Hepatic sinusoidal obstruction syndrome/veno-occlusive disease after hematopoietic cell transplantation: historical and current considerations in Korea
Article information
Abstract
Hepatic sinusoidal obstruction syndrome/veno-occlusive disease (SOS/VOD) is a rare but severe complication of hematopoietic cell transplantation (HCT) showing high mortality. Multiple risk factors for SOS/VOD were identified, but it is often confused with other hepatic complications due to nonspecific clinical features. Therefore, diagnostic and severity criteria have been revised several times. The European Society of Blood and Marrow Transplantation suggested a new guideline that excludes the standard duration of development within 21 days, emphasizes late-onset SOS/VOD, and suggests the importance of Doppler ultrasonography. The severity criteria were further subdivided for guidance to begin active treatment using defibrotide which was approved in Korea since 2016. In a phase 3 trial, defibrotide had superior 100-day survival, compared to best available treatments (38.2% vs. 25.0%). Although several studies of SOS/VOD in Korean patients have been performed after the implementation of HCT, most involved small number of pediatric patients. Recently, the Korean Society of Blood and Marrow Transplantation investigated the incidence of SOS/VOD in the Korean population, and several influential studies of adult patients were published. Here, we summarize recent issues regarding the mechanism, diagnosis, severity criteria, prevention, and treatments of SOS/VOD in Korean patients, as well as recent analyses of nationwide incidence.
INTRODUCTION
Hepatic sinusoidal obstruction syndrome or veno-occlusive disease (SOS/VOD) is a lethal complication that mainly develops after hematopoietic cell transplantation (HCT). The incidence of SOS/VOD varies according to study design and among patient cohorts; moreover, diagnostic criteria for both pediatric and adult populations have changed over time [1–8]. In Korea, there has been no nationwide report of the incidence of SOS/VOD, and few large transplantation cohort studies are available regarding post-HCT complications.
Risk factors for SOS/VOD involve transplant procedures, patient characteristics, underlying diseases, and hepatic concerns. Accordingly, traditional risk factors are wide-ranging and include allogeneic (allo)- or autologous (auto)-HCT, myeloablative conditioning (MAC) regimen (e.g., busulfan or total body irradiation [TBI]), unrelated donor or human leukocyte antigen-mismatched transplant, old age, advanced disease status, and previous liver disease (e.g., liver cirrhosis or active viral hepatitis) [6,7]. Recently, some novel agents have been shown to place patients at high risk for SOS/VOD [9–11].
Diagnostic criteria and severity stratification guidelines for SOS/VOD for both adult patients [7] and pediatric patients [8] were recently revised by the European Society of Blood and Marrow Transplantation (EBMT). However, older criteria, such as the modified Seattle [1, 2] or Baltimore [3] criteria, remain in use; these criteria include hyperbilirubinemia, hemodynamic instability (e.g., ascites or weight gain), and painful hepatosplenomegaly. The revised diagnostic criteria emphasize the speed of disease progression, time of onset (early vs. late), and hemodynamic instability as confirmed by Doppler ultrasonography. Older severity criteria [12] have been revised for more detailed stratification. Korean multicenter studies have revealed the need for early intervention to prevent progression to severe disease [13].
SOS/VOD should be actively managed according to disease severity due to its rapid progression and poor survival outcomes, particularly in patients with severe to very severe disease. In a phase 3 trial that enrolled patients with severe or very severe SOS/VOD, 100-day survival was significantly superior in patients who received defibrotide treatment, compared to patients who received conservative management [14]. Because defibrotide is the sole approved agent for the treatment of SOS/VOD, progression should be carefully monitored for early intervention. Preventive management might be the best approach to improve overall transplantation outcomes and prevent SOS/VOD onset. In this review, we summarize historical data regarding Korean patients with SOS/VOD data. We first reviewed 62 papers published from 1996 to 2020 and selected clinically relevant publications; we excluded pediatric-specific data, case reports, and conceptual in vivo studies. Because there have been several recently revised guidelines regarding the diagnostic and severity criteria for SOS/VOD, we provide consensus updates of these guidelines and describe modifications according to specific situations in Korea.
PATHOPHYSIOLOGY OF SOS/VOD
Major pathological changes due to SOS/VOD occur in the sinusoids, particularly zone 3 of the hepatic acinus [15]. Damage and thrombotic activation of sinusoidal endothelial cells lining the inner aspects of sinusoids occur during the early stages of SOS/VOD [16]. Destruction of the endothelium and entry of inflammatory cells and microbial products into the space of Disse cause vascular narrowing. The causes of sinusoidal endothelial damage are wide-ranging (Fig. 1). Previous chemotherapy or radiotherapy and current preconditioning regimens (e.g., alkylating agents or TBI) are toxic causes of SOS/VOD; these agents directly and indirectly attack endothelial cells, resulting in an inflammatory response and thrombotic activation [6]. Damaged hepatocytes and glutathione enzyme impairment system impairment lead to the accumulation of toxic metabolites that also contribute to endothelial destruction, as well as the entry of inflammatory cells and microbial products into the space of Disse [17,18]. Alloreactive complications after allo-HCT (e.g., graft-versus-host disease [GVHD]) also contribute to endothelial damage [19]. Moreover, granulocyte colony-stimulating factor and cyclosporine reportedly increase the expression levels of adhesion molecules, thereby aggravating inflammatory cell recruitment [20,21]. After endothelial damage, the extrinsic thrombotic pathway is activated by tissue factor activation, leading to clot formation. During the thrombotic process, increased levels of plasminogen activator inhibitor-1 (PAI-1) also interrupt the fibrinolytic pathway, thus promoting thrombotic sinusoidal occlusion [22].
