Efficacy and safety of mepolizumab in Korean patients with severe eosinophilic asthma from the DREAM and MENSA studies
Article information
Abstract
Background/Aims
The efficacy and safety of mepolizumab in patients with severe eosinophilic asthma has been evaluated in a global clinical trial programme. This post hoc analysis assesses the efficacy and safety of mepolizumab in Korean patients.
Methods
Data from Korean patients in the Phase III, placebo-controlled, randomised DREAM (MEA112997/NCT01000506) and MENSA (MEA115588/NCT01691521) studies were included. Patients ≥ 12 years old with severe eosinophilic asthma received mepolizumab (DREAM: 75, 250 or 750 mg intravenously [IV]; MENSA: 75 mg IV or 100 mg subcutaneously [SC]), or placebo every 4 weeks for 52 weeks (DREAM) or 32 weeks (MENSA). The primary outcome was the rate of clinically significant asthma exacerbations. Secondary outcomes included forced expiratory volume in 1 second (FEV1), Asthma Control Questionnaire (ACQ) and St George’s Respiratory Questionnaire (SGRQ) scores (MENSA only). Blood eosinophil counts (BEC) and safety were assessed throughout.
Results
Reductions in the rate of clinically significant asthma exacerbations were observed with the approved (100 mg SC) and bioequivalent (75 mg IV) doses of mepolizumab in Korean patients who participated in DREAM and MENSA. In MENSA, trends for improvements from baseline at week 32 in pre-bronchodilator FEV1 (75 mg IV group), ACQ-5 and SGRQ scores (in both treatment groups) were seen versus placebo in Korean patients. Incidence of on-treatment adverse events was similar in Korean patients versus non-Korean patients as were observed reductions from baseline in BEC.
Conclusions
Mepolizumab treatment provided clinical benefits for Korean patients with severe eosinophilic asthma; the safety profile is consistent with the overall population.
INTRODUCTION
Asthma is one of the most prevalent diseases in the Asia-Pacific region, accounting for over 30% of respiratory conditions [1]. Severe asthma accounts for approximately 5% to 10% of all asthma cases and is defined as asthma that requires treatment with high-dose inhaled corticosteroids (ICS) plus a second controller to maintain control, or asthma that remains uncontrolled despite this treatment [2-4]. The burden of severe asthma in Korea, in terms of use of healthcare facilities and asthma medications, is substantial [5].
Severe asthma is a heterogeneous condition and several clinical phenotypes have been identified, one of which is severe eosinophilic asthma [2,6,7]. This phenotype is characterised by elevated eosinophil counts and frequent exacerbations, and patients typically have a poor prognosis [2,7]. Currently, several biologic therapies are approved for treating patients with severe eosinophilic asthma [8], including mepolizumab, a humanised monoclonal antibody that targets interleukin-5 to reduce eosinophil levels [9]. In a global Phase III clinical trial programme, mepolizumab was shown to reduce clinically significant exacerbations, improve lung function, asthma control and health-related quality of life (HRQoL) in patients with severe eosinophilic asthma when used as add-on therapy to standard of care [10-12]. These studies have also demonstrated that mepolizumab is well-tolerated with a safety profile similar to placebo [10-12].
Although the majority of monoclonal antibody therapies do not display therapeutic differences across ethnic groups, there is some evidence that therapeutic responses may be influenced by variations in patient characteristics between different ethnic/racial groups, and in a few cases, this may lead to population-specific prescribing recommendations [13,14]. This post hoc analysis assessed the efficacy and safety of mepolizumab versus placebo using data from Korean patients enrolled in the Phase IIb/III DREAM (Dose Ranging Efficacy And safety with Mepolizumab in severe asthma) and Phase III MENSA (MEpolizumab as adjunctive therapy iN patients with Severe Asthma) studies to determine whether any therapeutic difference between Korean and non-Korean patients exists.
