Induction chemotherapy followed by concurrent chemoradiotherapy versus CCRT for locally advanced hypopharynx and base of tongue cancer
Article information
Abstract
Background/Aims
Clinical trials have not consistently supported the use of induction chemotherapy (IC) for locally advanced head and neck squamous cell cancer. Hypopharynx and base of tongue (BOT) cancer has shown relatively poor survival. We investigated the role of IC in improving outcome over current chemoradiotherapy (CRT) in patients with hypopharynx and BOT cancer.
Methods
Treatment-naïve patients with stage III/IV (M0) hypopharynx or BOT cancer were randomly assigned to receive CRT alone (CRT arm: cisplatin 100 mg/m2 on D1 3-weekly, two times plus radiotherapy 68.4 Gy/30 fractions on weekdays) versus two 21-day cycles of IC with TPF (docetaxel & cisplatin 75 mg/m2 on D1, and fluorouracil 75 mg/m2 on D1-4) followed by the same CRT regimen (IC arm). The primary endpoint was progression-free survival (PFS).
Results
This study closed early after enrollment of 36 patients (19 in the CRT arm, 17 in the IC arm). After a median follow-up of 47.2 months, there was no significant difference in PFS: the median PFS was 26.8 months for the CRT arm and was not reached for the IC arm (p = 0.13). However, the survival curves were widely separated with a plateau after 3 years, suggesting a potential survival benefit from IC: 3-year PFS rates were 45% and 68%, and 3-year overall survival rates were 56% and 86%, in the CRT and IC arms, respectively.
Conclusions
This study failed to demonstrate that induction TPF chemotherapy improves survival in patients with BOT and hypopharynx cancer. However, it suggested a favorable outcome with IC to this population.
INTRODUCTION
A role for induction chemotherapy (IC) in locally advanced squamous cell head and neck cancer (LASCC) has not been fully demonstrated. Two recent randomized phase III trials did not prove a survival benefit of IC compared to concurrent chemoradiotherapy (CRT) alone [1,2], however the failure of those studies could be attributed to methodological problems such as a, relatively low recruitment rate. Still, 40% to 60% of patients with LASCC experience locoregional or distant metastases after CRT [3] and the 3-year survival rate was less than 50% in a meta-analysis establishing CRT as the current standard of care [4]. Various studies to evaluate the efficacy and tolerability of proper combinations and/or schedules of IC have been tried. Among them, docetaxel (T) combined with cisplatin, and 5-fluorouracil (PF) (TPF regimen) showed superior survival compared with the PF regimen (cisplatin + 5-fluorouracil) [5-7].
We hypothesized that some subgroup populations could benefit from integrating IC into CRT alone. Previously, we confirmed that long-term outcome was significantly worse in patients with hypopharyngeal cancer compared to those with oropharyngeal cancer (3-year overall survival [OS] rate 52% vs. 75%, p = 0.001; 3-year progression-free survival [PFS] rate 42% vs. 72%, p < 0.001), although the overall response rate (ORR) after CRT was fairly good in both groups [8]. And another phase III trial comparing concurrent CRT versus IC followed by CRT reported a more pronounced benefit of IC with a TPF regimen in patients with non-oropharyngeal cancer compared to overall LASCC patients [9].
In the present study, we investigated whether IC with two cycles of TPF regimen before definitive CRT improved PFS in locally advanced (Stage III & IV [M0]) hypopharyngeal and base of tongue (BOT) cancer compared to definitive CRT alone.
METHODS
Patients and study design
This phase II randomized trial (NCT01312350) was conducted to test the efficacy of IC with a TPF regimen followed by concurrent chemoradiotherapy compared to CRT alone. Eligible patients were age ≥ 18 years with histologically confirmed stage III or IV (M0 disease) hypopharyngeal or BOT cancer according to the American Joint Cancer Committee (AJCC) staging classification 7th edition and had one or more measurable lesions. No previous chemotherapy, radiotherapy or surgery was allowed. Other key eligibility criteria included an Eastern Cooperative Oncology Group (ECOG) performance status of ≤ 2 and adequate bone marrow, renal and hepatic function. Patients with distant metastasis were excluded. All patients provided informed consent. This study was approved by the Institutional Review Board of Samsung Medical Center (IRB no.: 2010-10-028).