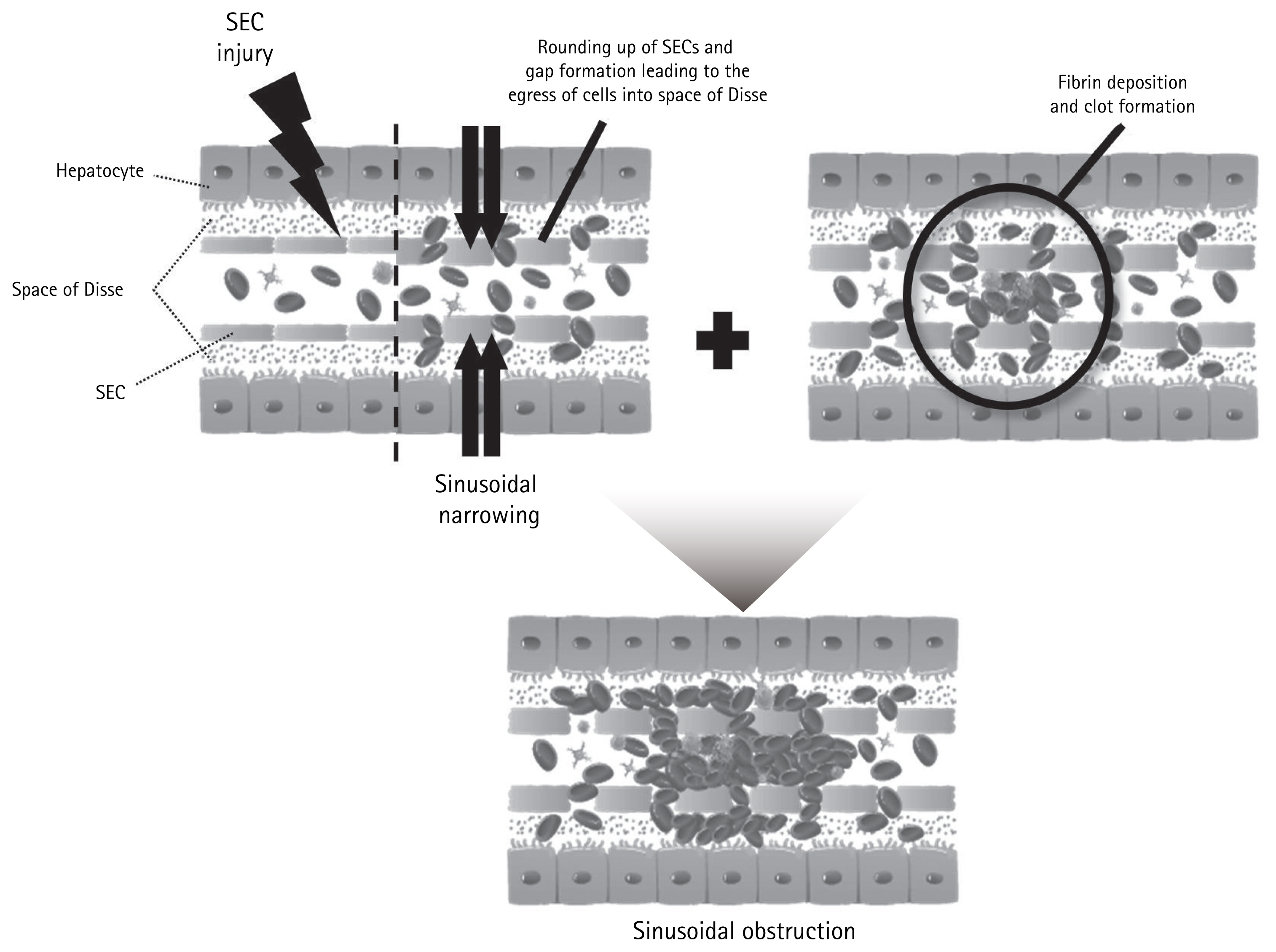
Pathophysiology of hepatic sinusoidal obstruction. Variable toxic agents cause sinusoidal endothelial cell (SEC) injuries, which accelerate sinusoidal narrowing and promote entry of inflammatory cells and microbial products into the space of Disse. Endothelial damage also activates the thrombotic pathway, which aggravates clot formation and fibrin deposition.
RISK FACTORS FOR SOS/VOD
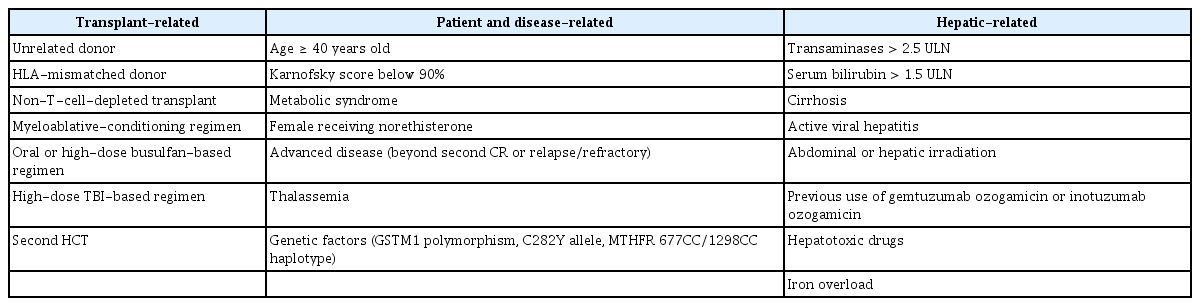
Risk factors for the development of SOS/VOD after HCT have been identified in many previous studies (Table 1). Hepatotoxic agents used for pre-HCT remission induction or preconditioning are major concerns. Inflammatory events that contribute to endothelial damage after HCT are additional risk factors; several patient-related factors contribute to increased susceptibility to hepatotoxicity. The EBMT previously suggested three risk factor subgroups: factors related to HCT; factors related to the patient’s susceptibility to SOS/VOD, including underlying disease; and hepatic-related factors (e.g., preexisting hepatic disease) [6,7]. Recent research has focused on several novel agents used for remission induction or GVHD prevention, which may be risk factors for SOS/VOD [9,10,23–26].
