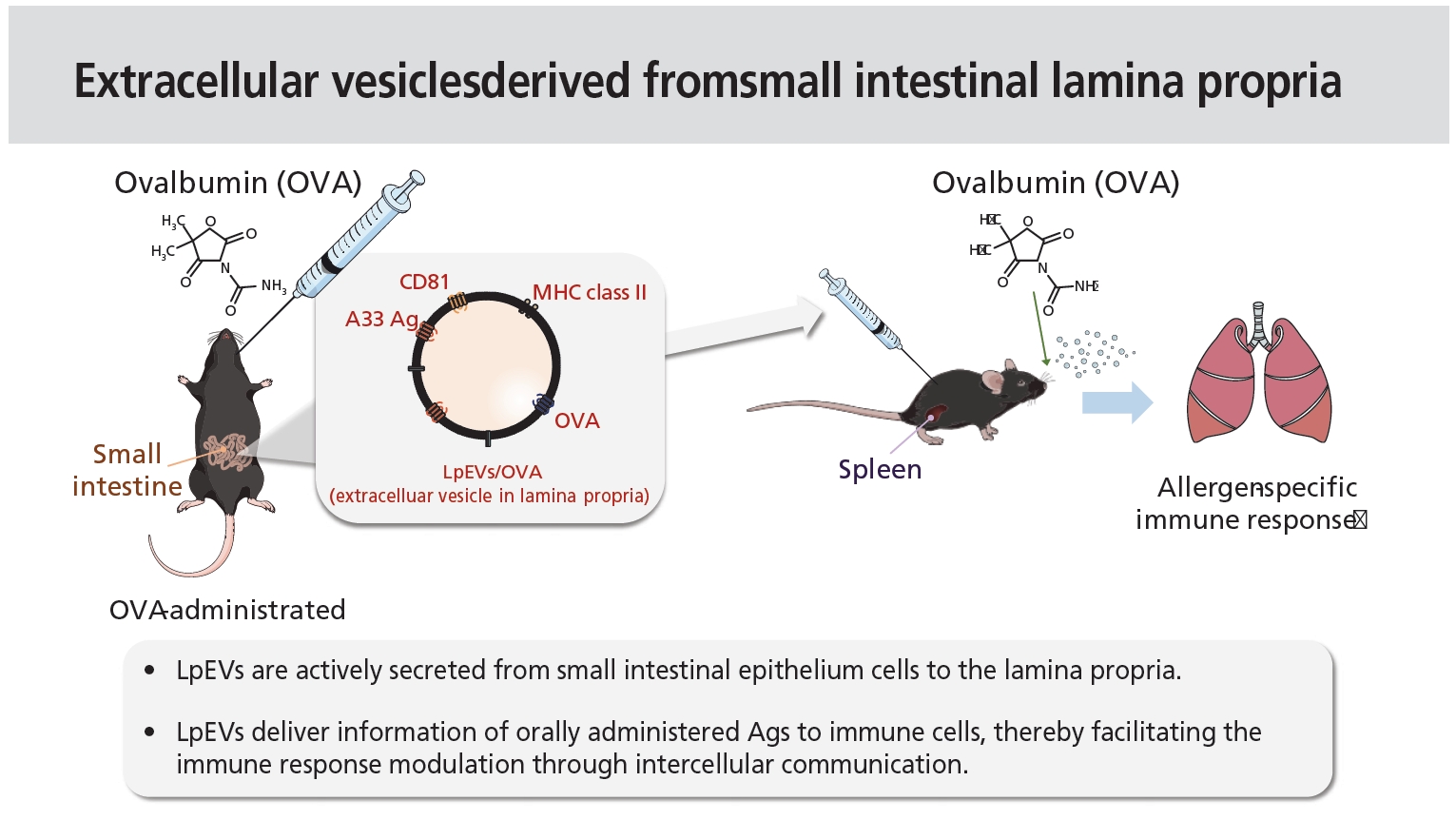
Extracellular vesicles derived from small intestinal lamina propria reduce antigen-specific immune response
Article information
Abstract
Background/Aims
Extracellular vesicles (EVs) are secreted from various types of cells and have specific functions related to their origin. EVs are observed in the small intestinal lamina propria (lpEVs), but their function remains unclear. This study aimed to investigate the role of lpEVs.
