Recent advances in airway imaging using micro-computed tomography and computed tomography for chronic obstructive pulmonary disease
Article information
Abstract
Chronic obstructive pulmonary disease (COPD) is a complex lung disease characterized by a combination of airway disease and emphysema. Emphysema is classified as centrilobular emphysema (CLE), paraseptal emphysema (PSE), or panlobular emphysema (PLE), and airway disease extends from the respiratory, terminal, and preterminal bronchioles to the central segmental airways. Although clinical computed tomography (CT) cannot be used to visualize the small airways, micro-CT has shown that terminal bronchiole disease is more severe in CLE than in PSE and PLE, and micro-CT findings suggest that the loss and luminal narrowing of terminal bronchioles is an early pathological change in CLE. Furthermore, the introduction of ultra-high-resolution CT has enabled direct evaluation of the proximal small (1 to 2-mm diameter) airways, and new CT analytical methods have enabled estimation of small airway disease and prediction of future COPD onset and lung function decline in smokers with and without COPD. This review discusses the literature on micro-CT and the technical advancements in clinical CT analysis for COPD. Hopefully, novel micro-CT findings will improve our understanding of the distinct pathogeneses of the emphysema subtypes to enable exploration of new therapeutic targets, and sophisticated CT imaging methods will be integrated into clinical practice to achieve more personalized management.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) imposes substantial social and economic burdens worldwide and causes considerable morbidity and mortality [1]. It is diagnosed by a combination of respiratory symptoms and airflow limitation, which are induced by complex structural alterations, including emphysema and airway disease, on spirometry. Structural evaluation was formerly based on histological assessment. However, given the invasiveness of sample acquisition and poor accessibility of histological samples in clinical practice, chest computed tomography (CT) is used widely for morphological evaluation of the parenchyma and airways [2,3]. Although CT exposes patients to radiation, this imaging technique enables three-dimensional structural assessment, which cannot be achieved easily with histology. Volumetric CT enables quantification of the extent of emphysema in the entire lungs and in specific lobes [4]. Emphysema severity on CT images is associated closely with impaired pulmonary function, symptoms, and health-related quality of life; more importantly, it can be used to predict future clinical outcomes, such as exacerbation and mortality [5–10]. Furthermore, visual CT assessment is used to classify emphysema as centrilobular emphysema (CLE), panlobular emphysema (PLE), and paraseptal emphysema (PSE) [3], which have distinct clinical features [11–13].
Like those of emphysema, structural airway changes of COPD can be evaluated by CT. However, its limited resolution hinders quantification of the dimensions of small (< 2-mm-diameter) airways, and these airways are important major pathological sites of COPD [14]. As an alternative, researchers have evaluated the central airways based on the assumption that small airway disease extends to the central airways in COPD [15]. The dimensions of the central airways on CT images are associated with pulmonary function, symptoms, and the risk of exacerbation in COPD [5,6,9,15–18]. Moreover, novel imaging equipment and analytical methods, including ultra-high-resolution computed tomography (U-HRCT), and nonrigid registrations of inspiratory and expiratory CT scans, have enabled the evaluation of non-emphysematous air trapping, which is induced mainly by small airway disease [19,20]. These findings have established the clinical relevance of airway imaging in living patients with COPD.
The use of micro-CT has been introduced in COPD research [21–24]. Although it cannot be performed on live patients, micro-CT generates high-resolution volumetric images of lung tissue samples and enables the three-dimensional morphological analysis of small airways such as terminal bronchioles (the last generation of conducting bronchioles) and transitional respiratory bronchioles (one level peripheral to the terminal bronchioles).
Based on the data obtained following recent advancements in CT and micro-CT imaging of the airways in COPD, this review describes our pathological understanding of airway trees ranging from the segmental bronchus to the terminal bronchioles, the evaluation of the airway trees in patients with COPD using clinical CT, and future perspectives for airway imaging studies. The use of CT images was approved by the Ethics Committee of Kyoto University Hospital (approval no. R1660), and the requirement for informed consent was waived because retrospective data were used.