Transplantation-related factors
With regard to transplantation-related factors, alloreactivity level is higher in allo-HCT than in auto-HCT [5,27]. However, rather than alloreactivity itself, some studies have demonstrated that high alloreactivity of unrelated or human leukocyte antigen-mismatched donor transplant is associated with the onset of SOS/VOD [28,29]. Furthermore, high alloreactivity was reportedly correlated with poor survival outcomes after SOS/VOD in Korean adults [13]. Another transplantation-related factor is the type of MAC regimen, including high-dose busulfan or TBI [27,28,30–33]. These preconditioning regimens damage the sinusoidal endothelium and hepatocyte glutathione system, thus causing an inflammatory response and coagulation. Busulfan-containing MAC regimens are associated with SOS/VOD, particularly when administered orally rather than intravenously [18,34,35], and when administered concomitantly with other alkylating agents [27]. The risk for SOS/VOD with busulfan is presumably dose-dependent; a Korean study demonstrated that a dose greater than 9.6 mg/kg significantly increased the risk for SOS/VOD [29,36], while a lower-dose busulfan regimen was less strongly associated with the development of SOS/VOD in an auto-HCT setting despite the use of an MAC regimen [37–40]. However, SOS/VOD developed despite reduced intensity conditioning (RIC) regimens in 1.6% to 8.9% of patients, and SOS/VOD reportedly developed later in patients with lower bilirubin levels, when these patients were compared to patients who received standard MAC regimens [28,34,41,42]. In the recent Harmony trial that analyzed the role of defibrotide in preventing the development of SOS/VOD in high-risk or very high-risk patients, eligibility criteria included an MAC regimen involving at least two alkylators or TBI plus at least one alkylator (ClinicalTrials.gov identifier: NCT02851407). In addition, advanced second or third allo-HCT is considered a high-risk factor [32], particularly for the development of severe to very severe SOS/VOD [29].
Patient- and disease-related factors
Well-known patient-related risk factors include young age (pediatric vs. adult), older age among adult patients, female sex, poor performance status, and presence of metabolic syndrome [6,7,27,32]. However, previous data suggested that only female patients who receive progestin therapy are at high risk [43], and Korean data have shown that male patients are susceptible to SOS/VOD. Although RIC regimens are associated with a lower risk for SOS/VOD development, compared to MAC regimens, RIC regimens are mainly considered in older patients or in patients with comorbid conditions (e.g., metabolic syndrome). Thus, candidates for RIC-HCT may have several adverse risk factors for SOS/VOD, such that the risk for SOS/VOD may not significantly differ according to RIC or MAC regimen [29].
Transplantation in patients beyond their second remission or with refractory disease status is also associated with a greater incidence of SOS/VOD [2,6,27,32]. A recent study of Korean adults showed that the very high-risk group (according to refined disease risk index [DRI] for allo-HCT) had a significant association with the development of SOS/VOD [29]. Very high-risk DRI consists of the blastic phase of chronic myeloid leukemia, relapsed or refractory acute lymphoblastic leukemia (ALL), progressive Burkitt lymphoma or non-Hodgkin lymphoma, and relapsed or refractory acute myeloid leukemia (AML) with adverse-risk cytogenetics [44]. For pediatric patients, specific diseases such as osteopetrosis, thalassemia major, and hemophagocytic lymphohistiocytosis are associated with a high risk for SOS/VOD [45–47].
Hepatic-related factors
Previous hepatic dysfunction may result from pre-HCT chemotherapy or abdominal irradiation in patients with hematological malignancies treated with HCT [2,27,48]. The threshold for previous hepatic dysfunction has not been precisely elucidated, but proposed risk factors for SOS/VOD include a transaminase level > 2.5-fold above the upper limit of normal (ULN) or bilirubin level > 1.5-fold above the ULN during the peri-HCT period, combined with a history of liver cirrhosis or active viral hepatitis [2,27]. However, multivariate analyses in a recent Korean single-center study showed that a history of significant liver disease or viral hepatitis was not significantly associated with the development of SOS/VOD [29]. Iron overload, as indicated by elevated ferritin level, has been proposed as a risk factor for liver toxicity, including the development of SOS/VOD [36,49–52].
High-risk therapies associated with SOS/VOD
Multiple therapeutic agents are reportedly associated with increased risks of SOS/VOD in both HCT and non-HCT settings, including cyclophosphamide, cytarabine, vincristine, methotrexate, busulfan, oxaliplatin, gemtuzumab ozogamicin (GO), and inotuzumab ozogamicin (INO) [53]. In an expanded-access study that evaluated the outcomes of pediatric patients with SOS/VOD who were treated with defibrotide, 12% had SOS/VOD associated with primary chemotherapy [54]. In the Harmony trial, which evaluated the prophylactic role of defibrotide in the adult allo-HCT setting, patients who had received immunotoxin therapy (e.g., INO for relapsed or refractory ALL [10] or GO for AML) were reportedly at high risk for SOS/VOD [25]. Sirolimus, which is used for GVHD prophylaxis, was associated with SOS/VOD development, particularly when used with a busulfan-containing regimen or concomitantly with calcineurin inhibitors and methotrexate [23,55]. Notably, patients who underwent sirolimus-based GVHD prophylaxis demonstrated later onset of SOS/VOD and less severe features (i.e., less severe hyperbilirubinemia and weight gain, as well as more frequent complete resolution of SOS/VOD) [24]. There have also been several reports of oxaliplatin-related hepatic SOS/VOD, particularly in patients with solid organ malignancies and hepatic metastasis [56–58].
