Acute pancreatitis and diabetes mellitus: a review
Article information
Abstract
Diabetes following acute pancreatitis (AP) is becoming increasingly recognized. It is unclear what subtype of diabetes mellitus (DM) occurs; however, type 3c diabetes mellitus (T3cDM) is gaining increasing recognition. T3cDM has differing pathophysiology than other subtypes of DM and therefore differing disease course and treatment. Current studies have examined the incidence and prevalence of DM following AP, and meta-analyses have shown around 15% develop DM at 1 year with an increasing proportion developing DM at 5 years. It has been observed that some patients have transient hyperglycemia following AP episode with a subset developing persistent impaired glucose metabolism; however, the exact timeline is not well defined. The data on risk factors for developing DM after AP is limited and mixed; however, it is likely that severity of AP may impact the propensity to develop DM. Screening guidelines have not been established following AP; however, screening 1-year post-event will likely capture a sizable proportion of newly developed DM. The endocrine and exocrine pancreas are closely linked, and studies have found significant overlap in dysfunction of both after AP. Finally, there are some data to suggest that diabetes predisposes patients to structural changes in the pancreas and increased risk of developing AP.
INTRODUCTION
Acute pancreatitis (AP) is common, and over the past decade there has been a trend towards increased number of admissions, but lowered mortality [1,2]. Specifically, in the United States, AP is responsible for 250,000 admissions each year and has shown an increase in 20% of admissions over the past 10 years [3]. The vast majority (80%) of admissions are mild, self-limited disease; however, long term consequences are still present [4]. One of those complications is endocrine dysfunction, and specifically impaired glucose metabolism or diabetes.
Diabetes is prevalent and its burden is felt worldwide. According to the World Health Organization, it affects around 422 million adults worldwide [5]. Type 2 diabetes mellitus (T2DM) is the most common sub-type; however, more and more recognition has been given towards other sub-types, namely diabetes related to disorders of the exocrine pancreas.
Diabetes of the exocrine pancreas or type 3c diabetes mellitus (T3cDM) is increasingly common and also under-recognized by providers [6]. One study found it to be more prevalent than type 1 diabetes mellitus (T1DM) [7], and T3cDM accounts for 5% to 10% of diabetes in the western population [8]. Furthermore, there is a well-established relationship between diabetes and chronic pancreatitis [9] as well as pancreatic cancer [9,10] but there is more and more emerging evidence for the association of diabetes with AP [11].
The goal of this review is to: summarize the existing literature on prevalence, natural history, risk factors of impaired glucose metabolism after AP; to explore the relationship with exocrine insufficiency; to discuss the potential bi-directional relationship between diabetes and AP; as well as to discuss the role of screening, diagnosis and treatment of diabetes in this cohort.
DIABETES OF THE EXOCRINE PANCREAS
Though it is not well established what sub-type of diabetes mellitus (DM) develops after AP, special consideration should be given to diabetes of the exocrine pancreas. Diabetes of the exocrine pancreas, otherwise known as T3cDM or “secondary pancreatic diabetes,” is an established clinical entity that is often under-recognized [11]. In a 2017 study, looking retrospectively at population level data from England, diabetes following pancreatic disease was more common than T1DM. Upon further analysis, the vast majority of those cases (87.8%) were identified as T2DM by clinicians [7]. T3cDM is more commonly characterized in the setting of chronic pancreatitis and pancreatic cancer as well as cystic fibrosis, hemochromatosis and prior pancreatic surgery [9]. It is less well characterized in AP, though still thought to occur [11].
Classification of T3cDM is important, as the proposed pathophysiology for T3cDM differs from T1DM and T2DM. The proposed mechanism involves inflammation, fibrosis, and sclerosis of pancreatic endocrine tissue (including cells that secrete glucagon, somatostatin, and pancreatic polypeptide), which leads to a reduction in total number of insulin producing islet cells and alteration of their function [11]. T3cDM affects all cells in the islets of Langerhans and therefore has features of both insulin resistance and insulin deficiency. Furthermore, several additional hormones are affected including glucagon, pancreatic polypeptide, incretin, adipokines (in the AP episode) leading to a unique clinical entity. This is characterized by a patient who has risk for hyperglycemic and hypoglycemic events with increased insulin requirements early in the disease course, but decreased risk of diabetic ketoacidosis [11].