METHODS
Study design and treatment
This was a post hoc subgroup analysis of all studies with Korean patient data available and included data from the Phase III, pivotal, placebo-controlled, randomised, double-blind, parallel-group, multicentre studies, DREAM (MEA112997/NCT01000506) and MENSA (MEA115588/NCT01691521) [10,11]. Both studies assessed the efficacy and safety of mepolizumab versus placebo in patients with severe eosinophilic asthma. Patients enrolled in DREAM were randomised (1:1:1:1) to receive intravenous (IV) mepolizumab 75, 250, or 750 mg, or placebo, plus standard of care, every 4 weeks for 52 weeks. In MENSA, patients were randomised (1:1:1) to receive IV mepolizumab 75 mg, mepolizumab 100 mg subcutaneously (SC) or placebo, plus standard of care, every 4 weeks for 32 weeks. The DREAM and MENSA studies were conducted in accordance with the ethical principles of the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice Guidelines and applicable country-specific regulatory requirements. All patients provided written informed consent. The study protocols for DREAM and MENSA: NCT01691521 were approved by local ethics committees.
Patients
Patients enrolled in DREAM and MENSA were ≥ 12 years old, had severe eosinophilic asthma, and a history of ≥ 2 exacerbations requiring systemic corticosteroids in the year prior to enrolment despite regular treatment with high-dose ICS in the 12 months prior to screening and additional controller(s). For DREAM, eosinophilic asthma was indicated as a sputum eosinophil count of ≥ 3%, fractional exhaled nitric oxide of ≥ 50 parts per billion, a blood eosinophil count of ≥ 300 cells/μL, or a prompt deterioration in asthma control after a ≤ 25% reduction in regular maintenance dose of ICS or oral corticosteroids (OCS) within the previous year. For MENSA, eosinophilic asthma was indicated as a blood eosinophil count of ≥ 300 cells/μL in the previous year or ≥ 150 cells/μL at screening.
Outcomes and assessments
The primary outcome was the annual rate of clinically significant exacerbations, which was defined as a worsening of asthma, which required the use of systemic corticosteroids for ≥ 3 days and/or hospitalisation/emergency department visits. Secondary outcomes included blood eosinophil counts, spirometry measurements and Asthma Control Questionnaire (ACQ)-5 (MENSA) or -6 (DREAM) scores, which were obtained every 4 weeks. In MENSA, St George’s Respiratory Questionnaire (SGRQ) scores were assessed at randomisation and at the exit visit (week 32). SGRQ scores were not available for the DREAM study.
Safety was evaluated by assessment of on-treatment adverse events (AEs), on-treatment serious AEs (SAEs) and immunogenicity. Levels of anti-mepolizumab antibodies were measured at weeks 0, 16, 32, and 40 in MENSA, and at weeks 0, 16, and 72 in DREAM.
Sample size and statistical analysis
This post hoc analysis was based on the Korean patients in the intent-to-treat (ITT) population from DREAM and MENSA, including all randomised Korean patients who received ≥ 1 dose of study medication. The analysis of exacerbations was performed using a negative binomial model with covariates of treatment group, baseline maintenance OCS therapy (OCS vs. no OCS), number of exacerbations in the previous year (as an ordinal variable), baseline percent predicted forced expiratory volume in 1 second (FEV1), and with inclusion of time on treatment as an offset variable.
Changes from baseline in FEV1, ACQ scores and blood eosinophil counts were analysed using mixed model repeated measures methods. SGRQ scores were analysed by analysis of covariance. Covariates of baseline value, baseline maintenance OCS therapy (OCS vs. no OCS), exacerbations in the year prior to the study (as an ordinal variable), baseline percent predicted FEV1 (except analysis of FEV1) and treatment were included in all analysis models, and visit plus interaction terms for visit by baseline and visit by treatment group were included in the mixed model repeated measures analyses. A pre-specified log transformation was applied to blood eosinophil count before analysis.