GO
GO is a humanized anti-CD33 monoclonal antibody linked to calicheamicin, which has been approved for the treatment of relapsed CD33+ AML. A phase 3, open-label study in 26 hematology centers in France (ALFA-0701) evaluated the efficacy and toxicity of adding low-fractionated-dose GO to standard chemotherapy (n = 140), compared to standard therapy alone (n = 140), in adults with AML [59]. In this study, SOS/VOD occurred in six of 131 (5.0%) evaluated patients who received GO; three (50.0%) of these six patients died. Another retrospective study evaluated 870 GO-treated patients (221 HCT recipients and 649 non-HCT patients). The overall incidences of SOS/VOD were 3.0% after GO monotherapy at doses < 6 mg/m2, 15% after GO monotherapy at a dose of 9 mg/m2, 28.0% after GO concomitant with thioguanine, and between 15% and 40% when HCT was performed at ≤ 3 months following GO. Death from SOS/VOD occurred in 33% of patients [60]. However, in another AML trial in older patients, GO monotherapy at doses of 6 mg/m2 on day 1 and 3 mg/m2 on day 8 was not significantly associated with the development of SOS/VOD [61]. Generally, lower doses of GO (median, 3 mg/m2) were not associated with SOS/VOD [62].
INO
INO is an antibody-drug conjugate composed of a humanized anti-CD22 monoclonal antibody conjugated to the cytotoxic agent calicheamicin. A phase 3 trial that compared INO with standard intensive chemotherapy in 326 patients with relapsed/refractory ALL reported that SOS/VOD occurred in 23 of 164 patients (14.0%) in the INO arm and in three of 143 patients (2.1%) in the standard therapy arm [63]. Based on data from this phase 3 trial, an expert panel of hematologists and transplantation physicians offered recommendations for preventing and monitoring SOS/VOD in patients receiving INO [64]. The key recommendations of this panel were the avoidance of HCT-conditioning regimens containing dual alkylating agents (e.g., thiotepa and melphalan); use of ursodiol prophylaxis in patients for whom HCT was considered; limitation of INO to two cycles if possible; and avoidance of hepatotoxic agents (e.g., azoles) in combination with high-dose alkylator-conditioning administration.
Korean data summary–risk factors
The incidence of SOS/VOD is much lower in patients who receive auto-HCT than in patients who receive allo-HCT, with respect to alloreactivity.
A myeloablative dose of busulfan (rather than TBI), male sex, and high-risk disease status (e.g., very high DRI) are the most influential risk factors associated with hepatic SOS/VOD after allo-HCT.
Antibody-calicheamicin conjugates such as GO or INO, which are associated with a high risk for SOS/VOD, are approved and widely used in Korean patients with acute leukemia.
INCIDENCE OF SOS/VOD
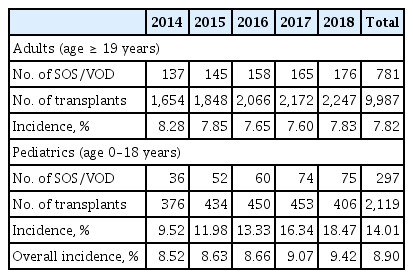
A meta-analysis of 27,269 transplants in Western countries calculated an overall incidence of SOS/VOD of 15% (range, 0.45% to 53.52%) [48]. Because there have been few nationwide incidence studies, we recently reviewed data from the Korean Health Insurance Review and Assessment Service, based on claims for reimbursements of test fees and drug prescriptions for each diagnostic category [65]. The Korean health insurance program covers the entire Korean population as a compulsory social insurance system. We searched the database from 2014 to 2018 to identify the number of transplants and incidence of SOS/VOD. Overall, 1,078 of 12,106 transplant patients (8.9%) developed SOS/VOD; 781 of 9,987 (7.82%) in adults and 297 of 2,119 (14.01%) in pediatric patients (Table 2). However, detailed subgroup analyses were not possible with this database; we also could not exclude the possibility of inaccurate coding. Thus, it was difficult to reassess the diagnostic and severity criteria for SOS/VOD, and to analyze detailed transplantation procedures.
A recent Korean single-center analysis of a large adult cohort showed that the incidences of SOS/VOD were 3.1% after allo-HCT and 0.6% after auto-HCT. In that study, allo-HCT was conducted under an SOS/VOD prophylaxis strategy using oral ursodiol with concomitant intravenous low-dose unfractionated heparin or prostaglandin E1. Because of the noncomparative retrospective design using data from a consecutive cohort collected over a 10-year period, the cause of low SOS/VOD incidence could not be clearly identified [29].
Korean data summary–incidence
From 2014 to 2018, according to nationwide Korean Health Insurance Review and Assessment Service data, the overall incidences of hepatic SOS/VOD were 7.8% to 8.9% in adults and 14.0% in pediatric patients.
Using a prophylactic strategy of oral ursodiol and intravenous heparin or prostaglandin E1 (PGE1), one Korean single-center study revealed that SOS/VOD incidence was 3.1% after allo-HCT.