The long-term management also differs in T3cDM. One study followed patients for up to 13 years and differentiated impaired glucose metabolism into T2DM and T3cDM. They found that all of the patients who had T3cDM eventually required insulin, where as those diagnosed with T2DM were predominantly controlled by oral medications [12]. This observation supports the proposed mechanism that T3cDM is due to inflammation, scarring and islet loss, leading to less insulin secretion, rather than predominant insulin resistance found in T2DM.
DIAGNOSTIC CRITERIA
The diagnosis of T3cDM has been difficult to distill. Currently similar diagnostic criteria for T2DM exist including: clinical symptoms of hyperglycemia and glucose of ≥ 200 mg/dL or asymptomatic individuals with at least two abnormal biochemical tests: fasting glucose ≥ 126 mg/dL, 2-hour glucose ≥ 200 mg/dL after 75-g oral glucose ingestion, or hemoglobin A1c (HbA1c) ≥ 6.5% [13]. However, the role of pancreatic dysfunction in diagnosis of T3cDM remains controversial. Many etiologies exist leading to T3cDM including: acute and chronic pancreatitis, pancreatic cancer, cystic fibrosis, hemochromatosis, etc. [11]. This makes including criteria based on pancreatic dysfunction and etiology difficult given the heterogeneity in disease and variable progression to T3cDM. For example, patients post-pancreatectomy may have an abrupt onset of T3cDM, whereas those with AP may have more subtle, slower progression.
Others have proposed targeting the characteristics specific to T3cDM which included: impaired beta cell function, lack of insulin resistance, deficiency of lipid-soluble vitamins A, D, E, and K, and impaired release of glucagon-like peptide-1 and pancreatic polypeptide [11]. Specifically, Ewald and Bretzel [14] proposed the following diagnostic criteria: (all of the following must be met)
- A diagnosis of diabetes mellitus
- Evidence of exocrine pancreatic insufficiency (fecal elastase 1 [FE1] < 200 μg/g or abnormal direct function testing)
- Abnormal pancreatic imaging (endoscopic ultrasound, magnetic resonance imaging, and computed tomography)
- Absence of T1DM associated autoimmune markers (antibodies against glutamine acid decarboxylase, islet cell antigen, or insulin) [14].
These criteria have undergone criticism for being particularly difficult to implement clinically [15]; however, they provide a potentially more specific approach to diagnosing T3cDM.
Finally, another study measured baseline and post-stimulation insulin and C peptide levels as a distinguishing marker for insulin resistance versus beta cell destruction. Amongst the small number of patients in the study, they found a trend towards lower C peptide and insulin levels in those who had severe AP (compared to mild disease); however, they also found an increase in C peptide and insulin levels in those who developed DM in general [16]. In general, the wide range of diseases that lead to T3cDM and the variable timeline of disease development makes it difficult to have clear cut diagnostic criteria. Currently, it is favored to first establish a diagnosis of DM, and then to pay particular attention to a patient’s pre-disposing conditions, namely, disease of the pancreas, to determine if their pathology more closely aligns with T3cDM versus other subtypes (T1DM or T2DM). Careful delineation of T3cDM from other subtypes is important to ensure optimal follow-up and treatment [7].
