Evidence based guidelines for the treatment of Helicobacter pylori infection in Korea 2020
Article information
Abstract
Helicobacter pylori infection is one of the most common infectious diseases worldwide. H. pylori is responsible for substantial gastrointestinal morbidity with a high disease burden. Since the revision of the H. pylori Clinical Practice Guidelines in 2013 in Korea, the eradication rate of H. pylori has gradually decreased with the use of a clarithromycin based triple therapy. According to a nationwide randomized controlled study by the Korean College of Helicobacter and Upper Gastrointestinal Research released in 2018, the intention-to-treat eradication rate was only 63.9%, which was mostly due to increased antimicrobial resistance to clarithromycin. The clinical practice guidelines for treatment of H. pylori were updated based on evidence-based medicine from a meta-analysis conducted on a target group receiving the latest level of eradication therapy. The draft recommendations developed based on the meta-analysis were finalized after expert consensus on three recommendations regarding the indication for treatment and eight recommendations on the treatment itself. These guidelines were designed to provide clinical evidence for the treatment of H. pylori to patients, nurses, medical school students, policymakers, and clinicians. These may differ from current medical insurance standards, and will be revised if more evidence emerges in the future.
INTRODUCTION
Helicobacter pylori infection is the most common cause of infectious disease in the world. Its prevalence varies worldwide, e.g., 11% in Northern Europe, 23.1% in Canada, and 30.0% in the United States, but compared to 72%–80% in South America and 91% in Nigeria [1]. The prevalence is 50% higher in Korea [2]. H. pylori causes progressive injury to the gastric mucosa and play an important role in gastrointestinal disease such as asymptomatic chronic gastritis, peptic ulcer disease, atrophic gastritis, intestinal metaplasia (IM), gastric mucosa-associated lymphoid tissue lymphoma, and gastric adenocarcinoma [3,4].
Many epidemiologic studies have shown the relationship between H. pylori and gastric cancer. The Asia–Pacific region (China, Japan, and Korea) which has a high risk of gastric cancer shows a high prevalence of H. pylori infection. In 2017, the standardized incidence of gastric cancer in Korea was 32.0 per 100,000 person, which was a leading cause of cancer second to thyroid cancer [4,5]. Considering the high prevalence and high fatality in advanced cases of gastric cancer, finding effective measures for primary or secondary prevention of gastric cancer is a public health priority. In 2013, the Japanese health insurance has begun covering eradication therapy for H. pylori-positive gastritis, even though there is no concrete evidence on the eradication therapy for H. pylori gastritis [6,7]. However, it is not clear whether there is a definite benefit compared to the harms due to their high cost and potential increase in antibiotic resistance with mass eradication therapy to H. pylori infection. In 2013, clinical practical guidelines for diagnosis and treatment of H. pylori infection were developed to address domestic situations with an adaptation process [8].
The present revision of previous guidelines intended to generate evidence by conducting a systematic review of the additive indication such as eradication therapy of H. pylori in patients with unexplained iron deficiency anemia (IDA), post-endoscopic resection (ER) of gastric adenoma and atrophy gastritis and/or IM.
For effective eradication, multi-antibiotics regimens with anti-secretory agents are used. Unsuccessful eradication is associated with high bacterial load, high gastric acidity, the virulence of Helicobacter strains and poor compliance. However, growing antibiotics resistance, particularly clarithromycin resistance seems to be the major cause of decreasing eradication rate [9].
In the last 20 years, a widespread use of antibiotics, such as clarithromycin for respiratory symptoms and levofloxacin for urinary infection, has increased the primary H. pylori resistance in many countries [10]. Systematic review revealed that the overall H. pylori antibiotic resistance rates were 17.2% (95% confidence interval [CI], 16.5% to 17.9%) for clarithromycin, 26.7% (95% CI, 25.2% to 28.1%) for metronidazole, and 11.2% (95% CI, 9.6% to 12.7%) for amoxicillin [10]. Based on these changes, European guidelines recommended to extend the standard triple therapy to 14 days where clarithromycin resistance was > 15% to 20% [11]. In Korea, the clarithromycin resistance rates rose from 9% in 1995 and 13.8% in 2003 to 16.7% in 2005, and 17.8% in a nationwide study in 2018 [2,12–14].
With dynamic changes of the epidemiology of H. pylori and increasing issue of antibiotics resistance, a new approach is needed for effective management. In these updated guidelines, we aimed to present the appropriate H. pylori treatment for Korean by conducting systematic review and meta-analysis to identify the clinical evidence for alternative treatments to the standard 7-day triple therapy.
METHODS
Guidelines development organization
The Steering Committee which consisted of the President and the executive members of the Korean College of Helicobacter and Upper Gastrointestinal Research established development strategies, appointed working group members, and approved budgets related to the project.