MICRO-CT
Micro-CT is a form of X-ray CT in which a sample is placed in the path of an X-ray beam to produce a projection image on detectors [25]. It enables the scanning of biological samples at spatial resolution as fine as 1 to 2-μm [26]. However, the size of target samples is limited by the need to balance the setting of a field of view with the target image resolution. Many studies have involved the scanning of lung tissues, rather than whole lungs, to achieve sufficient image resolution for evaluation of the small airways, including the terminal bronchioles [20,27–31]. Whereas Verleden et al. [32] scanned explanted whole lungs using micro-CT at 150-μm spatial resolution for the counting of the branches of the entire airway tree, this review focuses on micro-CT findings in tissue specimens removed from lungs with COPD.
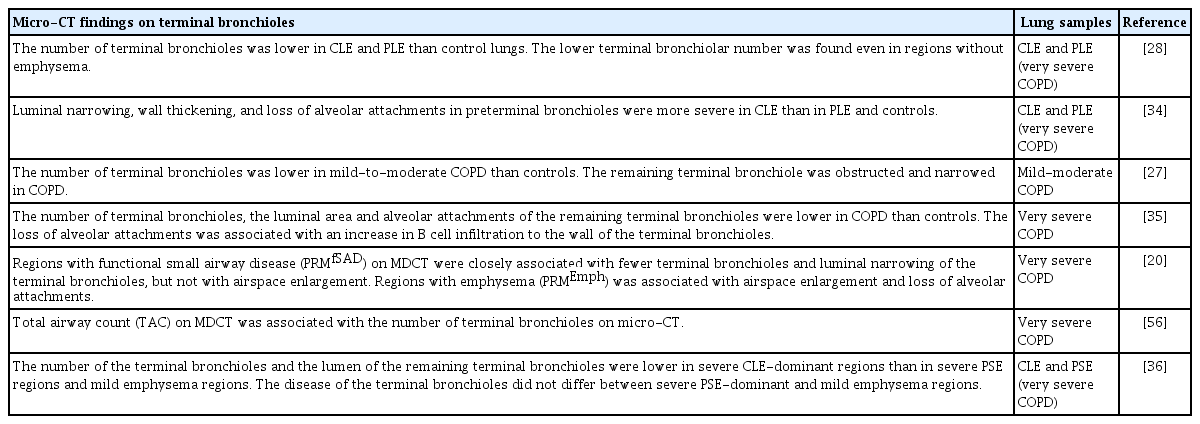
Table 1 summarizes the literature on micro-CT examination of the small airways in COPD. In 2011, McDonough et al. [28] first used micro-CT to evaluate the terminal bronchioles of lungs affected by COPD. The authors focused on the number of terminal bronchioles in severe COPD treated by lung transplantation in patients who had CLE or PLE associated with alpha-1 antitrypsin deficiency. Explanted lungs were inflated with air and frozen solid with liquid nitrogen vapor. Then, columnar tissue cores were removed from the lung slices, fixed, dried, and scanned using a micro-CT device at approximately 10-μm voxel resolution. Lungs with CLE and PLE had fewer terminal bronchioles than did control donor lungs (2,400 ± 600, 6,200 ± 2,100, and 22,300 ± 3,900, respectively). Moreover, when the tissue sample cores were grouped based on the mean linear intercept (a marker of airspace enlargement), samples with even minimal emphysema had fewer terminal bronchioles than did samples from control lungs. This finding suggests that a reduction in terminal bronchioles is not induced solely by extensive parenchymal emphysematous destruction, and that the loss of terminal bronchioles in the early stage of the disease.