INCIDENCE/PREVALENCE
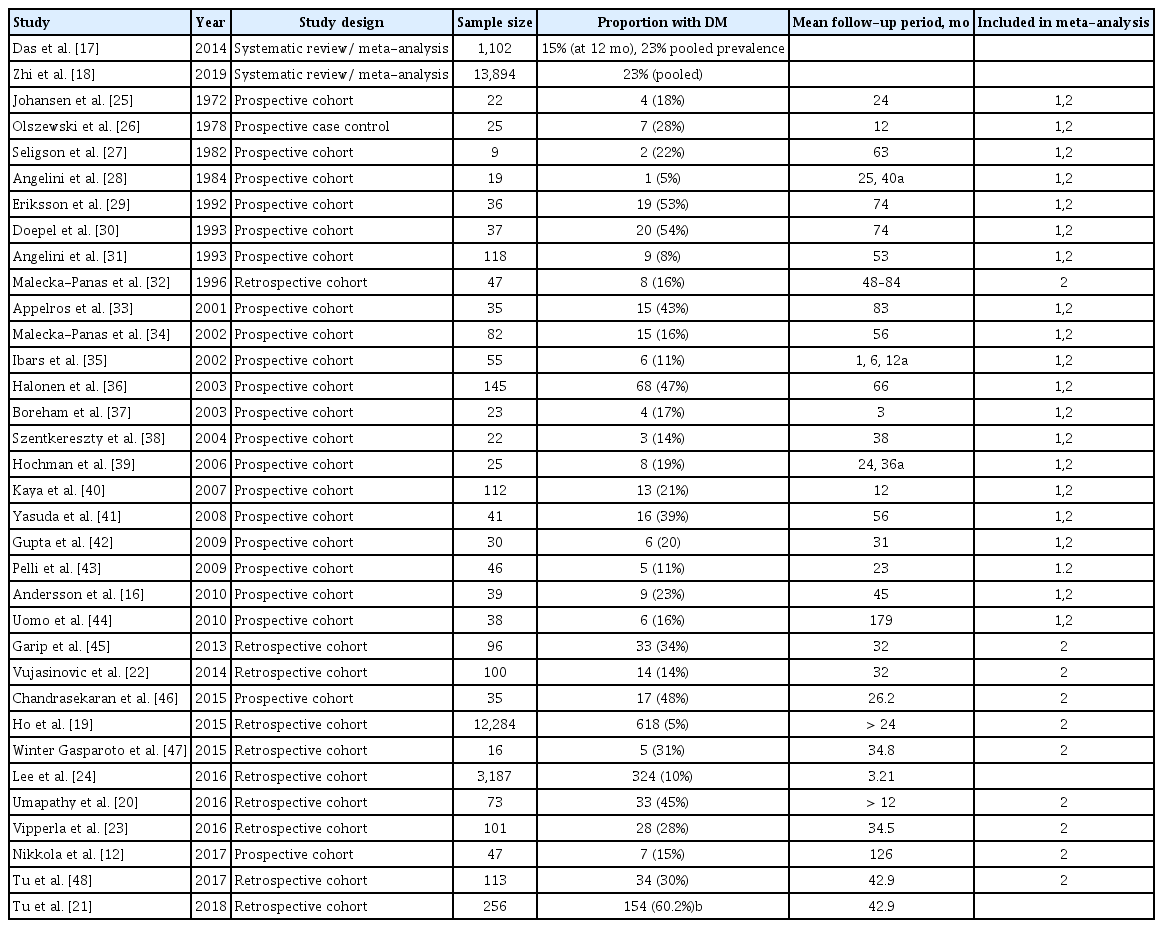
As a self-limiting disease, the concept that AP could lead to diabetes is one that is of increasing interest. Several studies have attempted to characterize the incidence and/or prevalence of new onset diabetes following a single AP event. These studies range from small, single-center cohorts to population level data to meta-analyses (Table 1) [12,16–48]. The incidence and prevalence vary widely in these studies. These differences likely stem from study design bias including but not limited to variable follow-up periods and tertiary referral bias.
An important study worth explicitly noting was a meta-analysis conducted in 2014 that showed an incidence of DM after 1 year of 15% and up to 23% after 5 years. This study specifically looked at incidence following the first episode of AP. Furthermore, the investigators subdivided impaired glucose metabolism into pre-DM, DM, and DM treated with insulin and found a pooled prevalence of 16%, 23%, and 15%, respectively over their study period [17].
Another meta-analysis conducted in 2019 showed a similar pooled incidence of 23% [18]. Upon further sub-analysis, the investigators found the pooled incidence within 5 years was 20% and after 5 years was 37%, showing a trend for increased development of endocrine dysfunction over time. This analysis, compared to the 2014 meta-analysis, used many of the same studies and also included additional studies up to 2017. Though there was heterogeneity in the data, it was similar to the previous meta-analysis and provides compelling evidence of incidence of DM following AP.
