardiac death.
With respect to puncture-site complications, local hematoma (EASY classification I) occurred in three patients (2.3%). One patient (0.8%) experienced puncture-related local numbness, but it improved after 3 months. There was no bleeding complication requiring surgery or transfusion. Furthermore, major bleeding, defined as Thrombolysis in Myocardial Infarction major bleeding or Bleeding Academic Research Consortium ≥ 3, was not observed (Table 4).
DISCUSSION
In this study on patients with STEMI undergoing primary PCI via the DRA, the success rate of snuffbox puncture was 92.8%. However, primary PCI via the DRA was successfully performed in all 128 patients. The snuffbox puncture time was 2.7 ± 1.6 minutes, and the percentage of the puncture time in the D2B time was 3.3%. The occurrence rate of puncture-site complications was low, and no major bleeding complication was observed. To our knowledge, this is the first study to report the feasibility of the DRA for primary PCI in the setting of STEMI.
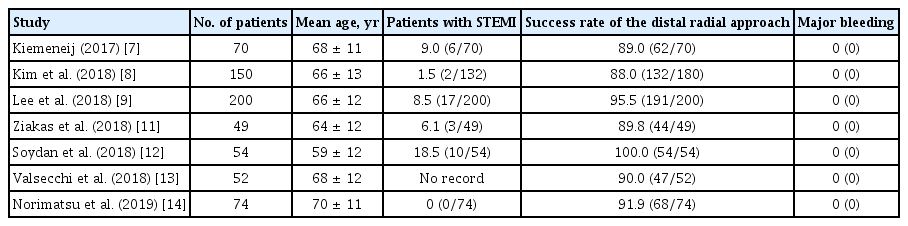
Recently, there have been several studies on the feasibility of the DRA as shown in Table 5. Kiemeneij [7] reported that the success rate of the DRA was 89.0% in 70 selected patients with a good pulsation of the distal radial artery. In our previous retrospective and prospective studies, the success rate was 88.0% (132/150) and 95.5% (191/200), respectively [8,9]. Several other studies have also reported an 89.8% to 100% success rate of the DRA [11-14]. However, these studies enrolled a small number of patients with STEMI. Considering the setting of STEMI, the success rate of the DRA (92.8%) in the present study may be acceptable. Interestingly, seven patients among 10 patients in whom the DRA failed were converted to the conventional radial approach. Even if the DRA failed, it could provide an opportunity for the conventional radial approach, which is recommended over the femoral approach in patients with acute myocardial infarction including STEMI, as described in the current guidelines [5,6]. With respect to the snuffbox puncture time, the mean puncture time in our study was 2.7 ± 1.6 minutes. In previous studies, snuffbox puncture time was checked 1.19 to 3.9 minutes [9,11,12]. Thus, the present study seemed to be acceptable snuffbox puncture time, but it is difficult to directly compare snuffbox puncture time between the present study and other studies as the definition of snuffbox puncture time was not suggested in previous studies. Further large-scale study of the DRA, having a unified definition, is needed to compare snuffbox puncture time in patients presenting with STEMI. Although there is some concern that this new puncture technique might delay the D2B time in patients presenting with STEMI, it was not observed in the present study. Moreover, the median percentage of the snuffbox puncture time in the D2B time was only 3.3% without delaying the D2B time to > 90 minutes. Given the snuffbox puncture success rate, the puncture time, and the effect of puncture time on the D2B time, the DRA might be feasible as an alternative access route in patients with STEMI.
As shown in Fig. 3, this study analyzed the percentage of the snuffbox puncture time in the D2B time according to the puncture time. A > 10% of the snuffbox puncture time in the D2B time was observed in two of 128 patients in whom the DRA was successfully used. On the other hand, the percentage was < 10% in all patients in whom a successful snuffbox puncture was achieved within 5 minutes. On the basis of our study results, delayed puncture (> 5 minutes) could be considered an indication of the need to change the puncture-site for the safety of patients.