This guideline included multidisciplinary processes by the Korean Society of Clinical Microbiology, the Korean Society of Pathologists, and the Korean Society of Gastroenterology. To establish the methodology for developing guidelines, two methodological experts (M.C. and Ein-Soon Shin, Research Agency for Clinical Practice Guidelines, Research Center, Korean Academy of Medical Sciences, Seoul) and Professor Soo Young Kim, a member of the Korean Medical Association’s Clinical Treatment Guidelines Development Committee, conducted four workshops on the literature search, quality assessment, meta-analysis, and methods of expert consensus.
In the course of developing or approving the guidelines, the members of the Working group were asked to confirm that they had no conflict of interest by accepting advice or employment from commercially relevant organizations, commercial ownership interests, research funds, and case fees, or intellectual property rights (e.g., patents, trademarks, licensing, or royalties) for drugs related to the development of the guidelines, and whether their families had the same relationship as those described above.
Patient’s preference and perspective
A web survey using structured questionnaires was administered to the largest Internet community associated with gastrointestinal diseases was to identify the experiences, expectations and preferences of patients in this guideline. A total of 233 subjects responded, 64.4% of whom were adult women and 57.5% of whom were H. pylori-positive. Among those who were positive for H. pylori, 86.7% wanted treatment, the reasons for which were for prevention of stomach cancer (44.6%), improvement of stomach symptoms (28.8%), and fear of transmitting infection to others (9.9%). The most worrisome aspect of the H. pylori treatment was 80.3% of the drug’s adverse reactions. Based on these points, the topic of adverse effect of eradication therapy and the impact of H. pylori eradication on gastric adenoma after ER of neoplastic lesions in the stomach, atrophic gastritis, and IM, and the precursor to gastric cancer were added as key questions.
Guideline development process
The scope of clinical practice guidelines was determined by deriving key questions tailored to the Population, Intervention, Comparator, Outcome (PICO) format using nominal group techniques in working group [15]. De novo method was implemented in this revision because the guidelines required the latest evidence as the dynamics of the H. pylori infection and related gastrointestinal diseases in Korea are changing rapidly.
Systematic review and meta-analysis
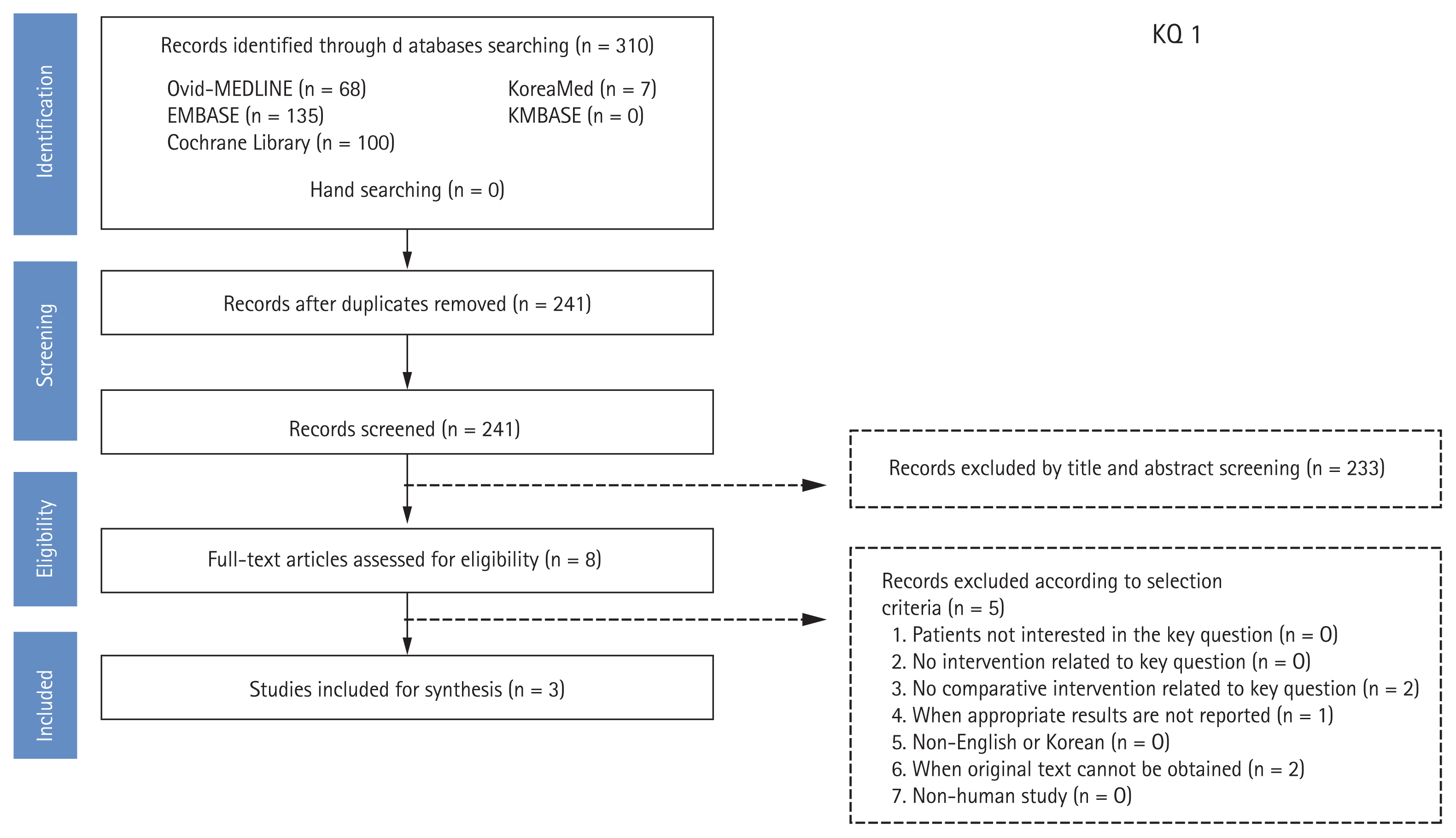
