Diagnostic index for acute eosinophilic pneumonia without bronchoscopy in military smokers
Article information
Abstract
Background/Aims
Acute eosinophilic pneumonia (AEP) is common among military smokers; however, bronchoscopy is required for the diagnosis. We aimed to derive and validate a scoring system to diagnose AEP without bronchoscopy.
Methods
We conducted a retrospective study including patients diagnosed with AEP or any other pneumonia among military smokers hospitalized in the Armed Forces Capital Hospital from 15 November 2016 through 25 December 2019. The patients were divided into derivation and validation groups according to their admission day. Patient symptoms, laboratory findings, and computed tomography findings were candidate variables. Least absolute shrinkage and selection operator (LASSO) regression was used to calculate the scores for each variable.
Results
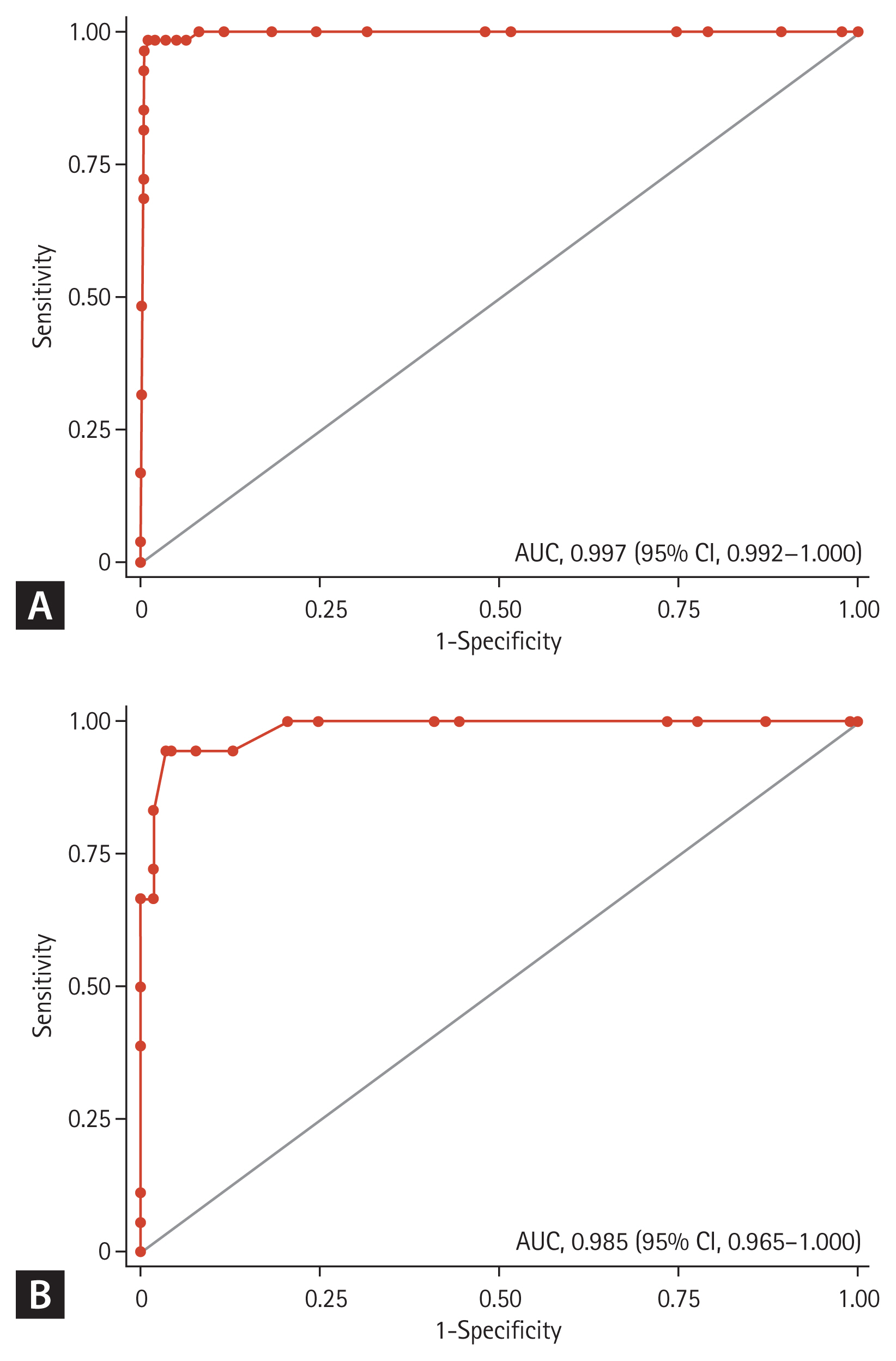
Among 414 patients, AEP was confirmed in 54 of 279 patients (19.4%) in the derivation group and in 18 of 135 patients (13.3%) in the validation group. Ten variables were selected using LASSO regression: new-onset or a recently increased smoking (≤ 4 weeks) (8 points), interlobular septal thickening (5 points), absence of sputum (3 points), ground glass opacity (3 points), acute onset (≤ 3 days) (2 points), dyspnea (2 points), chest pain (2 points), leukocytosis (2 points), bronchovascular bundle thickening (2 points), and bilateral involvement (2 points). The area under the receiver-operating characteristic curve of the score to diagnose AEP was 0.997 (95% confidence interval, 0.992 to 1.000) in the derivation group and 0.985 (95% confidence interval, 0.965 to 1.000) in the validation group.
Conclusions
We introduce a scoring system that can distinguish AEP from other types of pneumonia in military smokers without the need for bronchoscopy.
INTRODUCTION
Acute eosinophilic pneumonia (AEP) is a relatively rare respiratory disease, characterized by acute-onset dyspnea, fever, bilateral lung involvement, and lung eosinophilia [1]. The underlying pathophysiology of this disease is not fully known; it can be caused by various agents including smoking and medication [2]. AEP can progress rapidly and result in acute respiratory failure, which is life-threatening [3]. Fortunately, it responds well to systemic steroids [4,5].