Study treatment
Patients were randomly assigned to receive either IC followed by CRT (IC arm) or CRT only (CRT arm). IC consisted of two cycles of TPF regimen (docetaxel and cisplatin at 75 mg/m2 each on day 1, and fluorouracil [5FU] at 750 mg/m2 on D1 to 4, every 3 weeks), followed by CRT 3 to 5 weeks after the completion of IC. CRT was administered with cisplatin 100 mg/m2 on day 1 every 3 weeks up to a total of two to three times. Patients received radiotherapy using three-dimensional conformal radiotherapy or intensity-modulated radiotherapy techniques delivered at doses of 63 to 72 Gy in 30 to 35 fractions in 2.0 to 2.4 Gy per fraction over 6 to 7 weeks. For IC + CRT treated patients, neck computed tomography (CT) and positron emission tomography (PET)-CT were performed to assess the response to IC before starting CRT 3 weeks after completion of the TPF chemotherapy. If a patient had disease progression after IC, they were was referred to a multidisciplinary head and neck tumor board for further treatment. After completion of CRT in both the IC and CRT arm, a neck CT was performed after 1month and then every 3 months for 1 year and every 6 months thereafter. PET-CT was performed after 4 months and repeated every 6 months for 2 years and every 2 years thereafter until disease progression or death. If patients showed disease progression during or after study treatment, further treatment could be administered at the discretion of the investigators.
Endpoints
The primary endpoint was PFS, defined as the time from randomization to progression, secondary cancer or death resulting from any cause. Secondary endpoints included ORRs, OS, and toxicity. OS was calculated from the time of randomization to the date of death. PFS was set to be primary endpoint due to a surrogate marker of OS. These endpoints were measured in all registered patients (i.e., the intention-to-treat population). Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) and adverse events (AEs) were assessed according to National Cancer Institute criteria (CTCAE v3).
Statistical analysis
Survival rates were estimated by the Kaplan-Meier method and compared between the two treatment groups using the log-rank test. The sample size for the trial was derived from following assumption. Given a one-tailed alpha of 0.1 and power of 0.85, the 1-year PFS rate would be 65% and 80% in the CRT arm and IC arm, respectively, with an accrual rate of 29 patients per year and an additional follow-up period of 1.5 years. A 10% ineligible or non-assessable rate was assumed, resulting in the accrual goal of 100 total patients (50 patients for each arm). The significance of statistical tests was evaluated based on a one-tailed alpha value of 10% for the primary endpoint and a two-tailed alpha value of 5% for the other endpoints. Statistical analyses were performed using SPSS version 21 (IBM Co., Armonk, NY, USA). This trial was closed early due to slow recruitment.
RESULTS
Study patients
Between December 2010 and April 2015, a total of 41 patients were screened. Five patients were excluded during the screening period. We randomly assigned 36 patients to receive IC followed by CRT or CRT only (Fig. 1). Patient baseline characteristics are shown in Table 1 and were well balanced between the two groups. About twothirds of all patients were diagnosed with hypopharyngeal cancer and the rest were BOT cancers. All had squamous cell carcinoma histology, 28 patients (78%) had N2 to N3 disease and approximately 80% of patients showed clinical stage IV (A/B) disease (AJCC 7th edition).

Flow chart of study schema. CRT, chemoradiotherapy; IC, induction chemotherapy; ITT, intention-to-treat.
Efficacy
At the time of data analysis (February 2017), a total of 15 patients (42%) had disease progression or recurrent disease after partial response or complete response to treatment. After a median follow-up of 47.2 months, 1-year estimated PFS rates were 63.2% (95% confidence interval [CI], 0.415 to 0.848) in the CRT group and 76.5% (95% CI, 0.563 to 0.966) in the IC group, respectively. The median PFS was 26.8 months in the CRT arm and not reached in the IC arm (p = 0.131) (Fig. 2A). Two-year OS rates were 72.2% (95% CI, 0.515 to 0.929) and 86.9% (95% CI, 0.698 to 1.040) in the CRT and IC arms, respectively. Three-years after randomization, the survival curves widely separated with a plateau: 3-year PFS estimates were 45% and 68% (hazard ratio [HR], 0.55; 95% CI, 0.19 to 1.60), and 3-year OS estimates were 56% and 86% (HR, 0.35; 95% CI, 0.07 to 1.69), in the CRT and IC arms, respectively (Fig. 2B).