RESULTS
Patient population
In total, the ITT populations included 616 and 576 patients in the DREAM and MENSA studies, respectively. Of these, 24 (3.9%) and 45 (7.8%), respectively, were Korean. Across the two studies, three Korean patients were withdrawn, two from the DREAM study and one from the MENSA study. Both patients from DREAM withdrew consent, and the patient from MENSA withdrew because of an AE (patient was receiving placebo). Patient demographics and clinical characteristics were broadly similar in Korean and non-Korean patients, except there were fewer female patients, patients had a lower mean body mass index (BMI), a higher mean percent predicted FEV1, and lower mean ACQ-5 scores in the Korean group, compared with the non-Korean group (Table 1). Spirometry measurements at baseline, and disease characteristics were also similar between the Korean and non-Korean group across the two studies (Table 1).
Primary outcome
In DREAM, the annual rate of clinically significant exacerbations in Korean patients was numerically reduced with mepolizumab versus placebo in the 75 mg and 250 mg IV groups, but not in the 750 mg IV group (Fig. 1). Annual rates of exacerbations were 2.00, 1.26 and 3.28 in the mepolizumab 75 mg, 250 mg and 750 mg IV groups, respectively, compared with 3.01 in the placebo group. For Korean patients in the MENSA study, reductions in clinically significant exacerbations were seen in both mepolizumab (75 mg IV and 100 mg SC) treatment groups versus placebo (Fig. 1). Annual rates of exacerbations were 1.00 and 0.61 in the mepolizumab 75 mg IV and 100 mg SC groups, respectively, compared with 3.16 in the placebo group.

Annual rate of clinically significant exacerbations with mepolizumab versus placebo in Korean patients. DREAM, Dose Ranging Efficacy And safety with Mepolizumab in severe asthma; MENSA, MEpolizumab as adjunctive therapy iN patients with Severe Asthma; CI, confidence interval; IV, intravenous; SC, subcutaneous.
It is of note that in DREAM, five out of six Korean patients experienced ≥ 1 exacerbation in the placebo group, and although only three out of six patients who were receiving the highest dose of mepolizumab (750 mg IV) experienced ≥ 1 exacerbation (one of these patients had experienced a total of eight exacerbations). In MENSA, for Korean patients receiving the approved dose of mepolizumab (100 mg SC) four out of 15 patients experienced ≥ 1 exacerbation (one of whom experienced a total of seven exacerbations), compared with nine out of 15 patients in the placebo group (Supplementary Table 1).
Secondary outcomes
For the DREAM study, there were insufficient patient numbers to perform analyses of changes from baseline in FEV1, ACQ-5 scores and blood eosinophil counts. In the MENSA study, a trend for improvements from baseline in pre-bronchodilator FEV1 was seen in Korean patients receiving mepolizumab 75 mg IV compared with placebo, but not with mepolizumab 100 mg SC (Fig. 2). A trend for reductions (improvements) in ACQ-5 scores in Korean patients was also seen with mepolizumab 100 mg SC versus placebo at week 4 and with both doses (100 mg SC and 75 mg IV) at week 8, and was maintained until week 32 (except for week 12 in the 75 mg IV group) (Fig. 3). In addition, there were numerical reductions (improvements) from baseline in SGRQ scores seen at week 32 in both treatment groups versus placebo (adjusted change from baseline [95% confidence interval, CI] in 75 mg IV [n = 15]: –3.0 [95% CI, –13.8 to 7.8]; 100 mg SC [n = 15]: –0.2 [95% CI, –11.8 to 11.4]) (data not shown). Reductions from baseline in blood eosinophil counts for Korean patients were seen with both mepolizumab doses (75 mg IV and 100 mg SC), versus placebo at week 4, and these reductions were maintained until week 32 (Fig. 4).
