Colonoscopy quality in community hospitals and nonhospital facilities in Korea
Article information
Abstract
Background/Aims
High-quality colonoscopy is essential to reduce colorectal cancer-related deaths. Little is known about colonoscopy quality in non-academic practice settings. We aimed to evaluate the quality of colonoscopies performed in community hospitals and nonhospital facilities.
Methods
Colonoscopy data were collected from patients referred to six tertiary care centers after receiving colonoscopies at community hospitals and nonhospital facilities. Based on their photographs, we measured quality indicators including cecal intubation rate, withdrawal time, adequacy of bowel preparation, and number of polyps.
Results
Data from a total of 1,064 colonoscopies were analyzed. The overall cecal intubation rate was 93.1%. The median withdrawal time was 8.3 minutes, but 31.3% of colonoscopies were withdrawn within 6 minutes. Community hospitals had longer withdrawal time and more polyps than nonhospital facilities (median withdrawal time: 9.9 minutes vs. 7.5 minutes, p < 0.001; mean number of polyps: 3.1 vs. 2.3, p = 0.001). Board-certified endoscopists had a higher rate of cecal intubation than non-board-certified endoscopists (93.2% vs. 85.2%, p = 0.006). A total of 819 follow-up colonoscopies were performed at referral centers with a median interval of 28 days. In total, 2,546 polyps were detected at baseline, and 1,088 were newly identified (polyp miss rate, 29.9%). Multivariable analysis revealed that older age (odds ratio [OR], 1.032; 95% confidence interval [CI], 1.020 to 1.044) and male sex (OR, 1.719; 95% CI, 1.281 to 2.308) were associated with increased risk of missed polyps.
Conclusions
The quality of colonoscopies performed in community hospitals and nonhospital facilities was suboptimal. Systematic reporting, auditing, and feedback are needed for quality improvement.
INTRODUCTION
Colorectal cancer (CRC) remains a major cause of cancer-related deaths worldwide [1]. CRC screening programs aim to reduce CRC-related mortality through early detection of cancer [2]. As most CRC develops gradually from adenomatous polyps through the adenoma-carcinoma sequence, it has been suggested that detection and removal of pre-cancerous lesions by colonoscopy can prevent development of CRC and reduce mortality associated with CRC [3,4]. Colonoscopy is the most sensitive tool for colorectal adenoma detection, and the detected adenoma can be resected during the procedure [5].
Although colonoscopy is regarded as the standard to detect and prevent CRC, this procedure has some limitations. Back-to-back colonoscopy revealed that the miss rate for adenomas of any size was 20% [6]. In addition, there are marked variations in polyp detection, complete polyp resection, and cancer prevention effects among fully trained colonoscopists [7-9]. Therefore, a high-quality colonoscopy is necessary to reduce CRC-related mortality. The American Society for Gastrointestinal Endoscopy guidelines provide quality indicators including cecal intubation rate ≥ 90%, adequate bowel preparation ≥ 85%, adenoma detection rate (ADR) ≥ 25%, and average withdrawal time ≥ 6 minutes to ensure adequate colonoscopy quality [10].
Colonoscopy is now widely used in various clinical practice settings not only in academic medical centers, but also in community hospitals and nonhospital facilities. In Korea, the National Endoscopy Quality Improvement Program exists for colonoscopy quality control [11]. However, little is known regarding the substantive quality of colonoscopy in real-life practice settings. It is important to maintain a similar level of colonoscopy quality among various clinical care settings in a population-based screening program. Therefore, we aimed to evaluate the quality of colonoscopies performed in community hospitals and nonhospital facilities in real-life practice.
METHODS
Study population
In six tertiary care centers participating in the study, patients who were referred with a copy of colonoscopy images from community hospitals and nonhospital facilities between January 1st, 2015 and December 31st, 2018 were retrospectively identified. Colonoscopy data were collected using the Picture Archiving and Communication System of each participating center. We excluded patients aged 19 or younger, those with colonic stricture or obstruction, inflammatory bowel disease, previous history of colectomy, unknown source of colonoscopy, and those without time records in the photographs. The study protocol was approved by the Institutional Review Board of each participating hospital (Hanyang University Guri Hospital IRB No. 2018-05-038). Informed consent was waived by the board because only de-identified data were collected retrospectively.
Measurements and definition
Based on photographs of the colonoscopies, we assessed quality indicators including cecal intubation rate, withdrawal time, adequacy of bowel preparation, rate of complete photo-documentation, and number of polyps. Cecal intubation was defined as the presence of a closeup photograph of the cecum with appendiceal orifice. Withdrawal time was measured as cecal intubation time minus finish time minus time spent on biopsy or polypectomy. The adequacy of bowel preparation was evaluated using the Boston Bowel Preparation Scale (BBPS) [12], and a total score ≥ 6 was considered adequate bowel preparation. Complete photo-documentation was defined as the presence of the following representative landmark photographs: cecum, ileocecal valve, ascending colon, hepatic flexure, transverse colon, descending colon, sigmoid colon, and rectum.