DIAGNOSTIC CRITERIA FOR SOS/VOD
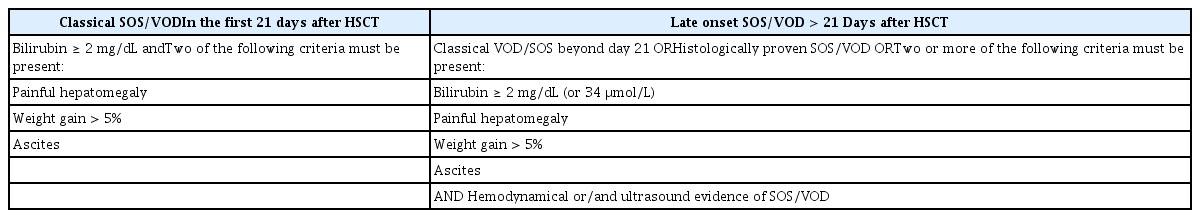
We have used two well-known sets of diagnostic criteria for an extended period, with minor modifications [2,66]. The first set is the Seattle criteria, reported by McDonald et al. [1] in 1984; the second set is the Baltimore criteria, reported by Jones et al. [3] in 1987. Several other diagnostic criteria have since been reported; depending on the criteria used, the reported incidence of SOS/VOD has varied considerably [5,13]. The original criteria consisted of three clinical features: hyperbilirubinemia (jaundice), painful hepatomegaly with/without tenderness, and hemodynamic instability presenting as ascites or weight gain. These clinical features are mainly due to hepatic sinusoidal obstruction and related portal hypertension—two of these conditions must be satisfied for the diagnosis of SOS/VOD. Moreover, bilirubinemia > 2 mg/dL is an obligatory marker in the Baltimore criteria. The original Seattle criteria stated that these features should be present within 30 days, while the modified Seattle and Baltimore criteria reduced this duration to 21 days post-HCT [67]. Some patients may have normal bilirubin levels, particularly pediatric patients or patients with late-onset SOS/VOD [68–70]. The EBMT suggested revised diagnostic criteria in 2016 that divided SOS/VOD into classic SOS/VOD (≤ 21 days after HCT) and late-onset SOS/VOD (> 21 days after HCT). A classic SOS/VOD diagnosis should be based on bilirubinemia ≥ 2 mg/dL, in addition to two of the following criteria: painful hepatomegaly, weight gain > 5%, or ascites. For late-onset SOS/VOD, classic criteria or proven liver histopathology are acceptable regardless of development > 21 days after HCT. Specifically, if hemodynamic changes are evident with or without ultrasonographic findings, hyperbilirubinemia is not an obligatory marker and merely two or more of the following criteria must be present: bilirubinemia ≥ 2 mg/dL, painful hepatomegaly, weight gain > 5%, or ascites (Table 3) [7]. Cairo et al. [71] recently developed further revised diagnostic criteria for SOS/VOD that emphasize the importance of early diagnosis using general clinical parameters regardless of age and duration of onset (Table 4).
Clinical features and laboratory findings
In contrast to other transplantation complications, the disease course of SOS/VOD is dynamic and rapidly progresses to multiorgan failure within a few days [5,68]. Thus, early diagnosis is important regardless of late-onset SOS/VOD, which might involve atypical clinical features. In the revised EBMT criteria, hyperbilirubinemia remains an obligatory marker for the diagnosis of classic SOS/VOD. However, we have observed SOS/VOD in patients with normal to low bilirubin levels, including after RIC allo-HCT, and in patients with late-onset SOS/VOD [31,42,67–70], suggesting that hyperbilirubinemia may not be obligatory for this group. Recent retrospective analyses that compared the characteristics of classic and late-onset SOS/VOD found that 30% of patients had late-onset SOS/VOD, refractory thrombocytopenia was present in up to 42% of patients with classic SOS/VOD, and 25% of patients with late-onset SOS/VOD did not have hyperbilirubinemia [69]. We frequently encounter difficulty controlling excess fluid volume despite aggressive diuretic therapy, which can present as sudden weight gain over a few days, followed by bilirubin elevation. In patients with progressive disease, acute renal failure occurs and urgent renal replacement is needed. Pre- or post-transplantation ferritin levels are associated with a high risk for hepatotoxicity, including risk for SOS/VOD [36,51]. Accordingly, some authors have proposed prophylactic iron-chelating agents, but randomized controlled trials are needed to evaluate the effectiveness of this treatment strategy. Because calcineurin inhibitors are generally metabolized in the liver, high serum levels of FK506 or cyclosporine may be observed in patients with SOS/VOD, despite dose reduction [72,73].
PAI-1 is generally increased in patients with SOS/VOD and reportedly participates in fibrinolytic shutdown and hypercoagulation; thus, it is useful for the differential diagnosis of patients with SOS/VOD and patients with elevated liver enzyme levels after transplantation [22,74,75]. Although PAI-1 and von Willebrand factor are generally increased in patients with SOS/VOD [76], their diagnostic roles in SOS/VOD have not been confirmed [77,78]. By contrast, protein C and antithrombin levels are generally reduced in patients with SOS/VOD, thus contributing to worsened hypercoagulability after transplantation [79,80]. According to a recent report, forkhead box P3 (FOXP3) polymorphisms can be used to predict the development of SOS/VOD after allo-HCT; they are also associated with GVHD and cytomegalovirus reactivation [81].