Other studies, however, show that DM is less common. Specifically, a large population-based study conducted using a national database in Taiwan showed only 5% developed endocrine dysfunction over a greater than 1 year follow-up period [19]. Various other studies show a wide range of incidence/prevalence. These studies have variable sample sizes, follow-up intervals and patient demographics, and exclusion criteria. Furthermore, some studies combined pre-diabetes and diabetes, whereas others separated these values. Finally, as physicians underrecognize T3cDM, the diagnostic criteria used in these studies varied making it difficult to compare results [6].
Given the data collected so far, it is difficult to ignore that a significant proportion of patients develop some form of impaired glucose metabolism after an episode of AP. The large meta-analyses have consistently shown close to a quarter of patients are diagnosed with diabetes at the 5-year mark, with the potential for even a greater portion after 5 years. Further studies are needed with strict inclusion and exclusion criteria as well as further characterization of what subtype of DM develops, to better characterize impaired glucose metabolism in this cohort.
NATURAL HISTORY
It is a known phenomenon that hyperglycemia occurs after critical illness [49]. When the body is under stress, especially in the setting of acute illness, it releases cortisol which stimulates gluconeogenesis in the liver and limits the uptake of glucose in the peripheral tissues leading to relative insulin resistance [50]. A study examined this phenomenon and found following Intensive Care Unit stay, stress induced hyperglycemia occurred in 17% of patients but only 4.8% of patients went on to develop T2DM. AP, leads to a similar acute illness and thus interferes with short- and long-term impaired glucose metabolism. This study exemplifies the complex natural history of DM in patients with critical illness and possibly identifies a similar process to what occurs following AP. It specifically highlights the distinction between transient hyperglycemia following critical illness and the development of chronic impaired glucose metabolism or diabetes.
In another study using a large population database in Taiwan, investigators evaluated the risk of developing DM after a first episode of AP. They found there were greater “odds” of developing insulin resistance in the first 3 months post-event (hazard ratio [HR], 5.9) compared to after 3 months (HR, 2.54). This demonstrates a previously discussed phenomenon of transient hyperglycemia post-event, followed by a smaller proportion going on to develop sustained impaired glucose metabolism [51].
In another study that included a cohort of severe AP patients, 45% developed new onset diabetes after their first episode of AP, with the vast majority developing it during the index admission [20]. In fact, the mean time from index admission to diagnosis of DM was 1 month. Development of DM was associated with the extent of necrosis in this study and brings up the question whether more severe AP episodes leads to more rapid development of T3cDM.
These studies highlight that the time course and natural history of diabetes following AP is not clearly defined. It appears to be on the orders of months to years, with a trend for increasing disease prevalence farther from the index AP episode (Fig. 1). This may suggest that the injury associated with AP may not be entirely self-limiting. Rather the injury may set in motion an inflammatory process with subsequent fibrosis with ongoing implications towards endocrine insufficiency. More research should be done to further delineate this time course, so surveillance in groups at higher risk of developing DM can be pursued.
PREDICTORS OF DIABETES OF THE EXOCRINE PANCREAS
Severity
The data have been mixed on whether severity of an AP episode increases the risk of developing T3cDM. We know historically from the data surrounding pancreatectomies that patients with DM prior to surgery often have worsening of their disease post-operatively as greater tissue loss occurs [52]. Similarly, for recurrent AP, one study evaluated computed tomography evidence of pancreatic volume loss in patients with a single episode of AP compared with recurrent pancreatitis. The investigators found total pancreatic volume was significantly reduced in those with recurrent AP and these patients also had a strong association with endocrine and exocrine insufficiency [53].
This, and other data, suggests that the theory of greater islet cell loss, leads to greater risk of developing T3cDM and impaired glucose metabolism. This has been supported by several studies [16,18,21–23]. Of note, some of these studies have predominantly severe cases, while others have a majority of mild cases, and often direct cohort comparison was difficult. The meta-analysis, however, was able to compare larger cohorts of severe AP and mild AP and found an incidence of DM of 39% compared to 14%, respectively [18].