Demographic data including age and sex were also collected. The practice setting in which each colonoscopy was performed was identified using the online hospital search provided by the Korean Ministry of Health and Welfare. Non-teaching medical institutions that do not provide inpatient care were classified as nonhospital facilities, and those that provide inpatient care were classified as community hospitals. Board certification of the endoscopist was assessed through an online search for board-certified endoscopists provided by the Korean Society of Gastrointestinal Endoscopy (KSGE). If a follow-up colonoscopy at the tertiary care center was performed, the number of newly detected polyps ≥ 5 mm in size was counted. The polyp miss rate was calculated as the total number of missed polyps / (total number of missed polyps + total number of polyps on initial examination) [13].
Statistical analysis
Continuous variables were expressed as mean ± standard deviation or as median (interquartile range [IQR]) and compared using a t test or Mann-Whitney test as appropriate. Categorical variables were expressed as numbers (with proportion) and compared using the chi-square test with Fisher’s exact test. Logistic regression models were used to evaluate risk factors for missed polyps. A p value < 0.05 was considered statistically significant. All statistical analyses were performed using R statistical language R Studio version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria) and IBM SPSS Statistics version 20.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Baseline characteristics
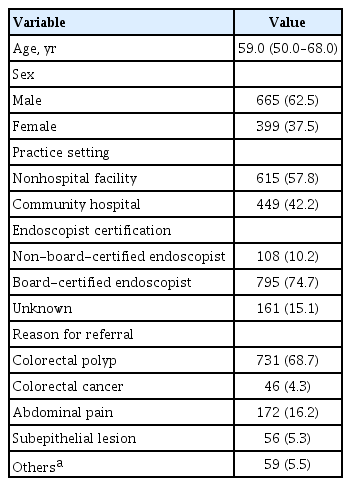
Data from a total of 1,184 colonoscopy procedures were collected; those of eight colonoscopies with colonic obstruction, 18 with inflammatory bowel disease, 58 with unknown source, and 36 without time records were excluded. Consequently, data from 1,064 colonoscopy procedures were analyzed. Of the colonoscopies, 57.8% were performed in nonhospital facilities and 42.2% in community hospitals. The median age of the patients was 59.0 years, and 62.5% were male. Of the endoscopists, 74.7% were board-certified. The reasons for referral were colorectal polyp (68.7%), CRC (4.3%), abdominal pain (16.2%), subepithelial lesions of the gastrointestinal tract (5.3%), and others (5.5%) (Table 1).
Measurement of quality indicators
The overall cecal intubation rate was 93.1%. The median withdrawal time was 8.3 minutes (IQR, 5.4 to 12.7), but colonoscopies with a withdrawal time < 6 minutes accounted for 31.3%. The percentage of colonoscopies with adequate bowel preparation (BBPS ≥ 6) was 96.1%. The mean number of photographs was 54.4, but complete photo-documentation was achieved in only 67.0% of exams. The presence of each landmark photograph was 93.1% in cecum, 78.1% in ileocecal valve, 95.7% in ascending colon, 89.3% in hepatic flexure, 96.7% in transverse colon, 95.9% in descending colon, 97.2% in sigmoid colon, and 96.1% in rectum. The mean number of polyps per exam was 2.6.
Comparing quality indicators according to practice setting, withdrawal time was significantly longer in community hospitals than in nonhospital facilities (median withdrawal time: 9.9 minutes vs. 7.5 minutes, p < 0.001; withdrawal time ≥ 6 minutes: 74.2% vs. 64.7%, p = 0.001). The percentage of colonoscopies with complete photo-documentation was significantly higher in community hospitals than in nonhospital facilities (73.9% vs. 62.0%, p < 0.001). More polyps were detected in colonoscopies at community hospitals than in those at nonhospital facilities (mean number of polyps: 3.1 vs. 2.3, p = 0.001) (Table 2).