Subsequently, Scott et al. [33] overcame the intrinsic problem that the difference in density between tissue and paraffin is too small to generate significant contrast for the detection of tiny structures on micro-CT images of formalin-fixed paraffin-embedded (FFPE) samples. The authors established a novel scanning condition that enabled micro-CT scanning of routinely prepared FFPE samples, which may include surgically resected specimens and small pieces of tissue obtained by transbronchial lung biopsy. Using this technique, Koo et al. [27] evaluated micro-CT images of FFPE tissue samples from lungs with mild COPD and demonstrated that these lungs had fewer terminal and transitional bronchioles than did control lungs, but that emphysematous changes on CT images did not differ between lungs with mild COPD and controls. Moreover, the authors’ use of micro-CT to identify the terminal bronchioles on histological sections revealed luminal obstruction in the remaining terminal bronchioles in lungs with mild and moderate COPD. These findings provide evidence that the loss of terminal bronchioles and remodeling of remaining terminal bronchioles are early pathological changes in COPD.
The same group performed three-dimensional structural evaluation of the terminal and preterminal bronchioles [34]. The authors established a method for computer-based quantitation of the lumen and wall areas and the number of alveolar attachments to the outer bronchiole walls. They found that the lumen area was smaller in lungs with CLE than in those with PLE and controls, whereas the number of alveolar attachments was similarly decreased in lungs with CLE and PLE compared with the controls. Moreover, wall thickness was associated closely with the number of alveolar attachments in the preterminal bronchioles in lungs with CLE, but not in lungs with PLE or controls. These findings suggest that airway remodeling damages the surrounding parenchymal tissues by destroying alveolar attachments in lungs affected predominantly by CLE.
Because micro-CT cannot provide information on molecular features, Vasilescu et al. [29] invented a method for the scanning of frozen tissue samples and their processing for histological assessment. Accurate matching of micro-CT images to histological sections has enabled the identification of target bronchioles on histology. Tanabe et al. [35] showed that B-cell infiltration of the terminal and preterminal bronchiole walls was associated with a reduction in the number of alveolar attachments. This finding supports the concept that airway inflammation disrupts the alveolar walls attached to the bronchioles.
Tanabe et al. [36] examined the microstructure of tissue samples obtained from explanted lungs with very severe COPD treated by lung transplantation. Ninety-six tissue samples were assigned to mild emphysema, PSE-dominant severe emphysema, and CLE-dominant severe emphysema groups (n = 32, each). The CLE-dominant samples had fewer terminal bronchioles and smaller lumen areas of remaining terminal bronchioles than did the PSE-dominant and mild emphysema samples, with no difference between the latter.
Overall, micro-CT has revealed that terminal bronchiole disease differs among CLE, PLE, and PSE. The loss of terminal bronchioles and the close relationship between wall remodeling and the loss of terminal bronchiole alveolar attachments may be early pathological features of CLE, whereas the terminal bronchioles are more preserved in PSE. These distinct pathological changes may explain the greater severity of airflow limitation and other symptoms in patients with CLE than in those with PSE.
U-HRCT
Because the collection of tissue samples for histology and micro-CT is too invasive for clinical practice, CT has been used to evaluate airway structure in patients with COPD. However, standard CT (512 × 512 matrix and ≥ 0.5-mm slice thickness) does not enable accurate evaluation of smaller (< 2-mm-diameter) airways due to its limited resolution. Oguma et al. [37] used an airway phantom of 2-mm lumen diameter and 0.5-mm wall thickness, and showed that the analysis of CT images obtained with a 512 × 512 matrix resulted in the overestimation of wall thickness and underestimation of lumen area.