Kaplan-Meier survival curves by treatment arm and corresponding p values. (A) Progression-free survival, (B) overall survival. IC, induction chemotherapy; CRT, chemoradiotherapy.
In 23 patients with hypopharynx cancer, IC tended to show more improvement in PFS compared to BOT cancer, although neither were statistically significant (p = 0.198 and p = 0.217) (Fig. 3). There was no significant difference in the overall incidence of distant failure between the two groups (p = 0.324). Two patients in the CRT arm and three in the IC arm initially progressed at distant metastatic sites.

Subset analysis of progression-free survival for patients with (A) hypopharyngeal cancer, (B) base of tongue cancer. IC, induction chemotherapy; CRT, chemoradiotherapy.
The ORR to IC was 94.1%; one patient (5.8%) achieved complete response, 88.2% had partial responses and one patient (5.8%) had stable disease. None of the patients progressed on IC. After completion of CRT, among all 36 patients, 20 reached complete response (55.6%), 14 had partial responses (38.9%) and one had stable disease (2.8%). Overall response rates were 100% in the CRT group and 88.2% in the IC group (p = 0.216). Five patients underwent salvage operations (four CRT arm, one IC arm) and four patients received salvage chemotherapy after progression.
Toxicity
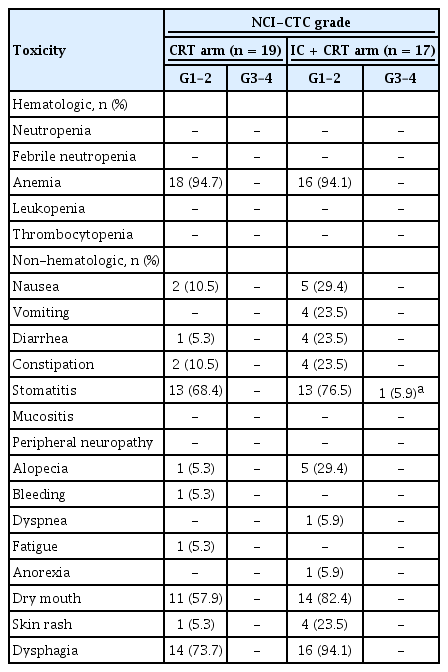
All patients were monitored for AEs and 94.4% (34/36) of them experienced an AE during the study period. There was only one case of grade ≥ 3 AE of stomatitis (5.9%) in the IC group during the CRT phase. The most common AEs experienced in both treatment arms were anemia, stomatitis, dry mouth, and dysphagia (Table 2). The addition of IC before CRT was relatively well tolerated and all AEs were manageable. Dose reductions of chemotherapy were required in 29.4% during IC and 5.9% during concurrent chemoradiotherapy only in the IC arm. Dose delays of at least one cycle of chemotherapy happened in 15.8% and 29.4% of patients during the concurrent chemoradiotherapy period in the CRT and IC arms, respectively (Table 3).
Treatment and drug delivery
The total number of cisplatin chemotherapy cycles administered was 47 (mean, 2.5) in the CRT arm and 42 (mean, 2.5) in the IC arm. Two cycles of planned IC were performed in all patients on the IC arm. The delivered relative dose intensities of radiation and cisplatin for CRT were similarly high in both groups (98.9%, 98.8% in the CRT am and 93.5%, 92.8% in the IC arm, respectively) (Table 3). The mean cumulative dose of cisplatin during CRT was 242 mg/m2 in the CRT arm and 213 mg/m2 in the IC arm (p = 0.096). Three chemotherapeutic agents were given to IC group patients with quite high dose intensities (≥ 95%).