Other studies, however, have shown no relationship between severity of AP and development of T3cDM [17,24,51]. These studies conclude that a mechanism, other than pure necrosis and cell loss, is at play. There are certainly limitations to these studies. In particular the meta-analysis [17] again included many studies with only severe cases of AP and was not able to do a sub-analysis based on severity of disease.
It is important to mention there has been an evolution in the classification of severity of AP: from Ranson’s criteria, to APACHE II, to Balthazar score, to the Atlanta classification of AP and BISAP score, to more recently revised Atlanta criteria [4]. Many of the studies reviewed used the Atlanta criteria to classify severity; however, others used the APACHE score, and others incorporated computed tomography data (Balthazar score) to assess pancreatic necrosis. This may have led to an inability to directly compare these studies and draw broad conclusions.
Given the evidence and data collected so far, it is very likely that severity of AP and total islet cell destruction and loss plays a part in the pathophysiology of T3cDM; however, it is also likely that this is not the sole risk factor or mechanism at play.
Etiology
Several studies examined etiology of pancreatitis and risk of developing T3cDM. It is well established that the three most common causes of AP are: gallstone, alcohol, and hypertriglyceridemia [4]. Several studies found that alcohol was associated with greater risk of developing T3cDM [18,19]. These studies postulate that alcohol’s effect on the pancreas directly and via its metabolites leads to multiple pathways of damage ultimately leading to atrophy, fibrosis, and premature activation of digestive enzymes. Furthermore, the specific activation of pancreatic stellate cells (by metabolites) leads to ongoing inflammatory response, fibrosis, and damage after the initial insult occurs [18]. Others, however, have found no significant association with etiology and development of T3cDM [16,17,22] suggesting confounding variables exist with alcoholic pancreatitis and development of T3cDM.
Other risk factors
There has been limited exploration of other risk factors associated with increased endocrine dysfunction after AP. One study explored a predictive model for developing diabetes post-AP and created a nomogram [54]. The investigators found that body mass index, age, glucose, triglycerides and low-density lipoprotein at time of admission were associated with increased risk of DM over a 3-month follow-up period. This study highlights other comorbid conditions that may contribute to worsened impaired glucose metabolism after an AP episode. Another smaller study monitored patients up to 3.5 years after AP and found that obesity and hyperlipidemia were risk factors [55]. For these risk factors it is difficult to distinguish between traditional risk factors for DM and their novel impact on T3cDM after AP. The studies conducted so far did not have control groups to distinguish natural progression to DM compared to development of DM after AP. Though the examination of risk factors for developing T3cDM is limited, it may begin to highlight particular patient populations who warrant closer follow-up after an AP episode.
CONCOMITANT ENDOCRINE AND EXOCRINE INSUFFICIENCY
Many studies explored both endocrine and exocrine impairment after AP, and some found significant overlap [12,23,56]. Some studies cite as high as 40% overlap [56] where as others have as low as 3% overlap [19]. Many of these studies used FE1 to measure pancreatic exocrine insufficiency; however, others used need for pancreatic enzyme replacement therapy [23] and a meta-analysis used a variety of measures (secretin-caerulein infusion testing, serum pancreolauryl testing, fecal elastase and fecal fat testing, self-reported need for enzyme replacement) [56].
For chronic pancreatitis, FE1 is a commonly used indirect measure of pancreatic exocrine function. For the diagnosis of chronic pancreatitis, it has increased sensitivity with increased severity of disease (63% mild, 100% moderate, 100% severe) and specificity of 93% [57,58]. FE1 has been subject to criticism as a test for exocrine function. Specifically, it is thought it is a useful tool in ruling out pancreatic exocrine insufficiency when you have a low pre-test probability, however, often leads to many false positives [59].
Furthermore, some definitions of T3cDM have even included the need for evidence of exocrine dysfunction [14]. It is therefore important to characterize the relationship between endocrine and exocrine dysfunction in patients after AP and to determine the best marker for disease overlap.