U-HRCT has recently become commercially available. It provides greater spatial resolution (0.14 mm/pixel) due to the use of 1,024 × 1,024 and 2,048 × 2,048 matrices with no increase in radiation exposure [24,38,39]. Studies performed with airway phantoms (2-mm-diameter, 0.5-mm wall thickness) have shown that the lumen area measurement error is minimal for U-HRCT performed with the 1,024 × 1,024 matrix, but not for a conventional 512 × 512-matrix scan, and that U-HRCT enabled the accurate quantification of lumen size in peripheral airways with diameters as small as 1 mm [21,22]. Whereas optical coherence tomography revealed small (< 2-mm-diameter) airways at the seventh (or higher) airway generation in healthy non-smokers [40], U-HRCT showed that the lumen diameters of more than half of sixth-generation airways were < 2 mm in COPD [22], consistent with the notion that airway narrowing is a feature of COPD [14,20,41–44]. Fig. 1 shows an example of cross-sectional U-HRCT images of the segmental (third-generation) to 10th-generation airways in smokers with and without COPD. The airway lumina were smaller in patients with than in those without COPD. Although central airways with diameters > 2 mm have been examined using conventional CT and the preterminal, terminal, and respiratory bronchioles have been examined using micro-CT and histology, much remains to be learned about the proximal airways with diameters of 1 to 2 mm. Further U-HRCT studies are needed to clarify the detailed morphological features of the proximal small airways.

Ultra-high-reso lution computed tomography images of airways in smokers with and without chronic obstructive pulmonary disease (COPD). (A) Airway tree in a smoker without COPD. Longitudinal paths for the airway tree were extracted, and (B) cross-sectional ultra-high-resolution computed tomography (U-HRCT) images of airways were constructed. (C) Airway tree and (D) cross-sectional images of the airways in a smoker with COPD. The lumina were smaller than in the smoker without COPD. The third-generation airway indicates the right lower posterior segmental airway. A phantom study [22] showed that the lumen area of airways with diameters > 1 mm can be measured accurately on U-HRCT images. Scale bar, 10 mm. FEV1, force expiratory volume in 1 second; FVC, force vital capacity.
UPDATE ON METHODS USED TO EVALUATE AIRWAY DISEASE ON INSPIRATORY CT IMAGES
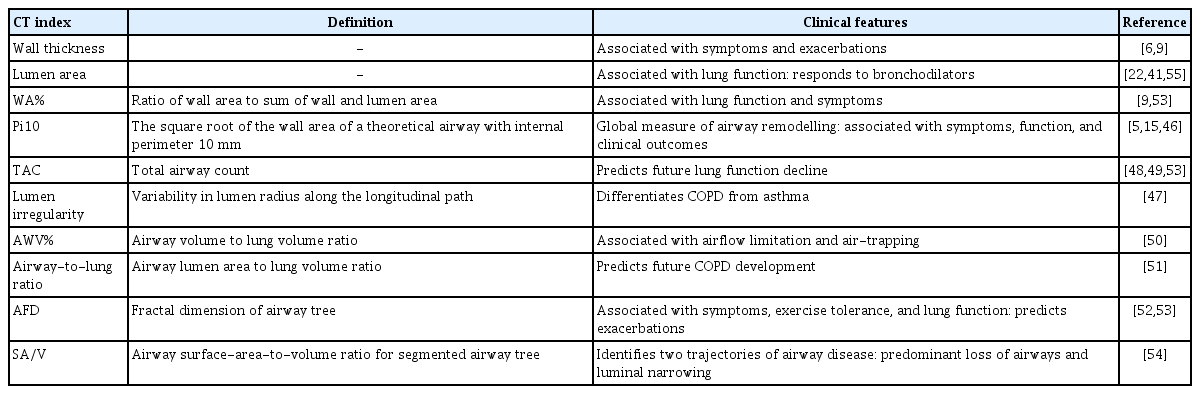
Inspiratory CT is used widely for patients with COPD for multiple clinical purposes, such as lung cancer screening [45]. In addition to methods for the direct measurement of the central airway wall and lumen sizes [9], several methods for evaluation of the extent of airway disease have been proposed (Table 2) [5,6,9,15,22,41,46–55]. Because the airway thickness wall is associated closely with the native airway lumen size, the square root of the wall area of a theoretical airway with an internal perimeter of 10 mm was proposed to represent the airway size-adjusted wall thickness, termed Pi10. Pi10 is calculated by plotting the internal perimeters of all measured airways against the square root of the airway wall area [5,15,46]. Moreover, Oguma et al. [47] measured the radius of the lumen along the longitudinal path of the right posterior lower bronchus. They expressed the radius as a function of the distance from the carina, obtained the regression line, and evaluated longitudinal airway lumen shape irregularity by calculating the coefficient of variation for deviations from the regression line. They observed more longitudinal lumen shape irregularity in COPD than in asthma, but more severe wall thickening and luminal narrowing in asthma than in COPD. These findings suggest the potential of combined cross-sectional and longitudinal airway structural analyses for the evaluation of airway diseases.