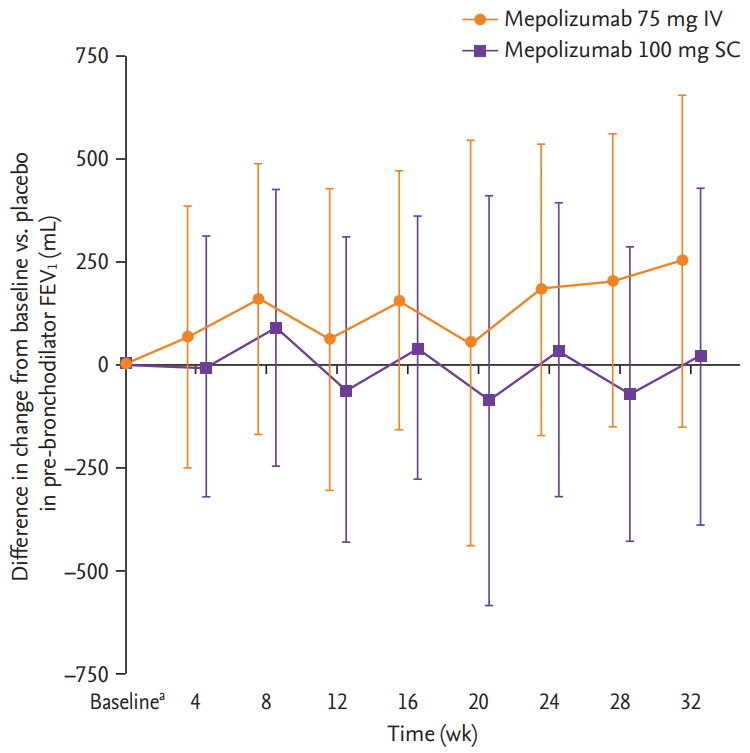
Difference in change from baseline in pre-bronchodilator forced expiratory volume in 1 second (FEV1) with mepolizumab versus placebo in Korean patients from the MENSA (MEpolizumab as adjunctive therapy iN patients with Severe Asthma) study. Error bars show 95% confidence intervals. Patient numbers for each group were: mepolizumab 75 mg intravenous (IV); 15 (all time points); mepolizumab 100 mg subcutaneous (SC); 15 (all time points), and placebo; 15 (baseline, weeks 8 and 16) and 14 (at weeks 24 and 32). aBaseline mean ± SD pre-bronchodilator FEV1 mL were 1,859 ± 623, 1,771 ± 599, and 1,902 ± 550 in the 75 mg IV, 100 mg SC and placebo groups, respectively.
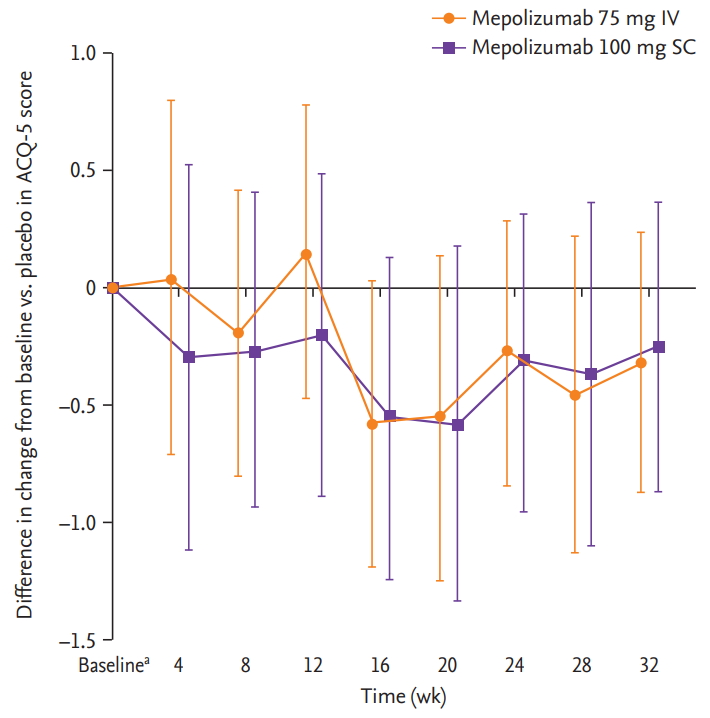
Difference in change from baseline in Asthma Control Questionnaire-5 (ACQ-5) score with mepolizumab versus placebo in Korean patients from the MENSA (MEpolizumab as adjunctive therapy iN patients with Severe Asthma) study. Error bars show 95% confidence intervals. Patient numbers for each group were: mepolizumab 75 mg intravenous (IV); 12 (baseline, weeks 8, 16 and 24), 11 (week 32); mepolizumab 100 mg subcutaneous (SC); 13 (baseline, weeks 8, 24 and 32), 12 (week 16), and placebo; 14 (baseline, weeks 8 and 16) and 13 (at weeks 24 and 32). aBaseline mean ± SD ACQ-5 scores were 1.6 ± 1.04, 1.1 ± 0.79, and 2.2 ± 1.2 for the 75 mg IV, 100 mg SC and placebo groups, respectively.