Scoring systems for diagnosis of SOS/VOD
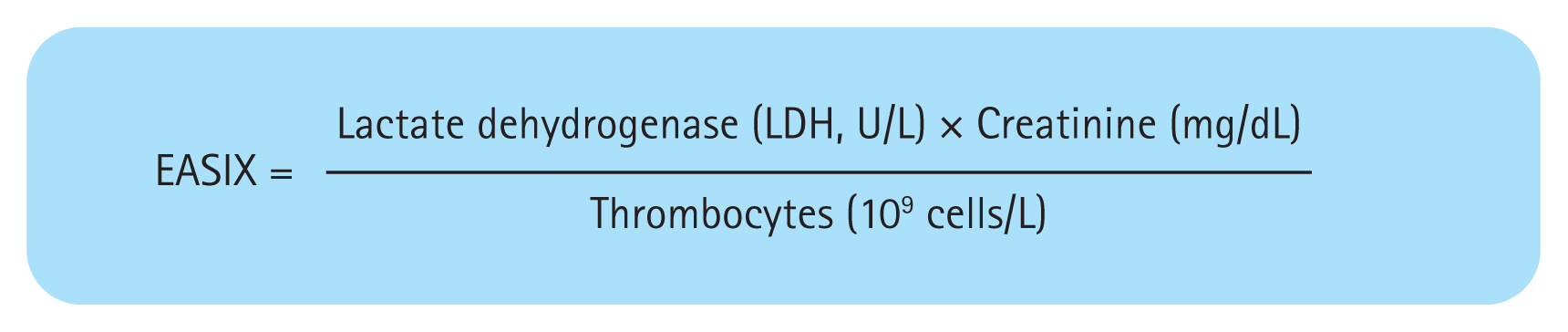
Clinical features and biomarkers are used for the diagnosis and prognostication of SOS/VOD in adults. The Center for International Blood and Marrow Transplant Research (CIBMTR) score [82] and Endothelial Activation and Stress Index (EASIX) score (Fig. 2) [83] are examples of validated tools that incorporate clinical features and biomarkers. The EASIX score has been used to predict the survival outcomes of patients with hematological malignancies, severe GVHD, or fluid overload. This score is based on lactate dehydrogenase and creatinine levels, as well as platelet count, at the time of transplantation (D+0); these comprise biomarkers of vascular endothelial dysfunction and abnormal activation [83–88]. The EASIX score may identify patients at high risk for SOS/VOD, and high EASIX scores are reportedly associated with poor survival outcomes and a high non-relapse mortality rate in patients with SOS/VOD [83]. By contrast, the CIBMTR score is complex and does not predict survival outcomes [82].
Imaging analyses
In patients with SOS/VOD, reduced or reversed portal vein flow and increased resistance index or peak systolic pressure (PSV) of the hepatic artery, gallbladder wall thickening, and ascites are statistically significant findings in Doppler ultrasonography. Those findings were significantly associated with progression to VOD in children with clinical suspicion of SOS/VOD after HCT [89,90]. Therefore, a scoring system was developed based on the following five parameters: portal vein velocity < 10 cm/sec, hepatic artery resistance index ≥ 0.75 or PSV ≥ 100 cm/sec, gallbladder wall thickening ≥ 4 mm, and presence of ascites. The overall relationships of ultrasound findings, based on this scoring system, and the diagnosis of SOS/VOD based on EBMT and Seattle criteria were fair based on calculation of the areas under the receiver operating characteristic curves (0.768 and 0.733, respectively) [90]. Preclinical data from Korean studies have demonstrated the possible utilities of intravoxel incoherent motion diffusion-weighted imaging, supersonic shear wave imaging, and dual-energy computed tomography for assessment of SOS/VOD in animal models [91–93].
Summary of changes in diagnostic criteria for SOS/VOD
Late-onset (> 21 days) or anicteric hepatic SOS/VOD should be considered, particularly after RIC allo-HCT. CIBMTR and EASIX scores may be helpful for more accurate diagnosis of SOS/VOD. Data from Korean pediatric patients suggest significant utilities of Doppler ultrasonography findings including portal vein velocity, hepatic artery resistance index, PSV, gallbladder wall thickening, and ascites.
TREATMENTS FOR SOS/VOD
The primary management strategy for SOS/VOD is symptomatic supportive care and prompt treatment initiation [6,68]. Supportive care includes fluid restriction and electrolyte balance, combined with careful use of diuretics. Drainage of pleural effusion or ascites may be helpful to avoid hypoxic events. When renal insufficiency is aggravated, renal replacement therapy should be readily available.
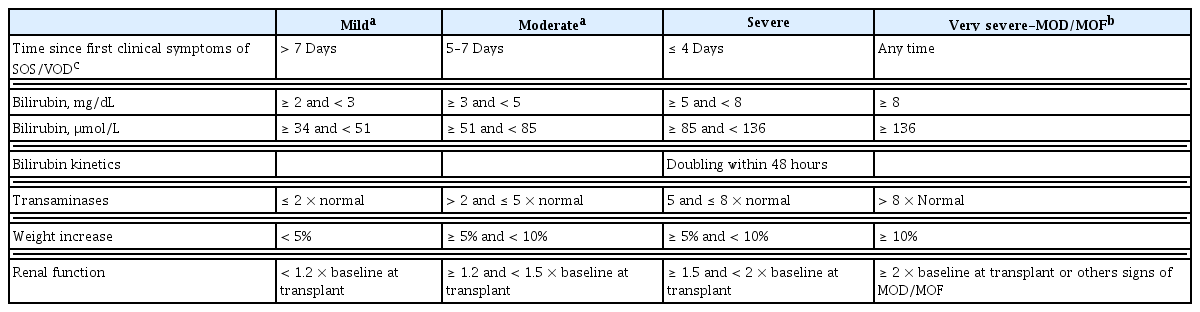
At present, the sole approved treatment agent for SOS/VOD is defibrotide [14,94,95] and its early application before deterioration results in more favorable treatment outcomes [96,97]. Defibrotide was approved by the Food and Drug Administration in June 2017 in Korea and the national insurance system approved its use in patients with very severe SOS/VOD. In 2020 in Korea, defibrotide is indicated when two or more of the following five criteria are satisfied: < 4 days elapsed since first clinical symptoms of SOS/VOD, total bilirubin ≥ 5 mg/dL, bilirubin kinetics doubling within 48 hours, transaminases > 5-fold above the ULN, and creatinine level ≥ 1.5-fold above baseline at transplantation. Defibrotide has anti-inflammatory and antithrombotic effects on the endothelium by attenuating intracellular adhesion molecular expression, clot formation, and extracellular matrix reactivity toward platelets [55]. Richardson et al. [96] conducted a phase 3 trial of defibrotide in patients with severe SOS/VOD, which revealed an improved response in 25.5% of patients in the defibrotide group, compared to 12.5% in the control group (p = 0.016). It also showed a 100-day survival rate of 38.2% in the defibrotide group, compared to 25.0% in the control group (p = 0.011). A recent report suggested that earlier use of defibrotide within 3 days after diagnosis can improve survival outcomes [96]. A Korean study also found that early defibrotide use within 5 days after diagnosis resulted in better 100-day survival, compared to later defibrotide use (74.5% vs. 43.5%, p = 0.044) [13].