Although certain computed tomography (CT) findings are recognised as key characteristics of AEP [6], it is difficult to fully distinguish AEP from other types of pneumonia in its early phase [4]. For an accurate diagnosis, bronchoscopic evaluation with bronchoalveolar lavage fluid is necessary to fulfil the diagnostic criteria [7]. Although systemic steroids may be considered for treatment in urgent settings without bronchoscopy, they must be used with caution in patients without an accurate diagnosis. In the recently published community-acquired pneumonia (CAP) guidelines [8], the use of systemic steroids is not recommended in patients with CAP.
Compared to the general setting, AEP was more commonly reported among military personnel [9–11]. Most of these patients were smokers [9,11,12]. However, early bronchoscopy may not be feasible in certain situations, such as in cases involving a shortage of medical equipment, unavailable medical staff, or acute deterioration of the patient [13]. Furthermore, bronchoscopy has become a high-risk procedure during the coronavirus disease pandemic as it provokes droplet formation [14]. Therefore, we aimed to derive and validate a scoring system that can distinguish AEP from other types of pneumonia without bronchoscopy in military smokers.
METHODS
Study subjects and definition of AEP
Patients hospitalized in the Armed Forces Capital Hospital from 15 November 2016 through 25 December 2019 were screened. The Armed Forces Capital Hospital is the highest-level referral center among 17 military hospitals in South Korea, and is the only center capable of performing bronchoscopy. Therefore, all patients suspected to have AEP are transferred to our center [10,12,15]. For this study, patients diagnosed with either AEP or pneumonia were selected. Because the aim of our study was to aid the diagnosis of AEP among military smokers, those who did not smoke at the time of admission were excluded. We also excluded patients without CT results, because chest CT findings are one of the key characteristics of AEP [6,16].