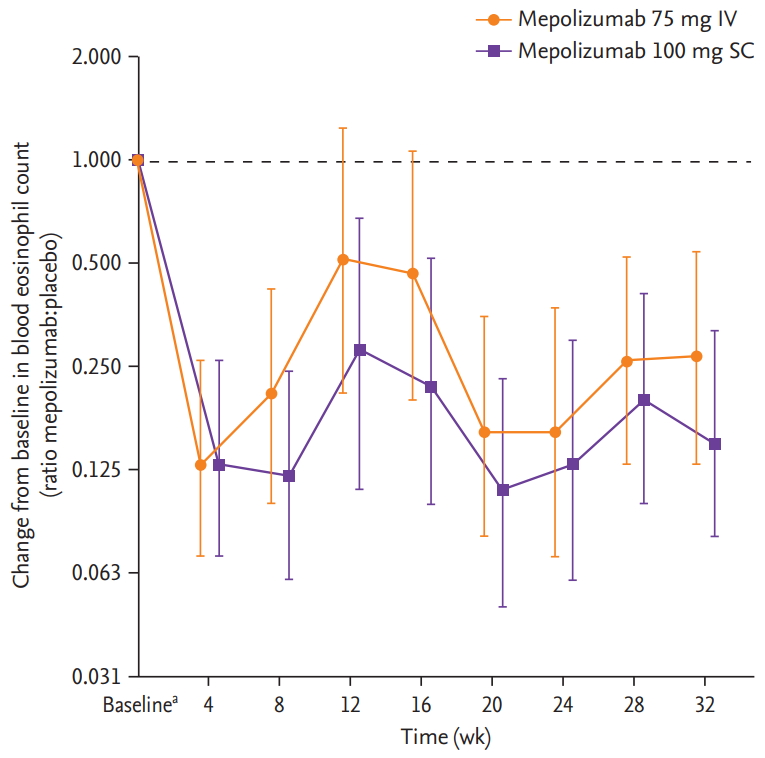
Ratio of change from baseline in blood eosinophil count with mepolizumab versus placebo in Korean patients from the MENSA (MEpolizumab as adjunctive therapy iN patients with Severe Asthma) study. Error bars show 95% confidence intervals. Patient numbers for each group were: mepolizumab 75 mg intravenous (IV); 15 (baseline, weeks 16, 24 and 32) and 13 (week 8); mepolizumab 100 mg subcutaneous (SC); 15 (all time points), and placebo; 15 (baseline) and 14 (weeks 8, 16, 24 and 32). aBaseline geometric mean ± SD logs blood eosinophil counts, in cells/μL, were 300 ± 0.82, 290 ± 1.05, and 240 ± 1.23 in the 75 mg IV, 100 mg SC and placebo groups, respectively.