Methods
LpEVs were isolated from antigen (ovalbumin [OVA])-fed mice (lpEVs/OVA), and administrated to the naïve mice for 5 days before induction of lung inflammation. Afterwards, the mice were sensitized and challenged with OVA to evaluate the role of lpEVs/OVA in the regulation of immune tolerance.
Results
The isolated lpEVs/OVA were sphere-shaped, bi-layered vesicles of approximately 50 to 100 nm in size. The vesicles expressed CD81, A33 antigen, and major histocompatibility complex (MHC) class II on the surface. When administrated to naïve mice, the lpEVs/OVA migrated to the spleen. Intraperitoneal lpEVs/OVA administration to naïve mice decreased the immune response against sensitized antigen in a CD4+FoxP3+T cell-dependent manner.
Conclusions
EVs are actively secreted from small intestinal epithelial cells to deliver information about orally administered antigens to immune cells, which will facilitate the modulation of the immune response by acting as an intercellular communicasome.
INTRODUCTION
Extracellular vesicles (EVs) are 50 to 200 nm lipid bilayer membrane vesicles released extracellularly after the fusion of multivesicular endosomes with cell membranes. EVs contain several molecules, including proteins, DNA, and RNA [1], and are released into the surrounding environment from several cells that are implicated in the immune response, including B and T cells, dendritic cells (DCs), and mast and epithelial cells. EVs have also been found in biological fluids, such as bronchoalveolar lavage (BAL) fluid, serum, urine, and breastmilk [2]. First, EVs were considered as non-functional cell fragments; however, recent evidence has shown that their proteic load depends on the status of the cell of origin, and that they hold different functions according to the condition of the host [3]. For example, Cancer cell-derived EVs transport the epidermal growth factor receptor (EGFR) and transfer it into normal cells to induce tumorigenesis [4]. Moreover, EVs derived from DCs induce T-cell activation by acting as a ligand [5]. Therefore, EVs can be considered as an insoluble molecular complex for intercellular communication.
The small intestine is involved in food digestion and nutrients absorption, and is always exposed to foreign pathogens and/or non-pathogenic antigens, thereby functioning as an immune-modulatory organ that maintains immune homeostasis against non-pathogenic antigens [6]. Microfold cells found in Peyer’s patches are gateways for the luminal antigens, by presenting antigens to DCs to modulate immune homeostasis [7]. However, mice without Peyer’s patches can maintain immune homeostasis against orally administrated non-pathogenic antigens. In this context, there are two types of cells known to be involved in immune homeostasis: DCs in the lamina propria, which extend their dendrites to the luminal side across the tight junctions between the small intestinal epithelial cells [8], and the small intestinal epithelial cells that line the small intestine. Recent evidence indicates that the latter engulf the luminal antigens by endocytosis and express major histocompatibility complex class II (MHC II) on their basolateral side [9–11]. The lamina propria is a thin layer of loose connective tissue that lies beneath the epithelium and is rich in immune cells, such as DCs, T and B cells, and even eosinophils [6]. The lamina propria has been studied as a secondary lymphoid organ that modulates the immune response. Recently, MHC II positive nanoparticles were detected in the lamina propria, but their functions remain unclear [9]. In this study, we isolated nanoparticles from the small intestinal lamina propria (SILP) and confirmed that they were EVs originating from small intestinal epithelial cells. Additionally, EVs from small intestinal epithelial cells (lpEVs) were found to express MHC II with orally administrated antigens (ovalbumin [OVA]) on the surface. Pre-injection of lpEVs from antigen-administered mice (lpEVs/OVA) reduced the antigen-specific immune response in naïve mice. These results suggest that lpEVs act as an intercellular communicasome, which transfers information from orally administrated antigens to other cells, thereby modulating the immune response.
METHODS
Mice
C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). OT-II TG mice were kindly donated by Dr. Jang (Pohang University of Science and Technology, Pohang, Korea). Mice were bred in special pathogen-free facilities at Pohang University of Science and Technology. All live animal experiments were approved by the Pohang University of Science and Technology Animal Use and Care Committee (Permit Number: 2010-02-0015) and performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the Animal and Plant Quarantine, Ministry of Agriculture, Food and Rural Affairs, Korea.