Data on the DRA for primary PCI remains limited, and only a few cases have been reported [15,16]. Furthermore, several studies have demonstrated that the diameter of the distal radial artery is relatively smaller than that of the conventional radial artery, thus raising the concern about whether the DRA can be as feasible as the conventional radial approach [8,9,13,14,17]. In our study, successful primary PCI was performed without a change in access-site in all 128 patients in whom successful snuffbox puncture was achieved. In addition, the present study showed the feasibility of the DRA for implantation of ≥ 2 stents, multivessel PCI, and imaging-guided PCI in patients with STEMI, by using 6-Fr guiding catheters similar to those used in the conventional radial approach. The feasibility of this new approach, similar to that of the conventional radial approach, could be explained by the choice of the left DRA in most of the present study population. Although most radial operators prefer the right radial approach because of the working position and for the operator’s convenience, the higher incidence of right subclavian tortuosity results in a longer fluoroscopic time and more contrast use [18]. Accordingly, the left DRA can facilitate easier catheter manipulation and save time and contrast in emergency situations, owing to the less tortuous subclavian artery.
Several studies have demonstrated that major bleeding did not occur in patients who underwent CAG and PCI via the DRA [7-9,11-14,19]. In the present study, new P2Y12 receptor inhibitors, including ticagrelor and prasugrel, were loaded before primary PCI in about 90% of patients and unfractionated heparin was used during primary PCI in all 128 patients. Furthermore, a glycoprotein IIb/IIIa inhibitor was used during primary PCI in one-third of the patients. Despite the potent peri-procedural antithrombotic treatment, there were no cases of major bleeding complication requiring blood transfusion or surgical repair. This study reaffirms the potential benefit of the DRA in terms of bleeding complication, as demonstrated in previous studies. Therefore, the DRA could be considered an alternative access route in patients with a high bleeding risk or those in whom bleeding complications need to be minimized.
There were several limitations in the present study. First, this study has the inherent limitations of retrospective studies with a small sample size. In addition, operators tried to perform the DRA in patients with well-palpable distal radial artery. Therefore, selection bias might have influenced the results and our results should be carefully interpreted. Second, the feasibility of the DRA in the setting of STEMI was evaluated without a control group. Therefore, large multicenter randomized trials comparing the DRA with the conventional radial approach are needed in the future. Third, two different types of puncture needles were used for the DRA according to the operator’s preference, and this might have affected the success rate and the snuffbox puncture time. Fourth, although a reduction in the risk of distal and conventional radial artery occlusion is a potential benefit of the DRA, the occurrence rate of radial artery occlusion was not evaluated in this study.
In conclusion, the DRA for primary PCI in the setting of STEMI was feasible in terms of the puncture success rate, puncture time, and procedure success rate on the basis of our experience. Moreover, this new approach showed a low occurrence rate of bleeding complications even in patients treated with potent antithrombotic medications. Further large randomized control trials are needed to confirm the feasibility and safety of the DRA for primary PCI.
KEY MESSAGE
1. On the basis of retrospective data at three hospitals from October 2017 to September 2019, the success rate of the distal radial approach for primary percutaneous coronary intervention, was 92.8% in patients with ST-elevation myocardial infarction.
2. The snuff box puncture time, defined as the time interval from local anesthesia induction to successful sheath cannulation, was 2.7 ± 1.6 minutes, and snuffbox puncture was performed within 5 minutes in 95.3% of patients.
3. Among total of 128 study population, the occurrence rate of puncture-site complications was only 3.1% including three cases of local hematoma (≤ 5 cm diameter) and one case of local numbness, but no major bleeding complication was observed.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by a grant from the National Research Foundation of Korea (NRF-2019R1A2C3003547) and by a grant from Chonnam National University Hospital Biomedical Research Institute (BCRI18015 & BCRI19009).