Safety
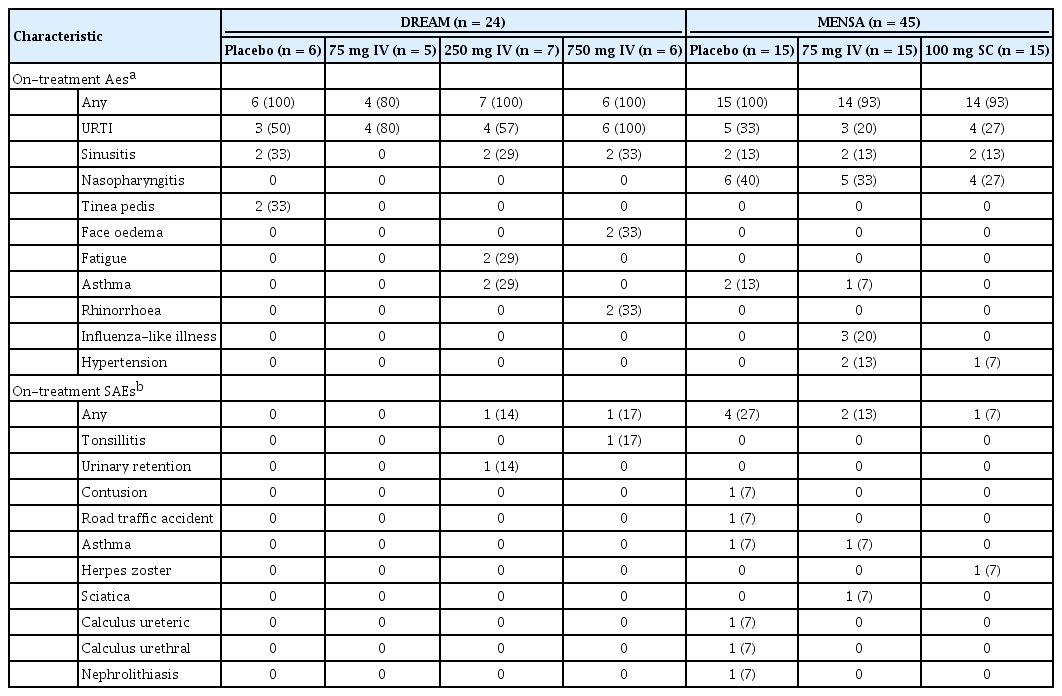
The most frequently reported on-treatment AEs in Korean patients across all treatment groups were upper respiratory tract infection (DREAM and MENSA), nasopharyngitis (MENSA) and sinusitis (DREAM [excluding the 75 mg IV group], and MENSA) (Table 2). The incidence of reported on-treatment SAEs in Korean patients was low across both studies, with four patients reporting an SAE in MENSA placebo, two in MENSA 75 mg, and one each in DREAM 250 mg, 750 mg and MENSA 100 mg groups (Table 2). One fatality across the two studies was reported, which was a Korean patient receiving placebo in the MENSA study. Overall, no anti-drug antibodies were detected in Korean patients at week 0 or at any time post-baseline. For both the DREAM and MENSA studies, no neutralising antibodies were detected in Korean patients during the treatment period.
DISCUSSION
This post hoc analysis of the pivotal Phase III DREAM and MENSA studies assessed, for the first time, the safety and efficacy of mepolizumab in Korean patients with severe eosinophilic asthma. We found that in Korean patients mepolizumab (100 mg SC and 75 mg IV) was associated with a reduction in the rate of clinically significant exacerbations compared with placebo; improvements in lung function, asthma control, and HRQoL were also seen, as well as reductions in blood eosinophil counts. These findings demonstrate that mepolizumab is a beneficial treatment for Korean patients with severe eosinophilic asthma.
Our results are broadly consistent with those seen in the overall ITT population in DREAM and MENSA as well as another Phase III clinical trial, where reductions in clinically significant exacerbations, trends for improvements in lung function and HRQoL were observed along with reductions in blood eosinophil counts [10-12]. It is important to note that in our analysis, Korean patients treated with the highest dose of mepolizumab (750 mg IV) did not show a significant reduction in exacerbations compared with placebo, which is in contrast to results seen in the overall ITT population in DREAM [11]. One patient in this treatment group may have unduly influenced these results having experienced a total of eight exacerbations during the study; however, the sample size was low and thus this result should be viewed with caution.