Reagents
Aluminum hydroxide, OVA, and protease K were purchased from Sigma-Aldrich (St. Louis, MO, USA). Phenylmethylsulfonyl fluoride (PMSF) was purchased from Bio-Rad Laboratories (Hercules, CA, USA). Antibodies used for detecting EVs by Western blot were the following: anti-mouse CD81 (Eat-2 clone; Biolegend, San Diego, CA, USA) and anti-mouse A33 antigens (Santa Cruz Biotechnology, Dallas, TX, USA). Antibodies used for fluorescence-activated cell sorting (FACS) analysis were obtained from eBioscience (San Diego, CA, USA).
Isolation of lpEVs and lpEVs/OVA
Naïve mice or mice orally administrated OVA were euthanized and their small intestines were isolated to collect EVs from the SILP or lpEVs/OVA, respectively. Lipids were removed along with the Peyer’s patch with scissors. The small intestine was divided into approximately 1 cm2 rectangular sized pieces and washed thrice with 30 mL of phosphate-buffered saline (PBS) solution. The washed tissues were digested with collagenase (400 U) and DNase I (100 μg/mL), and incubated for 30 minutes with stirring using a magnetic bar. The samples were then centrifuged at 500 ×g for 10 minutes and at 3,000 ×g for 20 minutes [12]. To isolate lpEVs, the supernatant was loaded onto 0.8 and 2.5 M sucrose cushions in PBS (pH 7.2) and then subjected to ultracentrifugation at 100,000 ×g for 2 hours. Afterwards, the interface between the 0.8 and 2.5 M sucrose layers was collected and diluted 10-fold with PBS. The sucrose cushion ultracentrifugation procedure was repeated. The collected interface was diluted 10-fold with PBS and subjected to ultracentrifugation again at 100,000 ×g for 2 hours. Finally, the remaining pellet was resuspended in PBS, and the protein concentration was measured using the Bradford dye assay (Bio-Rad Laboratories). LpEVs were analyzed by dynamic light scattering (DLS), Western blot, or by FACS analysis, as previously described [3].
Transmission electron microscopy
Purified EVs were applied to 400-mesh carbon-coated copper grids (Electron Microscopy Sciences, Hatfield, PA, USA). After allowing the EVs to absorb for 3 minutes, the samples were stained with 2% uranyl acetate (Ted Pella, Redding, CA, USA). Transmission electron microscopy (TEM) was performed using a JEM 1011 and 1010 microscope (JEOL, Tokyo, Japan) at an accelerating voltage of 100 kV.
In vivo evaluation of lpEVs function
To evaluate the activities of lpEVs and lpEVs/OVA in the development of an adaptive immune response to inhaled allergens (OVA), 6-week-old mice were intraperitoneally pre-administered 100 μg of lpEVs or lpEVs/OVA for 4 consecutive days. The mice were sensitized with 2 mg of aluminum hydroxide and 75 μg of OVA on days 7 and 14 after lpEVs or lpEVs/OVA administration. The mice were then challenged intranasally with 50 μg of OVA on days 21 and 22. Allergen-specific adaptive immune responses were evaluated 24 hours after the final allergen challenge.
Evaluation of BAL fluid cellularity and lung histology
BAL cellularity was analyzed as described previously [13]. Briefly, BAL cellularity was determined by counting 300 inflammatory cells after diluting the BAL-derived cell pellets with 50 μL PBS. Inflammatory cells were classified as macrophages, lymphocytes, neutrophils, or eosinophils. Lung sections were stained with hematoxylin and eosin (H&E) after pressure fixation with Streck solution (Streck Laboratories, La Vista, NE, USA) for histological evaluation. All sample slides were evaluated under the same magnification. Lung inflammation was assessed based on the degree of peribronchiolar and perivascular inflammation, as previously described [3].
Isolation of peritoneal cells and CD4+ helper T cells, and in vitro stimulation
Mice were euthanized by cervical dislocation, and 3 mL of PBS was injected into the peritoneum. The abdomens of the mice were massaged to release peritoneum cells into the PBS solution, which was harvested using a syringe. Next, the red blood cells were removed by incubating with ammonium chloride for 15 minutes. OT-II CD4+T cells were isolated from the spleen with microbead-conjugated anti-CD4 (Miltenyi Biotec, Bergisch Gladbach, Germany), and CD4+T cells were sorted using auto-MACS. The isolated peritoneal cells (1 × 106) were incubated with 100 μg of lpEVs or lpEVs/OVA, and isolated OT-II CD4+T cells (2 × 106) in 96-well plates for 71 hours. To evaluate the expression of the transcription factor forkhead box P3 (FoxP3), the co-incubated OT-II CD4+T cells were incubated in 96-well plates coated with anti-CD3 and anti-CD28 antibodies (1 μg/mL each; eBioscience) at 37°C for 6 hours. The FoxP3 levels were quantified by intracellular staining and FACS analysis.