Recombinant tissue plasminogen activator fibrinolytic therapy is not recommended due to the high risk of bleeding-related mortality [98]. However, a Korean study showed its possible utility when used at a low dose without concomitant anticoagulation or antiplatelet agents [99]. Steroid treatment was implemented to reduce inflammation when only supportive management was available. A few studies have suggested that steroids have treatment potential, but the effects might be complicated by difficulty distinguishing between hepatic GVHD and other hepatic complications [100–102].
Antithrombin III has been used for pre-emptive replacement or treatment of SOS/VOD, due to its anti-thrombotic effects. A study of pediatric patients showed that pre-emptive antithrombin III use failed to prevent SOS/VOD development. However, when used in combination with defibrotide, a good remission rate without severe bleeding complications was achieved [103]. A Korean multicenter study regarding antithrombin III monotherapy for SOS/VOD showed complete remission at standard dosing in 61.1% of patients, without significant bleeding.
Liver transplantation or a transjugular intrahepatic portosystemic shunt have been used in patients with very severe disease, leading to cure in a few patients following timely treatment. However, the possible benefits of these high-risk procedures should be weighed against their potential complications [104–106].
Korean data summary–treatments
-
In Korea, defibrotide was approved in 2017 for use in patients with severe and very severe SOS/VOD; it can be reimbursed when two or more of the following are satisfied:
- < 4 days elapsed since first clinical symptoms of SOS/VOD
- total bilirubin ≥ 5 mg/dL
- bilirubin kinetics doubling within 48 hours
- transaminases > 5-fold above the ULN
- creatinine level ≥ 1.5-fold above baseline at transplantation
A Korean multicenter study showed that early defibrotide initiation within 5 days after diagnosis resulted in better 100-day survival, compared to later defibrotide initiation.
Liver transplantation, transjugular intrahepatic portosystemic shunt, and the use of antithrombin III or recombinant tissue plasminogen activator have been discussed in several Korean clinical studies, but none of these approaches constituted sufficient curative treatment for SOS/VOD.
SEVERITY CRITERIA AND TREATMENT OUTCOMES
The prognosis of SOS/VOD depends on the extent of hepatic injury, subsequent liver dysfunction, and presence of multiorgan failure, which frequently occurs in patients with severe SOS/VOD. Severe SOS/VOD is associated with extremely poor survival, such that the all-cause mortality is > 80% at 100 days post-HCT [2,5,52,99,107]. Previously used severity criteria (mild, moderate, and severe) were developed based on classic SOS/VOD after MAC allo-HCT; these criteria have limited usefulness in determining proper early intervention [7,12]. A few patients were diagnosed with mild disease and had good treatment outcomes, while most patients in the severe group died of SOS/VOD [68,108]. Thus, intervention may be unnecessary for patients with mild to moderate SOS/VOD but insufficient for patients with severe SOS/VOD, leading to rapid progression to multiorgan failure despite defibrotide treatment [14,96,97,109]. The EBMT proposed revised severity criteria to guide therapeutic decisions based on increment rate and the levels of bilirubin, liver function, renal function, and weight gain. The revised criteria consist of four severity grades (mild, moderate, severe, and very severe) that emphasize the speed of deterioration; analyses using these criteria showed a need for early intervention in patients with rapid progression (Table 5) [6,7]. A Korean multicenter study validated the revised EBMT severity criteria in the context of 100-day survival and overall transplantation-related mortality. This retrospective analyses showed that the 100-day overall survival rates of patients with mild, moderate, and severe disease were 83.3%, 84.3%, and 94.6%, respectively, compared to 58.6% in patients with very severe disease. The 100-day non-relapse mortalities of patients with mild, moderate, and severe disease were 8.3%, 8.0%, and 2.7%, respectively, compared to 36.7% in patients with very severe disease. These data suggest that intervention should be initiated in patients with mild, moderate, or severe disease, thus preventing deterioration [13]. Cairo et al. [71] recently suggested another classification system using a common toxicity criteria for adverse events format, but these criteria require further validation in clinical trials (Table 6). This classification system includes clinical deterioration of the heart, lungs, and central nervous system [71].
Korean data summary–severity criteria and outcomes
Multicenter analyses in Korea validated the revised EBMT severity criteria, which showed significantly worse 100-day overall survival with high non-relapse mortality in patients with very severe SOS/VOD.
The data imply a need for initiation of active treatment before progression to very severe SOS/VOD.
PROPHYLAXIS OF SOS/VOD
Ursodiol has been shown to prevent SOS/VOD in several randomized studies [83,110–112]. A meta-analysis demonstrated that ursodiol had a significant preventive role (relative risk, 0.34; 95% confidence interval, 0.17 to 0.66), but no benefit was evident in patients receiving heparin prophylaxis [113,114]. In another study, the combination of ursodiol and a statin had a preventive effect, including among high-risk patients [83]. However, ursodiol must be administered orally, which is not possible in patients with nausea or severe mucositis. Heparin has also been evaluated in randomized controlled trials [115–117], but a recent meta-analysis and recent guidelines suggested no significant overall effect of heparin; they also revealed limitations in old previous randomized trials [26,118]. PGE1 was evaluated in a few studies, but most were small and used a retrospective design; randomized, controlled studies are required to robustly determine if PGE1 is an effective prophylactic option for SOS/VOD [52,119–121].