Diaz et al. [48] showed that the numbers of airways visible on inspiratory CT images was reduced in severe emphysema. Subsequently, Kirby et al. [49] showed that the total airway count (TAC) was lower even in mild COPD. Notably, a decrease in the TAC on CT images predicted future lung function decline independent of emphysema severity. Furthermore, the same group tested the hypothesis that the pathological process responsible for terminal bronchiole loss and remodeling extended into the central airways detectable on clinical CT scans [56]. They found that the TAC was associated with the number of terminal bronchioles, wall area percent (WA%), lumen circularity, and number of alveolar attachments for the remaining terminal bronchioles on micro-CT images. Thus, the TAC on inspiratory CT images could be useful for the assessment of airway disease from the segmental to the terminal bronchioles in COPD.
Long-term longitudinal observational studies have demonstrated that the trajectory of lung function is heterogeneous [57–59]. Not all subjects show accelerated lung function decline after normal lung growth. Abnormal lung growth increases the incidence of COPD, even in patients with normal lung function decline [57]. In particular, a mismatch between airway and lung sizes, termed dysanapsis, induced by abnormal lung growth in early life correlates closely with poor lung function [60,61]. In a volumetric CT study, the airway volume to lung volume ratio (AWV%) decreased with the increasing severity of COPD, and a decrease in the AWV% was associated with airflow limitation and air trapping independent of emphysema and the TAC [50]. Fig. 2 shows examples of cases with different airflow limitations, TACs, and AWV%s.
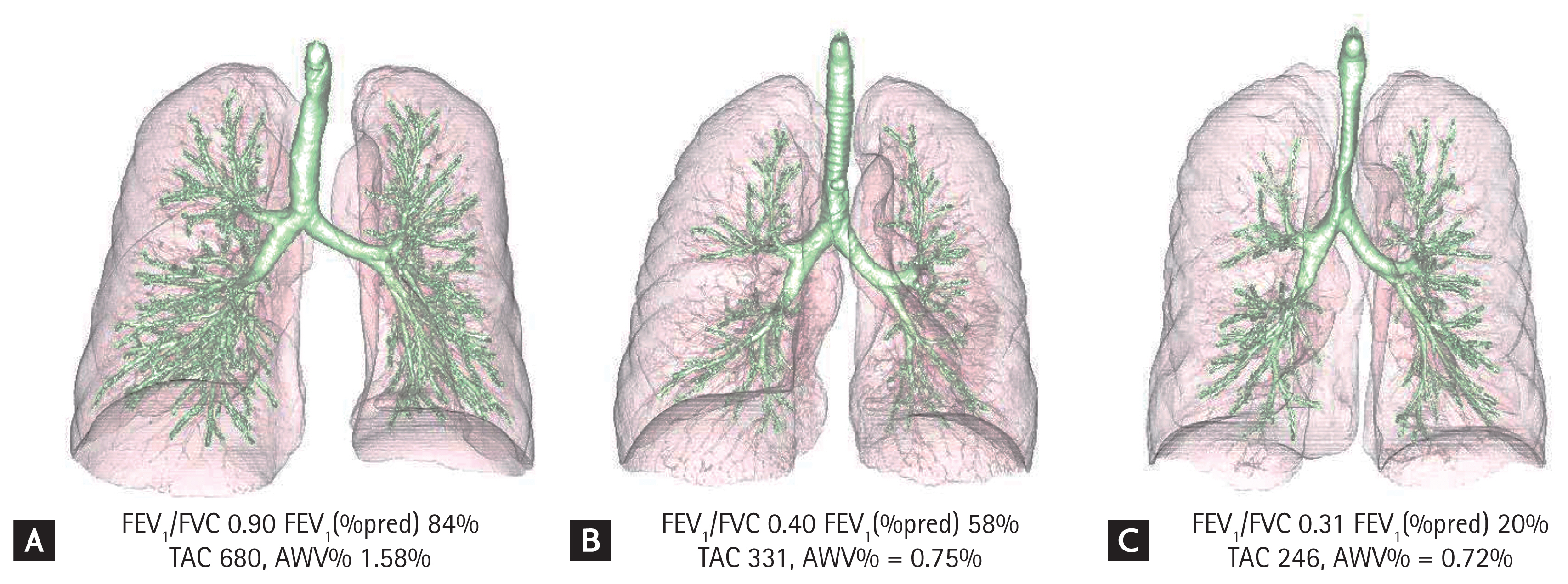
Airway trees and lungs in smokers with different degrees of airflow limitation. Airway trees and lungs were extracted from computed tomography images. (A) Smoker without airflow limitation. (B) Smoker with chronic obstructive pulmonary disease (COPD) and moderate airflow limitation. (C) Smoker with COPD and severe airflow limitation. The total airway count (TAC) and airway volume to lung volume ratio (AWV%) were lesser in case C than in case A. The AWV% was calculated using the volume of the airway tree, excluding the trachea. FEV1, force expiratory volume in 1 second; FVC, force vital capacity.