Cytokine assay
The levels of cytokines present in the BAL fluid and cell culture supernatants were measured by enzyme-linked immunosorbent assay, in accordance with the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA).
Latex beads labelling for FACS analysis
Aldehyde/sulfate latex beads (1 μg; Thermo Fisher Scientific, Waltham, MA, USA) were mixed with 100 μg of lpEVs/OVA suspension, and particle-free PBS was added up to a final volume of 1 mL. The mixture was left to incubate overnight at room temperature on a rotary wheel. Then, 100 μL of glycine was added and the mixture was incubated at room temperature for 30 minutes to block binding. The beads were pelleted by centrifugation at 5,000 rpm for 5 minutes, and the supernatant was removed. The pellet was washed with 1 mL of particle-free PBS. Finally, the pellet was resuspended and antibodies of interest were added according to standard FACS protocols.
Statistical analyses
Analysis of variance, Student’s t test, and Wilcoxon’s rank sum test were used to determine significant differences among treatments. Statistical significance was set at p < 0.05. R software version 3.6.3 (The R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analyses.
RESULTS
Characterization of nanoparticles from SILP
Nanoparticles isolated from the SILP of mice were observed by TEM, revealing that they were spherical bi-layered vesicles with approximately 50 to 100 nm in size, as measured by DLS. Based on protein concentrations measured using the Bradford assay, the amount of nanoparticles was approximately 200 μg/head. The expression of CD81, an EV marker, was detected in the nanoparticles. Other marker proteins were quantified to confirm the origin of the nanoparticles. A33 antigen, a marker for small intestinal epithelial cells, was highly expressed on the nanoparticles, whereas CD11c, CD11b, F4/80, and Ly6g were barely or not at all expressed. These data suggest that the nanoparticles from the SILP mostly originated from small intestinal epithelial cells (Fig. 1, Supplementary Fig. 1).
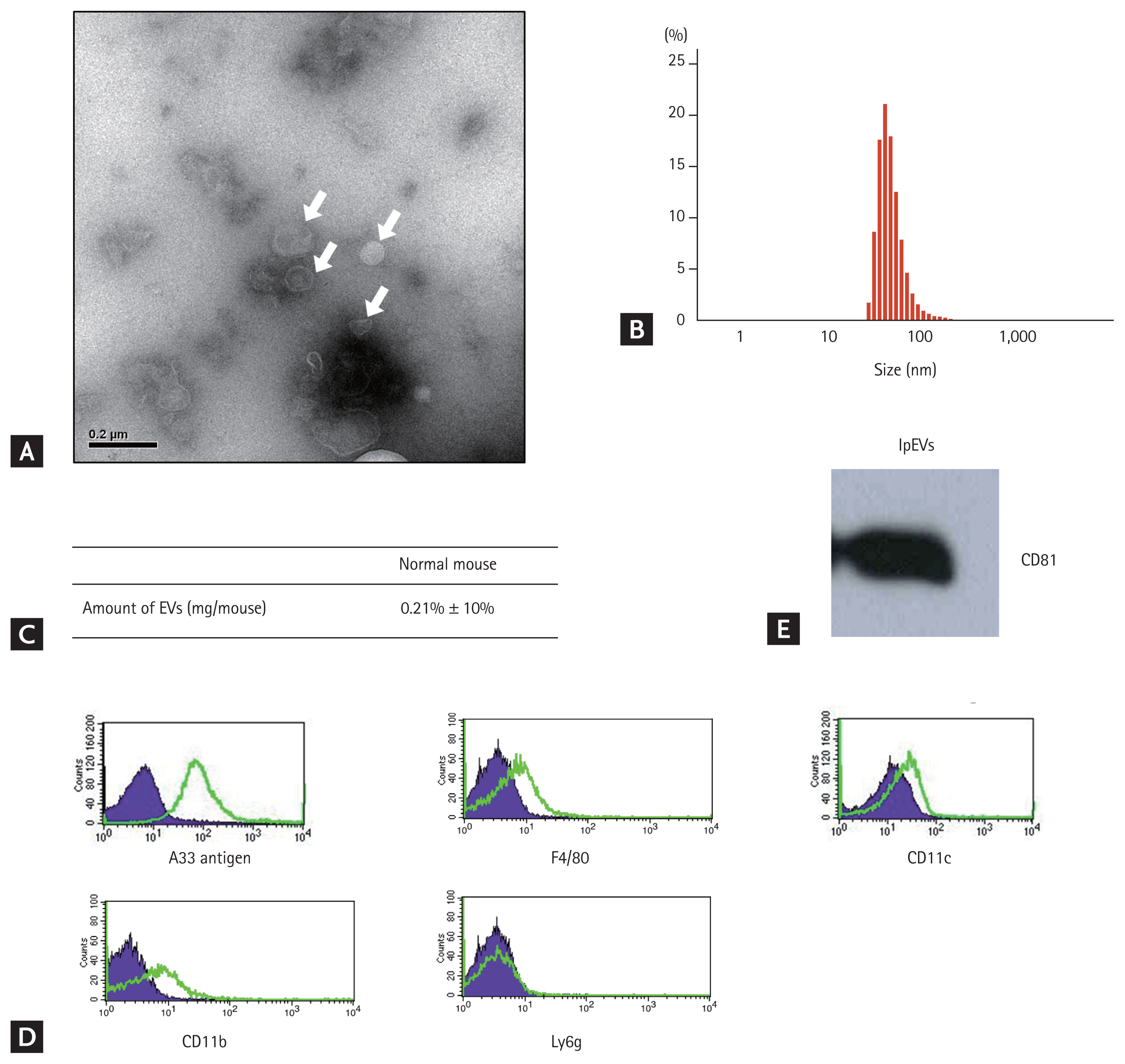