The diagnosis of AEP was confirmed according to the modified Philit criteria [7]: (1) acute respiratory illness (≤ 1 month); (2) pulmonary infiltrates on chest imaging; (3) pulmonary eosinophilia (> 25% eosinophils in the bronchoalveolar lavage fluid); and (4) absence of other specific pulmonary eosinophilic diseases. Some patients could not undergo bronchoscopy due to their urgent medical condition, unavailability of emergent bronchoscopy, or a temporary shortage of medical staff. Therefore, they were clinically suspected to have AEP and were treated accordingly. These patients were excluded because the fulfilment of the modified Philit criteria could not be assessed, nor could they be classified as having other pneumonias. Thus, we excluded patients whose diagnosis was inconclusive, who were transferred to other hospitals, and who died before the final diagnosis. The patients who were hospitalized from 15 November 2016 through 31 December 2018 were assigned to the derivation group, and those hospitalized after this period were assigned to the validation group.
Data collection
Data were collected retrospectively from the patients’ medical records; these included data on patient demographics, smoking history, comorbidities, and symptoms. Smoking history included conventional cigarettes only. We evaluated the duration (weeks), quantity (packs per day), and any change in habits of smoking. Patients who restarted smoking in the preceding 4 weeks after quitting for longer than 4 weeks were considered new-onset smokers, and patients who doubled their quantity of daily smoking within the preceding 4 weeks were considered to have increased their quantity of smoking. The comorbidities inspected were hypertension, diabetes mellitus, chronic lung disease (asthma, chronic obstructive pulmonary disease, bronchiectasis, or tuberculosis-destroyed lung), chronic liver disease, history of tuberculosis, history of AEP, and any type of allergy. Symptoms consisted of dyspnea, fever, chills, night sweats, cough, sputum, chest pain, rhinorrhea, myalgia, fatigue, palpitation, and sore throat, along with the onset of the chief complaint. Laboratory findings that could be commonly evaluated were extracted: white blood cell count, neutrophil count, lymphocyte count, eosinophil count, platelet count, and C-reactive protein levels.
The included chest CT findings were ground glass opacity, interlobular septal thickening, bronchovascular bundle thickening, pleural effusion, bilateral involvement, consolidation, and centrilobular nodules [6,17,18]. The CT findings were independently inspected by two blinded researchers (S.P. and D.H.) and any discrepancy was resolved by another researcher (H.J.K.).
Our study was conducted in accordance with the amended Declaration of Helsinki and was approved by the Institutional Review Board of the Armed Forces Capital Hospital (protocol number: AFCH-20-IRB-025). The requirement for informed consent was waived because of the retrospective design of the study, but the records were anonymized prior to the analyses. There were no relevant missing data found during the data collection process.
Construction of the scoring system
The scoring system was constructed according to the recent recommendation for the development and reporting of prediction models in respiratory medicine [19]. The candidate variables included the majority of the inspected characteristics. Since this scoring system was designed for bedside use, continuous variables were transformed into generally acceptable forms. The white blood cell count was transformed to leukocytosis (≥ 10,000/μL) or not, platelet count to thrombocytopenia (≤ 150,000/μL) or not, eosinophil count to eosinophilia (≥ 500/μL) or not, and neutrophil and lymphocyte counts to the neutrophil-to-lymphocyte ratio. The smoking history was simplified to ‘new-onset or a recent increase in the quantity of smoking (≤ 4 weeks)’ [20,21]. The onset of the chief complaint was simplified as acute onset (≤ 3 days) or otherwise. Consequently, a total of 36 candidate variables were included to construct the scoring system (Supplementary Table 1).
For variable selection and score derivation, the least absolute shrinkage and selection operator (LASSO) regression was used [22]. With LASSO regression, a simplified list of variables was derived with 10-fold cross-validation, and the penalized coefficients were calculated. To simplify the coefficients for use in the scoring system, they were multiplied by 5 and rounded to the nearest integer.