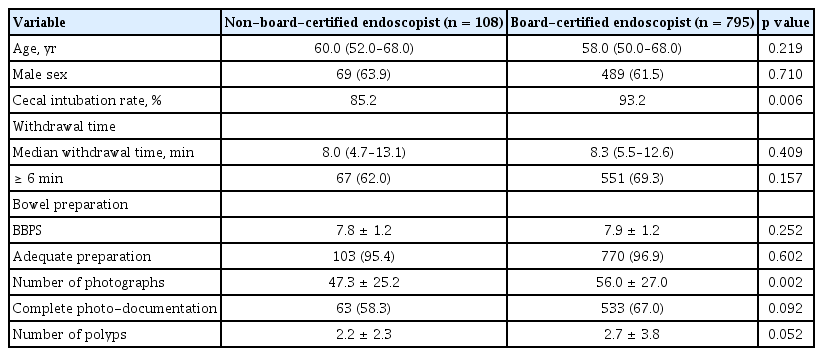
Using data from 903 colonoscopies performed by an identified board-certified endoscopist, we compared quality indicators according to board certification of the endoscopist. Board-certified endoscopists had a significantly higher rate of cecal intubation and larger number of photographs than non-board-certified endoscopists (cecal intubation rate: 93.2% vs. 85.2%, p = 0.006; mean number of photographs: 56.0 vs. 47.3, p = 0.002). Although board-certified endoscopists showed a higher rate of complete photo-documentation and a larger number of polyps than non-board-certified endoscopists, the differences were not statistically significant (Table 3).
In addition, colonoscopies with a withdrawal time < 6 minutes had lower rates of cecal intubation and complete photo-documentation and fewer polyps per exam than colonoscopy with a withdrawal time ≥ 6 minutes (cecal intubation rate: 88.6% vs. 95.2%, p < 0.001; complete photo-documentation rate: 54.1% vs. 72.9%, p < 0.001; mean number of polyps: 1.8 vs. 3.0, p < 0.001).
Follow-up colonoscopy and missed polyps
A total of 819 follow-up colonoscopies were performed at the tertiary care centers with a median interval of 28 days; 2,546 polyps were detected at baseline and 1,088 polyps were newly identified at follow-up colonoscopies (polyp miss rate, 29.9%). Among newly detected polyps, 35 were histologically identified as adenocarcinoma.
Table 4 shows the univariable and multivariable logistic regression analyses for risk of missed polyps. Older age (adjusted odds ratio [OR], 1.032; 95% confidence interval [CI], 1.020 to 1.044) and male sex (adjusted OR, 1.719; 95% CI, 1.281 to 2.308) were significantly associated with an increased risk of missed polyps. The number of polyps at baseline was associated with missed polyps (unadjusted OR, 1.058; 95% CI, 1.008 to 1.111), but the result was not significant on multivariable analysis (adjusted OR, 1.014; 95% CI, 0.972 to 1.057).
DISCUSSION
In the present work, we found that the quality of colonoscopies performed in community hospitals and nonhospital facilities was suboptimal. Approximately one-third of colonoscopies were withdrawn within 6 minutes and had incomplete photo-documentation. Although the overall rate of cecal intubation exceeded 90%, the cecal intubation rate of non-board-certified endoscopists was 85.2%. Moreover, a large number of polyps were newly detected on follow-up exams within a short period of time.
According to previous studies conducted in Spain and the United States comparing the quality of colonoscopies between various practice settings, both academic and non-academic centers provided high-quality colonoscopies [14,15]. Our results, unlike previous studies, showed that the quality of colonoscopies in community hospitals and nonhospital facilities was suboptimal. In addition, community hospitals tend to perform colonoscopies of higher quality than nonhospital facilities. This disparity may be difficult to explain, given that there are numerous factors affecting the quality of care in medical practice environments [16]. In Korea, there is no medical budget for quality of care, and reimbursement for medical care is relatively low for nonhospital facilities. Indeed, the cost of a colonoscopy in Korea is approximately $72.6 (1100.3 Korean won = 1 US dollar by an annual exchange rate in 2018) [17], which is significantly lower than the cost of approximately $899 in the United States [18]. In addition, small-volume endoscopy units lack staff fully responsible for endoscopy and quality management. Meanwhile, it is also necessary to consider the possibility that excessive endoscopic workloads may result in suboptimal quality. Previous studies on the relationship between colonoscopy volume and quality have been inconclusive [19,20]. However, a recent study on the capacity for colonoscopies in Korea showed that there is a heavy colonoscopy workload and lack of capacity [21]. Further research and discussion on appropriate cost and colonoscopy workload is needed.
Interval CRCs, generally defined as CRCs after index colonoscopy before the next surveillance schedule, account for 1.8% to 9.0% of all CRCs [22]. Although the etiology of interval CRCs is unclear, the majority of interval CRCs originates from missed lesions [23]. Non-gastroenterologists and incomplete exams are associated with an increased risk of interval CRCs [24]. In our results, non-board-certified endoscopists had a higher rate of incomplete exams than board-certified endoscopists, although it was not an independent risk factor for missed polyps. It is worth noting that KSGE provides board certification to both internists and surgeons who have completed training at a designated training endoscopy center and have passed a qualifying examination [25]. In addition, to maintain qualifications, endoscopists should complete an annual education program including quality management [25]. This suggests that qualified endoscopists who were systematically trained in endoscopy and quality management are important for high-quality colonoscopies, regardless of their specialty in medical practice.