Isolation and characterization of nanoparticles from small intestinal lamina propria. (A) Transmission electron microscopy images of the nanoparticles derived from small intestinal lamina propria (white arrows). (B) Size of the nanoparticles derived from the lamina propria measured by dynamic light scattering. (C) Quantity of nanoparticles from the lamina propria, determined based on the protein concentrations measured using the Bradford assay. (D) Western blotting assessment of the host cell marker protein CD81 within nanoparticles from the lamina propria. The blot was scanned using a Samsung SCX-4623 device and photographs were cropped using Microsoft PowerPoint. Full length blot is presented in the Supplementary Fig 1. (E) Expression of the small intestinal epithelial marker A33 antigen and inflammatory cell marker proteins, F4/80, CD11c, CD11b, and Ly6g within the nanoparticles determined by flow cytometry. The original data is shown in Supplementary Fig. 1. EV, extracellular vehicle; lpEV, extracellular vehicle from small intestinal epithelial cell.
Characterization of EVs from SILP of OVA-administered mice
Orally administered antigens have been observed from endocytic vesicles of small intestinal epithelial cells after 1 hour. To identify whether orally administrated antigens reside in EVs of the lamina propria, we isolated EVs 1 hour after oral administration of OVA twice at 1 hour intervals (Fig. 2A). Since nanoparticles of the lamina propria are known to express MHC II, we quantified MHC II and OVA in EVs from OVA-administered mice. MHC II was detected on these EVs, and the expression was increased compared with that in naïve mice. OVA was detected only in EVs from OVA-administered mice (Fig. 2B and 2C, Supplementary Fig. 2). These results indicate that orally administered antigens are gathered by small intestinal epithelial cells and released to the basolateral side with MHC II loaded EVs. However, it remains unclear whether OVA and MHC II form a complex.

Isolation and characterization of small intestinal lamina propria-derived nanoparticles (extracellular vehicles from small intestinal epithelial cells [lpEVs]) from ovalbumin (OVA)-fed mice. (A) Protocol for in vivo oral administration of OVA and isolation of lpEVs. (B) Flow cytometry data of lpEVs and lpEVs/OVA for analysis of the major histocompatibility complex II (MHC II). (C) Western blotting assessment of OVA levels in lpEVs or lpEVs/OVA. The blot was scanned using a Samsung SCX-4623 device and cropped with Microsoft PowerPoint. Full length blot is presented in the Supplementary Fig. 2.