In Korean patients from the DREAM and MENSA studies, the majority of patients reported ≥ 1 on-treatment AE across the different treatment groups (with frequencies of any AEs ranging from 80% to 100%). This is consistent with those reporting such events in the overall ITT population of MENSA (78% to 84% in the mepolizumab groups; 83% in the placebo group) and DREAM (78% to 82% in the mepolizumab groups; 77% in the placebo group) [10,15]. The most commonly-reported AEs in the overall ITT population were broadly similar to those in Korean patients, although the incidence of headaches appeared to be less common in Korean patients. In addition, the incidence of SAEs was broadly similar to that reported in the overall ITT population in the DREAM and MENSA studies [10,11] and there were no fatalities considered related to treatment. During the treatment period, no anti-drug antibodies were detected in Korean patients, which is in line with the low immunogenic response observed in the mepolizumab clinical trial programme. Taken together, these results demonstrate that mepolizumab is well-tolerated in Korean patients, with no additional safety concerns identified.
Racial differences have been reported to influence treatment responses in patients with asthma. For example, in an analysis of 1,200 patients with asthma from 10 trials, African Americans were shown to have an increased rate of treatment failures (defined as asthma exacerbations, worsening of lung function, increased use of asthma medication or physician-determined) compared with Caucasians when treated with long-acting b-agonists [16]. However, it is not known whether these differences are a result of genetic, environmental or socioeconomic factors or inherent pathophysiologic or pharmacogenomic differences [16]. Obesity has also been found to be associated with increased asthma severity and poor asthma control [17]. However, weight and body mass index may play less of a role in Asian populations as they tend to be lower than in other populations [18]. To date, there is limited information on the efficacy and safety of mepolizumab in specific population cohorts. However, a prospective, open-label study of Japanese patients (n = 32), and a post hoc analysis of Japanese patients from the MENSA study (n = 50) have demonstrated that mepolizumab treatment (100 mg SC and 75 mg IV) is associated with reductions in the rate of clinically significant exacerbations and improvements in lung function in patients with severe eosinophilic asthma [19,20], similar findings to those reported here for Korean patients. Together, these studies do not suggest the presence of clinically relevant inter-ethnic differences in response to mepolizumab treatment.
In this post hoc analysis, the main limitation was the small number of Korean patients in each treatment group, and for the DREAM study this meant that there were insufficient patient numbers to perform formal analyses relating to change from baseline in pre-bronchodilator FEV1, ACQ-5 and blood eosinophil counts with mepolizumab versus placebo. These small sample sizes may reduce the generalisability of the results observed to the wider Korean population. Nonetheless, our findings provide valuable insights into the use of mepolizumab in Korean patients.
In conclusion, this post hoc subgroup analysis of the DREAM and MENSA studies demonstrates a clinically relevant reduction in the rate of asthma exacerbations with mepolizumab compared with placebo in Korean patients with severe eosinophilic asthma. Trends for improvements in lung function, asthma control and HRQoL were also observed, and the safety profile in Korean patients was consistent with that seen in the overall study population. Taken together, these results indicate that treatment with mepolizumab is of clinical benefit in Korean patients with severe eosinophilic asthma.
KEY MESSAGE
1. Post hoc analysis of data from the Phase III studies, DREAM (Dose Ranging Efficacy And safety with Mepolizumab in severe asthma) and MENSA (MEpolizumab as adjunctive therapy iN patients with Severe Asthma), evaluated the efficacy and safety of mepolizumab in Korean patients with severe eosinophilic asthma.
2. Here, we demonstrate that mepolizumab reduces the rate of clinically significant asthma exacerbations, improves lung function, and is well-tolerated in this patient population.
Notes
Namhee Kwon, Soung-Jun Min, Frank C. Albers, Steven W. Yancey, and Bhabita Maye are employees of GSK and hold stocks/shares. Hae-Sim Park, Mi-Kyeong Kim, and Choon-Sik Park have no financial or other issues that might lead to conflict of interest.
Acknowledgements
This post hoc analysis and the parent studies (DREAM, MEA112997/NCT01000506 and MENSA, MEA115588/NCT01691521) were funded by GlaxoSmithKline (GSK). The study nurse and institution service funded by the Korean government (KHIDI [HI16C0992]) also played a role in the conduct of the DREAM and MENSA studies
Editorial support (in the form of writing assistance, including development of the initial draft, assembling tables and figures, collating authors comments, grammatical editing and referencing) was provided by Sarah Farrar, Ph.D., from Fishawack Indicia Ltd., and was funded by GSK.