Validation of the score
Each patient’s total score was calculated using the derived scoring system. The total score was used to evaluate its performance by calculating the area under the receiver-operating characteristics curve (AUC) to predict the diagnosis of AEP for the derivation and validation groups. To evaluate the goodness-of-fit of our model, a calibration plot was obtained and the Hosmer-Lemeshow test was performed for both groups.
Our study was performed in accordance with the Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD) statement [23]. The TRIPOD checklist is available in Appendix 1.
RESULTS
Patient selection and characteristics
During the study period, 1,178 patients were screened. After excluding the non-eligible patients, 414 patients were included in our study and were divided into the derivation (n = 279) and validation groups (n = 135); 54 of 279 patients (19.4%) and 18 of 135 patients (13.3%) were diagnosed with AEP in the two groups, respectively (Fig. 1).

Flowchart of the patient selection process. AEP, acute eosinophilic pneumonia; CT, computed tomography.
All the patients were male, with a median age of 20 years (interquartile range, 20 to 21). The median duration of smoking was 37.5 months (interquartile range, 12.0 to 62.5), which was shorter for those diagnosed with AEP than for those diagnosed with other types of pneumonia (median 0.8 months vs. 48.0 months, p < 0.001). Fifty-seven of 72 patients (79.2%) diagnosed with AEP were classified as new-onset smokers, as compared to 13 of 342 patients (3.8%) diagnosed with other types of pneumonia (p < 0.001). The distribution of comorbidities did not differ between patients diagnosed with AEP and those without. The patients diagnosed with AEP had a higher white blood cell count (median 14,015/μL vs. 7,260/μL, p < 0.001), neutrophil count (median 10,940/μL vs. 5,175/μL, p < 0.001), lymphocyte count (median 1,375/μL vs. 1,230/μL, p = 0.042), platelet count (median 241 × 103/μL vs. 189 × 103/μL, p < 0.001), and higher levels of C-reactive protein (median 7.9 mg/dL vs. 5.4 mg/dL, p = 0.006). Radiographic findings differed significantly according to the diagnosis of AEP. Among the patients with AEP, ground glass opacity (100.0% vs. 77.5%, p < 0.001), interlobular septal thickening (94.4% vs. 6.1%, p < 0.001), pleural effusion (36.1% vs. 7.6%, p < 0.001), bronchovascular bundle thickening (33.3% vs. 1.5%, p < 0.001), and bilateral involvement (98.6% vs. 37.1%, p < 0.001) were more commonly found than in patients diagnosed with other types of pneumonia. The prevalence of consolidation (40.3% vs. 87.1%, p < 0.001) was lower in patients with AEP. Results from microbiological evaluation were less likely to be positive in patients with AEP compared to those without AEP in both bacterial (56.9% vs. 75.0%, p = 0.002) and viral origin (19.3% vs. 73.4%, p < 0.001) from respiratory specimens (Table 1). Details of the microbiological evaluation are presented in Supplementary Table 2.
Patients in the derivation and validation groups revealed similar characteristics, although there were several differences. Patients in the derivation group had a higher incidence of interlobular septal thickening (24.4% vs. 15.6%, p = 0.041) and bilateral involvement (51.3% vs. 40.7%, p = 0.045). Patient symptoms and laboratory findings did not differ between the two groups (Supplementary Table 3).
Derivation of the score
After LASSO regression, 14 variables were selected from the 36 candidate variables. The selected variables were new-onset or a recent increase in the quantity of smoking (≤ 4 weeks), interlobular septal thickening, ground glass opacity, the presence of sputum, bronchovascular bundle thickening, dyspnea, chest pain, leukocytosis (≥ 10,000/μL), bilateral involvement, acute onset (≤ 3 days), fever, fatigue, pleural effusion, and a higher neutrophil-to-lymphocyte ratio. The penalized coefficients were calculated, multiplied by 5, and subsequently rounded off to the nearest integer. After this process, the coefficients of four variables (fever, fatigue, pleural effusion, and the neutrophil-to-lymphocyte ratio) became zero and were excluded from the final score (Table 2).