More recently, Smith et al. [51] used a large dataset from the Multi-Ethnic Study of Atherosclerosis Lung Study (n = 2,531), the Canadian Cohort of Obstructive Lung Disease (n = 1,272), and the Subpopulations and Intermediate Outcome Measures in COPD Study (n = 2,726) to evaluate dysanapsis by quantifying the ratio of the average central airway lumen diameter to the cube root of lung volume (airway-to-lung ratio). The authors found that dysanapsis characterized by a lower airway tree caliber relative to lung size was associated with a greater risk of COPD. Subsequently, a study performed using a combination of CT and micro-CT [62] showed that central airway dysanapsis on CT images was associated with terminal bronchiole size on micro-CT images, and suggested that dysanapsis causes increased resistance of the central and peripheral airways and enhances susceptibility to COPD.
Instead of counting all branches of airway trees (i.e., TAC calculation) or measuring the airway tree size, Bodduluri et al. [52] performed a morphological analysis of airway trees with calculation of the airway fractal dimension (AFD). Lower AFDs were associated with airflow limitation, impairments of health-related quality of life and exercise capacity, longitudinal lung function decline, exacerbation, and mortality. The simulation revealed that a reduction in the AFD reflected airway loss and luminal narrowing. Bhatt et al. [53] compared the associations of the WA%, Pi10, AFD, and TAC with chronic bronchitis symptoms, and found that higher AFD and TAC values were associated with a lower risk of persistent chronic bronchitis. The same group used the airway surface-area-to-volume ratio (SA/V) to distinguish subjects with predominant airway loss from those with luminal narrowing [54]. With a simulation, the authors showed that a reduction in the SA/V reflected airway loss, rather than luminal narrowing. Their longitudinal data further showed that the mortality rate was higher in subjects with predominant airway loss than in those with predominant luminal narrowing.
NONRIGID REGISTRATION OF INSPIRATORY/EXPIRATORY CT PARAMETERS
Methods for the analysis of the central airways have improved substantially, enabling the estimation of small airway disease in COPD [15,56]. Additionally, U-HRCT has enabled the direct evaluation of small (1 to 2-mm-diameter) airways [22]. Moreover, technical advancements have enabled the separate localization of emphysema and non-emphysematous air trapping in the lungs through the use of nonrigid registration for inspiratory and expiratory CT [19,63,64].