LpEVs/OVA reduce allergen-specific immune response
To evaluate the functions of lpEVs in OVA-administered mice (lpEVs/OVA), we pre-injected lpEVs/OVA into the intra-peritoneum of naïve mice for 5 days because there is no method available to control the release of lpEVs. Three days after pre-injection, C57BL/6 mice were sensitized with aluminum hydroxide and allergen (OVA), while challenged with OVA alone (Fig. 3A). Examination of BAL cellularity revealed that the infiltration of inflammatory cells, specifically eosinophils, into the lungs was significantly reduced in mice pre-injected with lpEVs/OVA compared with mice pre-injected with lpEVs or positive control (Fig. 3B). The lung inflammatory score, which was based on the peribronchiolar and perivascular infiltration of inflammatory cells, was found to be lower in mice pre-injected with lpEVs/OVA than in mice pre-injected with lpEVs or positive control (Fig. 3C and 3D). In this context, cytokines, which promote infiltration of inflammatory cells, were quantified in BAL fluid samples. Pro-inflammatory cytokine tumor necrosis factor-alpha (TNF-α) and interleukin 5 (IL-5) were reduced only in the lpEVs/OVA pre-injected group (Fig. 3E). CD4+ memory T cells are important mediators that induce an adaptive immune response to inert allergens. Therefore, we evaluated the pattern of CD4+T cells present in the lung tissue. The CD4+T cells were isolated from the lung tissue of each group of mice and stimulated in vitro with CD3 and CD28. Interferon-gamma (IFN-γ) or IL-17, which represent Th1 or Th17 immune responses, were not detected in any group, whereas IL-4, which represents the Th2 immune response, was secreted in mice pre-injected with lpEVs or positive control (Fig. 3F). These findings indicate that lpEVs were derived from orally administered antigens and that the anti-inflammatory functions depend specifically on the antigens. However, the underlying anti-inflammatory mechanisms remain unclear.

Protective role of small intestinal lamina propria-derived nanoparticles (extracellular vehicles from small intestinal epithelial cells [lpEVs]) from ovalbumin (OVA)-fed mice on the development of allergen-specific hyper-response. (A) Experimental protocol. (B) Bronchoalveolar lavage (BAL) cellularity. (C) Representative lung histology (H&E staining, ×100; a, NT, negative control; b, PT, positive control; c, lpEVs; d, lpEVs/OVA). (D) Inflammatory score based on the peribronchiolar and perivascular infiltration of inflammatory cells in the lung tissue. (E) In vivo levels of tumor necrosis factor-alpha (TNF-α) and interleukin 5 (IL-5) at 24 hours after final challenge. (F) Levels of Th2 (IL-4) cytokine in culture medium, 6 hours after incubation of lung CD4+T cells with anti-CD3 and anti-CD28 antibodies. ap < 0.05 compared to PBS group.
Migration of lpEVs/OVA to the spleen and fusion with CD11c+ cells
To trace the location of lpEVs/OVA within the body, lpEVs/OVA were prepared from transgenic (TG) mice expressing green fluorescent protein (GFP) and pre-injected into wild-type (WT) naïve mice three times at 24 hours intervals. On the day after the final injection, the mice were euthanized and their organs were collected for GFP analysis. GFP signal was detected only in the spleen of lpEVs/OVA-injected mice (Fig. 4A and 4B), whereas no signal was evident in mice pre-injected with lpEVs or in the liver and mesenteric lymph nodes of lpEV/OVA pre-injected mice. To determine the mechanism of lpEVs/OVA migration and whether the lpEVs/OVA migrated alone, peritoneal cells were isolated from GFP TG mice and injected into WT mice for 3 days at 24 hours intervals. As a means of stimulation, lpEVs or lpEVs/OVA were injected 1 hour after each injection. GFP was observed only in the lpEVs/OVA-injected group, but not in lpEVs-injected mice (Fig. 4C and 4D). These data suggest that lpEVs/OVA do not migrate alone; although, how lpEVs/OVA internalize the peritoneal cells and migrate to the spleen warrants further investigation.

Migration of small intestinal lamina propria-derived nanoparticles (extracellular vehicles from small intestinal epithelial cells [lpEVs]) from ovalbumin (OVA)-fed mice to the spleen after internalization by peritoneal cells. (A) Experimental protocol for lpEVs/OVA tracing. (B) Immunohistochemistry of the liver, mesenteric lymph node, and spleen derived from mice injected lpEVs or lpEVs/OVA isolated from green fluorescent protein (GFP) transgenic mice (×100). (C) Experimental protocol for the mechanistic study of lpEVs/OVA migration. (D) Immunohistochemistry of the spleen derived from lpEV or lpEVs/OVA-injected mice administrated peritoneal cells isolated from GFP transgenic mice (×100). GFP positive peritoneal cells are depicted by arrows. mLN, mesenteric lymph node; IP, intraperitoneal.