DISCUSSION
We initially designed this phase II trial to evaluate the efficacy of adding IC to concurrent CRT in a specific patient subset of BOT or hypopharynx cancer. While the TAX (Cisplatin and Fluorouracil Alone or with Docetaxel in Head and Neck Cancer) 324 trial suggested that TPF IC was superior to a PF regimen with improved survival, we noticed that the 3-year OS rate in the TAX 324 trial, which included about 50% oropharyngeal cancer patients, was 62% in the TPF IC group [6], but only 49% in a subgroup analysis of hypopharynx cancer patients [10]. Such an inferior outcome for hypopharyngeal cancer was similar to our previous study [8] where the 3-year OS rate was 52% in the hypopharynx group compared to 75% in the oropharynx group (HR, 2.21; 95% CI, 1.38 to 3.56; p = 0.001) after treatment with upfront CRT.
Hypopharynx and BOT cancers have been regarded as good candidates for organ preservation, and so multidisciplinary treatments including chemotherapy, and radiotherapy rather than surgery have been evaluated. IC before CRT demonstrates a superior organ preservation rate in hypopharynx and BOT cancer (3-year PFS: 52%) [11] and the TPF regimen showed a better ORR and a 3-year larynx preservation rate of 70.3% compared to the PF regimen [12,13].
We administered two cycles of IC with the TPF regimen as previously reported [7]. In both treatment arms, concurrent chemoradiotherapy was delivered with a 3-weekly cisplatin dose of 100 mg/m2, a current standard treatment. Importantly, the study treatment was tolerated well without any Gr4 toxicities even after the completion of chemoradiotherapy. In the present study, which was prematurely closed due to slow accrual with a small number of patients, the primary endpoint of 15% improvement in 1-year PFS was not met. However, 1-year PFS rates were 63.2% (95% CI, 0.415 to 0.848) in the CRT group and 76.5% (95% CI, 0.563 to 0.966) in the IC group, which supports the trend favoring IC. However, we could not validate the result due to early closure of the study. While the present study was ongoing, results from related randomized phase III trials (PARADIGM and DeCIDE) did not demonstrate the superiority of IC over CRT. There has also been a trend in favor of chemoradiotherapy alone. Accordingly, co-investigators hesitated to participate in our trial. In addition, among all head and neck squamous cell carcinoma, hypopharynx and BOT cancers are diagnosed infrequently and incidence rates have recently been decreasing [14].
Given that more than 50% of enrolled patients had oropharynx cancer in both the PARADIGM and De-CIDE study and the outcome of non-significant trends for improved survival in the non-oropharynx subgroup in the DeCIDE analysis [1,2], we hypothesized that IC might be useful for a selective subset of LASCC patients with poor prognosis.
Although PFS and OS in the two treatment arms of the present study were not significantly different, there was a trend for improved survival with TPF IC (HR, 0.35; 95% CI, 0.07 to 1.69), reaching a plateau after 3 years. The OS rate of 86% at 3 years in the IC group compares favorably to other reported data [1,2], particularly given that 78% of cases (28 of 36) had stage IVA/B disease in the current study.
The relatively small number of patients and early termination of this study limits interpretation, but patients in both groups showed good adherence during the treatment phase, which led to a higher relative mean dose intensity of chemotherapy and radiotherapy. Among about 35% of patients with BOT cancer in both group, human papillomavirus (HPV) status were not evaluated which has been regarded as an important prognostic factor in HNSCC.
In summary, our study failed to demonstrate a significant improvement of PFS by adding IC before chemoradiotherapy, and concurrent CRT remains the standard of care with IC remaining investigational. Considering the PFS trend favoring IC, further prospective randomized clinical trials in a large number of patients are warranted in specific subset of LASCC patients to improve poor outcomes.
KEY MESSAGE
1. Hypopharynx and base of tongue cancer (BOT) shows relatively poor survival.
2. Induction docetaxel, cisplatin and fluorouracil chemotherapy did not prolong progression-free survival compared to concurrent chemoradiotherapy alone among hypopharynx and BOT cancer patients; however, after 3-year, favorable outcome with induction chemotherapy was sustained.
Notes
Keunchil Park has received honoraria from Abbvie, Astellas, Astra Zeneca, Boehringer Ingelheim, Clovis, Eli Lilly, GSK, Hanmi, Kyowa Hakko Kirin, MSD, ONO, and Roche. Keunchil Park’s institution received research funding from Astra Zeneca.