The concept that low-attenuation regions on expiratory CT images reflect air trapping is not new [65]. However, air trapping in lungs with COPD can be affected by multiple factors, such as emphysema and small airway disease. Galban et al. [19] established parametric response mapping (PRM), which nonrigidly registers inspiratory CT to expiratory CT parameters on a voxel-to-voxel basis, and defined voxels with CT < −950 HU on registered inspiratory CT scans and < −856 HU on expiratory CT scans as indicating functional small airway disease (PRMfSAD). Regions with PRMfSAD corresponded to non-emphysematous air trapping. Vasilescu et al. [20] performed CT and micro-CT examinations of lungs from patients with severe COPD treated by lung transplantation and control subjects. By registering regions of interest on CT images to the tissue sample locations used for micro-CT, they associated regions with PRMfSAD with disease of the terminal bronchioles, but not with the extent of emphysema on micro-CT images. This finding indicates that non-emphysematous air trapping on inspiratory/expiratory CT images is induced by small airway disease. The use of this technique has shown that non-emphysematous air trapping on CT images is a radiological precursor of emphysema [66], is associated with physiological indices of small airway disease [63,67], and predicts longitudinal lung function decline [68]. A very recent study showed that airway-dominant COPD, defined as a higher WA% of the segmental airways, was associated with smaller right main and intermedius bronchi, but not non-emphysematous air trapping on CT images, whereas emphysema-dominant COPD was associated with increased non-emphysematous air trapping [69]. These findings are consistent with those of Young et al. [70], who suggested that central airway disease precedes peripheral lung disease in a portion of smokers with COPD.
The nonrigid registration of paired inspiratory and expiratory CT parameters has been also used to calculate local respiratory volume changes in COPD and asthma [71–73]. Bhatt et al. [74] showed that respiratory volume changes in normal regions near emphysema are associated with subsequent lung function decline in smokers. Chae et al. [75] showed that the extent of low ventilation areas measured on paired inspiratory and expiratory CT scans was associated with functional impairments and emphysema in patients with COPD. Haghighi et al. [76] performed an imaging-based cluster analysis of 406 former smokers using CT indices such as lung shape, central airway branching angle, wall thickness, lumen diameter, airway circularity, emphysema, functional small airway disease, and local respiratory volume changes. The analysis yielded four clusters; in terms of airway morphology, cluster 1 was characterized by airway wall thickening, whereas cluster 4 was characterized by airway wall thinning, which reflects the heterogeneity of airway structural changes in smokers.
CONCLUSIONS
In this review, we summarized recent advancements in methods for the evaluation of airway disease in COPD. Micro-CT has enabled three-dimensional visualization of the transitional, terminal, and preterminal bronchioles and clarification of the pathological roles of small airway disease in different emphysema subtypes. In CLE, terminal bronchiole disease appears to be an early pathological change, and the loss of alveolar attachments may link small airway disease and emphysema. The terminal bronchioles are more preserved in PSE than in CLE, suggesting that potential therapeutic targets for these emphysema subtypes differ. Moreover, the introduction of U-HRCT and the technical progress in the analytical methods of multidetector computed tomography (MDCT) have enabled us to understand the complex structural alteration of airway trees and even estimate the extent of disease in terminal bronchioles that cannot be visualized directly by MDCT. Because we still need to determine the responses to pharmacological and non-pharmacological interventions and establish preventive strategies for COPD development, future studies should investigate whether different interventions induce distinct structural changes in airway trees, and whether the detailed morphology of the airway tree predicts the response to an intervention and allows for personalized management of COPD.
Notes
Conflict of interest
This work was partially supported by a grant from the Fujifilm Corporation. No potential conflict of interest relevant to this article was reported.