Induction of antigen-specific CD4+FoxP3+T cells by lpEVs/OVA
Pre-injection of lpEVs/OVA reduced the antigen-specific immune response. This can occur via several mechanisms. Among them, the small intestine can induce the development of antigen-specific regulatory T cells (Tregs). Thus, we evaluated the effect of lpEVs/OVA on antigen-specific Treg development using CD4+T cells expressing the OVA-specific T-cell receptor (OT-II CD4+T cells). Peritoneal cells isolated from WT mice were co-incubated with OT-II CD4+T cells and lpEVs or lpEVs/OVA. After 72 hours, the transcription factor FoxP3 (which is a marker of Treg) was found to be only expressed by OT-II CD4+T cells co-incubated with lpEVs/OVA, but not in cells co-incubated with lpEVs (Fig. 5A). Furthermore, CD3 and CD28 re-stimulation of co-incubated OT-II CD4+T cells with lpEVs/OVA induced the secretion of IL-10 (Fig. 5B). Altogether, these findings indicated that lpEVs/OVA transfer of antigens (OVA) to peritoneal cells activated these cells and induced differentiation from naïve T cells into CD4+FoxP3+T cells.

Induction of CD4+FoxP3+T cells via extracellular vehicles from small intestinal epithelial cells (lpEVs)/ovalbumin (OVA) occurs in an antigen-specific manner. (A) Intracellular staining of forkhead box P3 (FoxP3) transcription factor. Flow cytometry data were derived from CD4+ gated spleen cells. (B) Secretion of the immune-modulatory cytokine interleukin 10 (IL-10) quantified from culture supernatant of re-stimulated cells with anti-CD3 and anti-CD28 antibodies for 6 hours. ap < 0.05 compared to phosphate-buffered saline group.
DISCUSSION
Recent evidence has indicated that EVs are secreted from several types of cells and are found in various biological fluids, such as BAL fluid, milk, urine, and even stools [3,14–16]. EVs are insoluble complex structures that are actively released. They contain proteins, DNA, RNA, and other molecules, and they are not the dust of cells or apoptotic bodies, contrary to previously believed. In this context, nanoparticles expressing MHC II have been found within the SILP [9]. However, to date, their characteristics and functions were unknown. The present data show that nanoparticles isolated from the SILP are EVs that originate from small intestinal epithelial cells. Furthermore, intraperitoneal injection of EVs derived from mice orally administered antigens reduced the antigen-specific Th2 immune response, which mimics the immune response induced by airway exposure to antigens. To the best of our knowledge, our study is the first to demonstrate the role of lpEVs, which may be critical to modulate the immune response by functioning as an intercellular communicasome [17].
MHC II nanoparticles from the SILP can be difficult to isolate; thus, their characteristics remain unclear. Herein, we describe a protocol to isolate the nanoparticles from the lamina propria and characterize them. These nanoparticles were found to be spherical lipid bilayer vesicles with approximately 100 nm in size. They contain the EV marker protein CD81 and the intestinal epithelial cell marker A33 antigen [2,18]. These findings suggest that the nanoparticles on the lamina propria are EVs derived from intestinal epithelial cells.
Microfold cells of Peyer’s patches or DCs are involved in the mechanism of antigen uptake. Recent studies have suggested that small intestinal epithelial cells engulf luminal antigens by endocytosis, process and release them into the basolateral side of the small intestine [9]. IN the present study, we revealed that EVs from the SILP of OVA-administered mice contained OVA peptide and MHC II. However, our data did not show whether the OVA peptide was loaded on MHC II. Nonetheless, the data indicated that pre-injection of lpEVs/OVA, and not lpEVs, to the peritoneal cavity of naïve mice reduced the allergen (OVA)-specific immune response. Collectively, these findings suggest that, although it remains unclear whether the OVA peptide is loaded on MHC II, lpEVs/OVA transport information of orally administrated antigens and transfer this information to other cells, which fulfill an adaptive immune response, similar to an intercellular communicasome.