The suboptimal colonoscopy quality seen in community hospitals and nonhospital facilities in our results calls for strengthening quality management systems. In Korea, the National Cancer Screening Program (NCSP) provides fecal occult blood testing for adults 50 years of age or older and colonoscopies for positive cases [26]. For endoscopy quality management, NCSP operates an Endoscopy Quality Improvement Program, and revised quality indicators were recently published by the KSGE [11]. Quality indicators consist of 34 items in six areas of the workforce, process, facilities and equipment, outcome, reprocessing, and sedation [11]. However, endoscopy quality evaluation criteria only assess the overall quality of the institution, not the performance indicators of the individual endoscopist. Therefore, an individual endoscopist’s cecal intubation rate, mean withdrawal time, and ADR are not measured. In addition, measurement of quality indicators depends on voluntary reporting by the endoscopist, and most end up with a document review without a field investigation. This suggests that current quality assessment and audit systems may be ineffective in real-life practice settings.
Poor-quality colonoscopies may cause not only interval CRCs and CRC-related deaths, but also overutilization, subsequent complications, and increased medical costs [27]. The cost for improving colonoscopy quality may be lower than the medical costs brought on by overutilization and subsequent complications associated with poor-quality colonoscopies. Cost-effective methods for quality improvement need to be discussed. In the United States, the Center for Medicare & Medicaid Services has a Physician Quality Reporting System in which physicians voluntarily report endoscopy quality indicators and receive incentives [28]. In Spain, all screening colonoscopy data are coded by the coordinating staff in the Basque Country CRC Screening Program. In this way, data on colonoscopy quality indicators including ADR, cecal intubation rate, complications, and bowel preparation quality can be collected [14,29].
Ultimately, a comprehensive approach is needed to further improve the quality of colonoscopies in Korea. The first step is to refine the reporting system. According to a study conducted in Ontario, Canada, a considerable proportion of the colonoscopy reports submitted by the endoscopists in the field were inappropriate in quality [30]. Feedback is another important factor associated with quality improvement [31]. Providing appropriate feedback rather than auditing alone contributes to quality improvement, and providing incentives further increases effectiveness [31]. In addition, because it is not realistic to field investigate all institutions, an electronic database of colonoscopy quality indicators needs to be established. Pathological results after polypectomies should be collected to automatically calculate ADR and provide periodic feedback. Above all, to create a virtuous circle for quality improvement, it is important to encourage endoscopists to participate in the process of reporting, auditing, and providing feedback. To do so, the reporting system should be easy to implement, and appropriate feedback and incentives need to be provided.
This study has some limitations. Because colonoscopy data were retrospectively collected, we were not able to identify the indications for colonoscopy. Therefore, this study included colonoscopies of various indications besides screening colonoscopies. It may be inappropriate to directly apply our results to quality indicators of screening colonoscopies. Second, we measured withdrawal time as cecal intubation time minus finish time minus time spent on biopsy or polypectomy, which was an arbitrary measurement. In fact, withdrawal time ≥ 6 minutes is a meaningful indicator in a negative study. Third, we were not able to investigate individual endoscopist ADR, which is an important indicator for endoscopist performance. The retrospective nature of the study limits the data collection; many colonoscopy pathologic reports were not available and a follow-up colonoscopy was not performed in all cases. Therefore, a complete assessment of adenoma detection could not be achieved. Fourth, bowel preparation was evaluated by the investigators, not by the operators. We used BBPS as a measure of bowel preparation, which relies on the degree of bowel visualization after sufficient washing and aspiration. Our method was limited in assessing bowel preparation accurately, because it was not possible to confirm that the pictures that the investigators used to evaluate bowel visualization were taken after sufficient washing and aspiration. Finally, we could not measure the level of experience of the endoscopist, nor the capacity and colonoscopy volume of each endoscopy unit. Future studies regarding colonoscopy quality indicators in non-academic centers including detailed information about endoscopists and endoscopy units are needed.
In conclusion, our results showed that the quality of colonoscopies performed in community hospitals and nonhospital facilities was suboptimal. Systematic reporting, auditing, and feedback are needed to establish a virtuous circle for colonoscopy quality improvement.
KEY MESSAGE
1. The quality of colonoscopies performed in community hospitals and nonhospital facilities in Korea was suboptimal. Several quality indicators did not meet the guideline recommendations, and a considerable number of polyps were missed.
2. Systematic reporting, auditing, and feedback are needed to improve the quality of colonoscopies.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2018R1A2B6004475).