Several Korean population studies have reported that the agents described above reduced the incidence of SOS/VOD in both pediatric [52,121] and adult patient populations [29]. Ursodiol and intravenous heparin or PGE1 were administered in a large cohort of 2,572 adult patients. The overall incidence of SOS/VOD was 3.1% and significant bleeding complications occurred in < 3% of patients. In that study, PGE1 was selectively used in patients with low platelet counts at the time of preconditioning, who were at high risk for SOS/VOD. However, there were no significant differences in SOS/VOD incidence or bleeding complications, compared to patients who received heparin [29].
At present, defibrotide is the sole agent used for prevention of SOS/VOD, based on data from a phase 3 trial of pediatric patients treated with MAC-HCT [66]. Per-protocol analyses revealed an SOS/VOD incidence of 11% in the defibrotide group, compared to 20% in the control group (p = 0.0225). A recent clinical trial to evaluate the preventive role of defibrotide in adult patients is currently underway (ClinicalTrials.gov identifier: NCT02851407). A Korean study reported that SOS/VOD developed in one (2.0%) of 49 transplant recipients; 34 (69.4%) of those 49 patients were at high risk for SOS/VOD [122].
Transplantation specialists from 20 institutions in Korea were surveyed to collect their perspectives regarding SOS/VOD prophylaxis and treatment strategies. The survey revealed that 70% of institutions implemented SOS/VOD prophylaxis with selective administration in high-risk patients (approximately 80% of patients). Ursodiol, low-dose unfractionated heparin, or PGE1 were the most widely used agents [29].
Korean data summary–prophylaxis
In Korea, oral ursodiol, heparin, or PGE1 are used for prophylaxis of SOS/VOD in approximately 70% of transplantation centers, but none of these treatments are significantly preventive.
One large single-center study in Korea revealed that a combination of oral ursodiol and intravenous heparin/PGE1 might be preventive against SOS/VOD.
PEDIATRIC PATIENTS
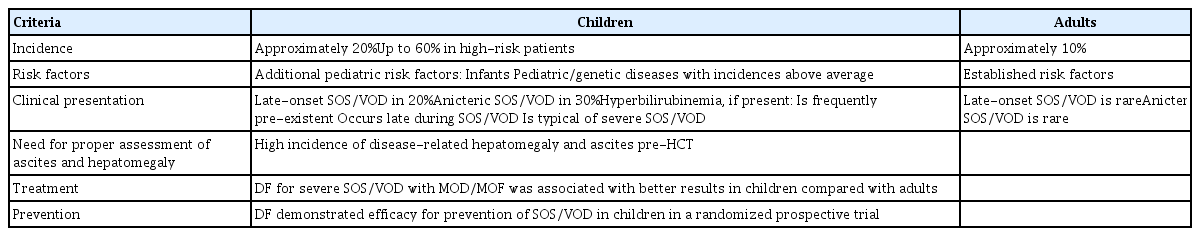
SOS/VOD develops in pediatric patients more frequently and has distinct characteristics, compared to SOS/VOD in adult patients (Table 7) [8]. Treatment-related risk factors are similar between pediatric and adult patients, but patient-related factors (e.g., genetic predisposition or age at primary disease onset) differ between children and adults. Specific diseases that place children at high risk for SOS/VOD include infantile osteopetrosis, congenital macrophage activation syndrome, neuroblastoma treated with high-dose therapy, and sickle cell anemia; notably, sickle cell anemia is rare in Korea. SOS/VOD can occur after chemotherapy or radiotherapy outside of the transplantation setting, and anicteric or late-onset SOS/VOD is frequently observed. Suggested diagnostic parameters in adults (e.g., weight gain > 5%, hyperbilirubinemia, and right upper quadrant abdominal pain) are difficult to apply in pediatric patients. Thus, the EBMT diagnostic criteria are distinct for pediatric patients. Similar to adult patients, there is no limitation regarding time of onset; however, the criteria include an increase in bilirubin level from baseline, weight gain > 2%, and imaging tools for the identification of hepatomegaly and ascites. A bilirubin level > 2 mg/dL and renal function < 30 mL/min are considered severe SOS/VOD in children. Additional criteria used in the pediatric population include a paracentesis requirement, persistent thrombocytopenia, coagulation abnormality, and pulmonary, or central nervous system functional impairment.
Summary of SOS/VOD in pediatric patients
In pediatric patients, anicteric or late-onset SOS/VOD is more frequently observed.
Additional criteria are needed for severity grading–paracentesis requirement, persistent thrombocytopenia, coagulation abnormality, and pulmonary or central nervous system functional impairment.
CONCLUSIONS
Various risk factors including transplantation setting, disease status, comorbid conditions, and toxicities of novel agents must be considered to prevent and treat SOS/VOD. The presence of anicteric SOS/VOD, particularly in patients with late-onset SOS/VOD, is an important diagnostic consideration. Several endothelial activation biomarkers or imaging tools should be used for rapid and accurate diagnosis. Although defibrotide is the sole approved treatment for SOS/VOD and is used in Korea, it has important limitations (e.g., risk-benefit ratio and cost). There remain many challenges regarding SOS/VOD. More clinical trials and large retrospective reports are needed to reduce the incidence of SOS/VOD and improve transplantation outcomes.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
The authors acknowledge Seonghee Jung from HANDOK Inc. for the coordination of references collection and manipulating the figures and tables.