EVs have various functions depending on the type or status of cells. Because there is no method to control the release of lpEVs in vivo, we pre-injected lpEVs/OVA or lpEVs into the peritoneal cavity of naïve mice and induced allergen-specific lung inflammation to evaluate the functions of these EVs. Pre-injection of lpEVs/OVA, but not lpEVs, reduced lung inflammation, specifically eosinophil inflammation and Th2 immune response. In this context, it has been reported that endocytosis of luminal antigens can produce an intracellular signaling switch to activate cells, and that the components of EVs are different depending on the cellular status [19]. Therefore, we suggest that lpEVs/OVA are secreted depending on an active mechanism and have specific functions to modulate the immune response.
In the human body, the secondary lymphoid organs, such as the spleen, mesenteric lymph node, and even the liver, are involved in modulating immune tolerance [20,21]. We tried to trace lpEVs/OVA localization to clarify how they modulate the immune response. Thus, we isolated lpEVs/OVA from GFP TG mice, which express GFP protein spontaneously. GFP signals were observed only in the spleens of mice pre-injected with lpEVs/OVA, but not lpEVs. However, how lpEVs/OVA migrate to the spleen remains to be investigated. Our previous data showed that EVs are rapidly internalized, within at least 6 hours [3]. Therefore, we hypothesized that lpEVs/OVA pre-injected into naïve mice may migrate to the spleen with other carriers present in the peritoneal cavity. To clarify whether lpEVs/OVA migrate alone, peritoneal cells were isolated from GFP TG mice and pre-injected into WT mice for 3 days. One hour after each injection, lpEVs or lpEVs/OVA were administrated to the WT mice that had been pre-injected with peritoneal cells. GFP signals were observed only in the lpEVs/OVA-injected group but not in the lpEVs-injected group; therefore, we suggest that lpEVs/OVA migrate to the spleen with peritoneal cells to modulate the immune response.
The peritoneum harbors various immune cells, including B and T cells, macrophages, and other granulocytes, to protect the host when leakage of the gastrointestinal tract occurs [22]. Among them, macrophages are one of the most important immune cells to fulfill the innate immune response. Recent evidence indicates that peritoneal macrophages migrate to the spleen, a process that is related not only to the innate immune response but also to the adaptive immune response [23]. Our data showed that lpEVs/OVA, not lpEVs, can induce CD4+Foxp3+T cells, which are from OVA-specific T-cell receptor TG mice. In addition, lpEVs/OVA activated peritoneal macrophages to induce the secretion of Treg-polarized cytokines IL-10 and TGF-β.
To illustrate how lpEVs/OVA are internalized by macrophages and how macrophages migrate to the spleen, some possible explanations could be drawn from previous studies. Several types of interactions between EVs and recipient cells have been proposed based on indirect evidence and in vitro studies. These interactions include the adhesion of EVs to the recipient cell surface through lipids, ligand-receptor interactions, and the subsequent internalization of whole EVs into the endocytic compartments of cells. Recent studies have indicated that EVs derived from intestinal epithelial cells have several types of adhesion molecules on their membranes. Moreover, we previously demonstrated that EVs treated with protease could not internalize into recipient cells [3,9,24]. Thus, it can be concluded that, although we could not identify the molecule that is detrimental for the internalization of lpEVs into the recipient cells, a membrane protein on the lpEVs may be the essential for the internalization by peritoneal macrophages. Although our study targeted EVs originating from small intestinal epithelial cells, it is possible that EVs may also be secreted from DCs or macrophages, which are abundant in the lamina propria. To overcome this limitation, additional studies should explore the potential role and immuno-regulatory effect of DCs- or macrophage-derived EVs in the lamina propria.
In conclusion, our present data suggest that lpEVs, which are EVs actively secreted from small intestinal epithelium cells to the lamina propria, deliver information regarding orally administrated antigens to immune cells, which will in turn facilitate the modulation of the immune response by acting as an intercellular communicasome. The exact mechanisms underlying this process still need to be further explored.
KEY MESSAGE
1. Extracellular vehicles from small intestinal epithelial cells (lpEVs) are actively secreted from small intestinal epithelium cells to the lamina propria.
2. LpEVs/ovalbumin are internalized by peritoneal cells that migrate to the spleen, although its underlying mechanism remains unclear.
3. LpEVs deliver information of orally administered antigens to immune cells, thereby facilitating the immune response modulation through intercellular communication.
Notes
Conflict of interest
This work was done regardless of MD healthcare. And the author who belong to MD healthcare has no conflict of interest with this work. No other author has reported a potential conflict of interest relevant to this article.
Acknowledgements
We thank Sung Won Kim for improving the use of English in the manuscript.