SCREENING RECOMMENDATIONS
There is no consensus on when or who to screen for impaired glucose metabolism after AP. One can extrapolate from the chronic pancreatitis consensus guidelines and consider screening yearly with either fasting glucose or HbA1c [23,60]. Additionally, it is recommended to pay particularly close attention to those with recurrent episodes or severe episodes [23]. This proposed follow-up timeline will likely capture a significant number of patients, and will avoid premature capture of the cohort who experience transient hyperglycemia following AP. There are groups who may warrant closer follow-up, namely, those with severe episodes or recurrent episodes [23]. It appears, however, that as an increasing proportion of patients develop DM at 5 years, there must be a balance between capturing patients post-event versus general population screening for DM. More research must be collected on this timeline, and what particular risk factors predispose an individual to developing T3cDM.
DIABETES AS AN ETIOLOGY FOR ACUTE PANCREATITIS?
A less well-established and more controversial concept that is important to mention is the bidirectional relationship between AP and diabetes. It is established that AP leads to DM; however, the reverse is less well studied.
One study examined this, using population level data derived from the Taiwan National Health Insurance claims database. The investigators first looked at the risk of developing AP in those with DM and compared those to controls. They found an increased HR of 1.72 of developing AP in those who were diabetic, and this was even higher if they had a history of ‘hyperglycemic crisis” (HR, 6.32). This study also found a similar relationship between developing DM after AP that many other studies have found (HR, 2.15) [24]. This study proposed that given the higher HR in those with a history of hyperglycemic crisis, there might be a “severity-response” relationship. Several mechanisms were proposed in this study including: (1) chronic hyperglycemia leads to increased reactive oxygen species, increased lipid peroxidase which may lead to AP episodes; (2) association with comorbid conditions such as obesity, hyperlipidemia, and gallstones which can precipitate AP; (3) cellular mechanisms including enhanced ryanodine receptor function leading to alterations in cellular mechanisms, specifically calcium and is a similar pathway involved in AP and DM [24]. Other studies have also supported this association. One study in particular showed a HR of 1.49 even after controlling for common comorbidities such as age, gender, obesity, smoking, alcohol use, or gallbladder disease [61].
Another study approached this question by examining the structural changes that occur in the pancreas as a result of DM [62]. They found pancreatic weight and volume were decreased in those with T1DM (no significant decrease in T2DM), and at autopsy, the investigators found fibrosis with minimal inflammatory changes and no duct abnormalities in these patients. Additionally, these patients were largely asymptomatic, despite having reduced FE1 levels. This study highlights a disease entity separate from chronic pancreatitis. This suggests pancreatic fibrosis and exocrine dysfunction exists separately (or on a continuum) from chronic pancreatitis and occurs most predominantly in those with T1DM. Though this cohort did not develop AP episodes, this study does highlight the presence of structural changes within the pancreas that may increase a patient’s risk for developing AP, further showing the complex interplay between the endocrine and exocrine pancreas.
CONCLUSIONS
In conclusion, DM (including T3cDM) and impaired glucose metabolism is common and increasingly recognized following AP. Among the types of DM, T3cDM is an increasingly recognized entity and has been found following AP. Though the diagnostic criteria have varied over time and it is largely underrecognized, its unique disease profile warrants further attention. These patients typically require insulin earlier than those with T2DM and often have difficult to manage hypo- and hyperglycemic episodes. Several large studies estimate prevalence of about 15% at 1 year and even greater proportion of cases at 5 years. Severity appears to affect propensity of developing diabetes, and several studies have found that alcohol may also be correlated. Physicians should be aware and aim to screen patients yearly following AP episode, and pay particular attention to those with severe episodes, alcoholic pancreatitis, and diabetes risk factors. Finally, there are data suggesting diabetes leads to structural changes in the pancreas potentially predisposing to AP, further highlighting the complex interplay between AP and the endocrine pancreas. This review highlights that diabetes following AP is an increasingly recognized clinical entity; however, currently the data are limited and heterogeneous and future studies are needed to clarify the existing gaps in knowledge.
Notes
No potential conflict of interest relevant to this article was reported.