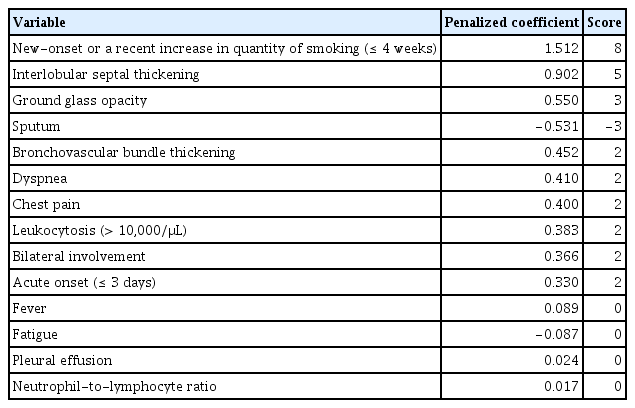
Calculated penalized coefficients of variables selected by least absolute shrinkage and selection operator
The scores were organized as follows: 8 points for new-onset or a recent increase in the quantity of smoking (≤ 4 weeks); 5 points for interlobular septal thickening; 3 points for both the absence of sputum and ground glass opacity; and 2 points each for acute onset (≤ 3 days), dyspnea, chest pain, leukocytosis (≥ 10,000/μL), bronchovascular bundle thickening, and bilateral involvement (Table 3). The score distribution ranged from 0 to 31 points. We named this scoring system ‘AEP Diagnostic Index for Military Smokers,’ which was abbreviated as ADIMS. The ADIMS score was calculated for each patient and showed a similar distribution among the derivation and validation groups (Supplementary Fig. 1).
Validation of the score
The AUC of the ADIMS to distinguish AEP from other types of pneumonia was 0.997 (95% confidence interval [CI], 0.992 to 1.000) in the derivation group, and 0.985 (95% CI, 0.965 to 1.000) in the validation group, which refers to an excellent performance (Fig. 2). We evaluated the calibration plots and Hosmer-Lemeshow tests, which revealed that the ADIMS fit well (Supplementary Fig. 2).
Sensitivities and specificities
Various cut-off values were used to calculate the sensitivities and specificities to correctly diagnose AEP. With cut-off values of ≥ 18, 96.30% of patients could be correctly classified. The cut-off of ≥ 18 points showed a sensitivity of 94.44% and a specificity of 96.58% (Supplementary Table 4).
DISCUSSION
This is the first study to introduce a scoring system, the ADIMS, which can distinguish AEP from other types of pneumonia without bronchoscopy. The ADIMS showed excellent discrimination performance in both the derivation and validation groups. The cut-off of ≥ 18 points showed a high sensitivity and specificity in distinguishing AEP from other types of pneumonia in young male military smokers.
The presenting manifestations of the patients with AEP in our study were similar to those reported previously among other study populations [2,9,10,20,24]. Symptoms of dyspnea, cough, chest pain, fever, and fatigue, along with bilateral parenchymal infiltrates were commonly observed in studies from the United States [2,9]. A study from Japan reported that the symptoms of fever, dyspnea, and cough were common, while sputum was not [20]. Previous reports from our center at a different time period also confirmed similar characteristics, including cough, dyspnea, fever, diffuse ground glass opacity, interlobular septal thickening, pleural effusion, and leukocytosis [10,24].
The excellent discrimination performance of the ADIMS can be explained by the distinct features of AEP compared to those of other types of pneumonia. Primarily, AEP is well known to be associated with a recent change in smoking habits [25,26]. New-onset smoking, along with restarting or increasing the amount of smoking, was commonly observed among patients with AEP [20]. In a previous study, patients who smoked a cigarette every hour for a duration of 4 hours suffered from cough and dyspnea within 12 hours of exposure, along with a decline in the partial pressure of oxygen and vital capacity [20]. On the other hand, the association between a recent change in smoking habits and CAP has not been established, despite the higher risk of CAP among smokers and ex-smokers [27].
The recognizable radiographic features of AEP also play an important role in enhancing the performance of the ADIMS. As previously reported [17], among 29 patients with AEP, ground glass opacities and interlobular septal thickening were present in more than 90%, with more than 70% presenting with bilateral involvement. Such diffuse bilateral lung involvement along with pleural effusion was also emphasized in an earlier study [18]. This differs from other commonly encountered pneumonia types such as CAP. Focal airspace consolidation is the most common radiographic abnormality found in CAP [28], especially in cases of bacterial origin [29].
Dyspnea, chest pain, and the absence of sputum were selected as key symptoms in the ADIMS. Since our study population included young military patients (median age 20) with minimum underlying comorbidities, the risk of deterioration due to CAP was very low [28]. Therefore, dyspnea, which implies extensive involvement of the lungs, was relatively rare in patients with other types of pneumonia compared to that in patients with AEP. This was also true for chest pain, which implies chest tightness or discomfort resulting from acute respiratory difficulty. The presence of pleural effusion may induce pleuritic chest pain, which is common in AEP but only observed in approximately 20% of hospitalized patients with CAP [30]. This is in accordance with the incidence of chest pain related to other types of pneumonia found in our study (19.0%). The absence of sputum was another important aspect. Although sputum can be present in any type of respiratory disease, the purulence and color may imply an infectious origin [31,32].
Leukocytosis was the only laboratory parameter selected in the ADIMS. Peripheral neutrophilic leukocytosis in the early course of disease is a well-known characteristic of AEP [5,7,33–35]. The exact cause for this phenomenon is not fully understood; however, the increased production of interleukin-8 by bronchial epithelial cells following cigarette exposure may be associated with such peripheral neutrophilic leukocytosis [35].
We would like to make some recommendations in order to utilize the ADIMS wisely. First, the ADIMS should be considered in young military smokers with suspected lower respiratory tract disease. Second, it should be an alternative diagnostic method when bronchoscopic evaluation is not possible. When bronchoscopy is feasible, the modified Philit criteria should be the gold standard for diagnosing AEP. Third, an ADIMS score of ≥ 18 seems to be an appropriate cut-off value considering the high sensitivity (94.44%) and specificity (96.58%) at that point. When the cut-off is achieved, the administration of systemic steroids should be considered in these patients.
Several limitations should be recognised. First, a detailed history regarding heat-not-burn and electronic cigarettes was not included in our study. Although both smoking products can be possible causes for AEP [36], they were excluded because it was not possible to assess the relative importance of each agent. In addition, the standardized quantification of electronic cigarettes is impossible, considering the diverse ingredients in the devices [37]. Second, our study was confined to young male military smokers and could not evaluate exposure to certain drugs or toxins. This was inevitable due to the single center retrospective design in a military hospital. Although AEP is common in young military smokers, future studies are required to validate the ADIMS in other populations.
In conclusion, we developed and validated the ADIMS, a simple scoring system that can efficiently distinguish AEP from other types of pneumonia without bronchoscopy in military smokers. The model was well-fit and revealed excellent performance. The ADIMS can be used as a reasonable alternative to diagnose AEP when bronchoscopic evaluation is not feasible.
KEY MESSAGE
1. Among 414 patients with suspected acute eosinophilic pneumonia or other types of pneumonia, acute eosinophilic pneumonia was confirmed in 62 (15.0%) patients.
2. Recent change in smoking habits, chest computed tomographic findings, patient symptoms, and some laboratory findings could distinguish acute eosinophilic pneumonia from other types of pneumonia in military smokers.
3. The scoring system can be a useful alternative to diagnose acute eosinophilic pneumonia among military smokers, when bronchoscopic evaluation is not feasible.
Acknowledgments
This work was supported by the Korean Military Medical Research Project funded by the ROK Ministry of National Defense (ROK-MND-2020-KMMRP-024). The sponsor had no role in the study.
Notes
No potential conflict of interest relevant to this article was reported.