A systematic review was conducted at each PICO. Dr. M.C. of the National Evidence-based Healthcare Collaborating Agency and a working group member of each subject selected appropriate search key words and conducted a literature search from July to August 2018 using the Ovid-MEDLINE, EMBASE, Cochrane Library, KoreaMed, and KMBASE databases; the key words were listed in the appendix. The common inclusion criteria for the studies were as follows: (1) adult subjects or patients as the study population; (2) written in English or Korean; (3) systematic reviews and meta-analyses, randomized or non-randomized trials, and observational studies; (4) published between 2008 and 2018; and (5) studies with proper results (e.g., symptom improvement, development of gastric cancer, eradication rates) reported. The common exclusion criteria were as follows: (1) studies on children or teenagers; (2) studies with no proper results reported; (3) duplicated publication; (4) impossible to obtain original text; and (5) expert opinion, case series or report, narrative review, or guidelines. Two independent members reviewed the literatures and selected the final studies according to the inclusion and exclusion criteria. The literature selection process was summarized in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) plot for each PICO. An example of the process of literature selection for key question 1 is shown in Fig. 1. The quality of finally selected studies was assessed using quality assessment tools according to the study design. Randomized controlled trials (RCTs) were evaluated using the Cochrane risk of bias (RoB) tool [16], while non-randomized clinical studies were assessed using the Risk of Bias Assessment Tool for Non-Randomized Study (RoBANS) [17]. If the assessments were not consistent as the two paired working members, the two members and the chairman coordinated a final evaluation.
Elicitation of recommendations: Level of evidence and grade of recommendation
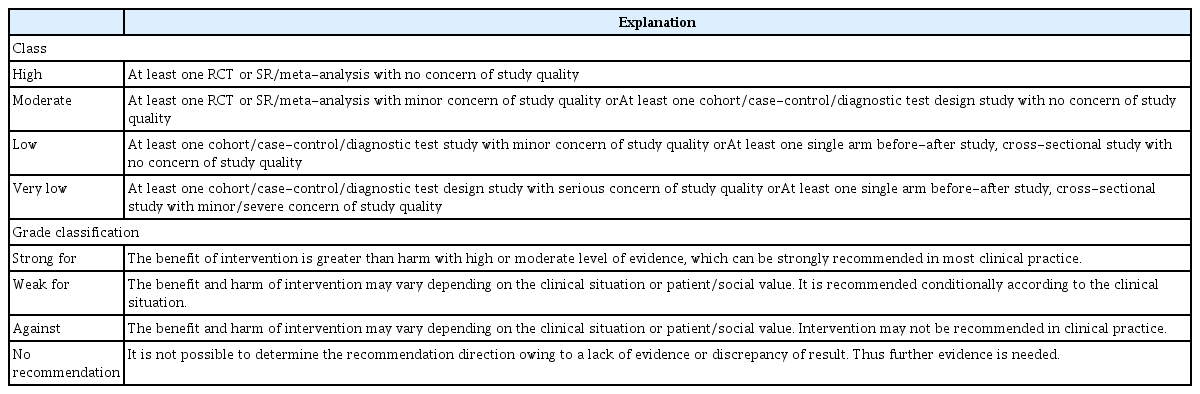
After systematic literature review, the evidence table was organized and meta-analysis was conducted. Evidence profiles were created based on ‘Grading of Recommendations, Assessment, Development and Evaluation’ (GRADE) (Table 1) [18]. GRADEpro software was used to rank the quality of evidence according to four categories: high, moderate, low, and very low. The quality assessment of the evidence was then used to determine the strength of the supporting evidence that informs a recommendation [19]. The recommendations presented in this guideline are summarized in Table 2.
A literature search was conducted to utilize resources and economic evaluation. The cost of H. pylori eradication, which uses antibiotics and proton pump inhibitors (PPIs) for 7 to 14 days, was not significantly different between the treatment methods. The tailored therapy based on H. pylori susceptibility to antibiotics may be cost-effective in a high clarithromycin-resistant region compared to standard empirical triple therapy. There are reports that tailored treatment is superior in terms of cost-effectiveness if the eradication rate of H. pylori is lowered below 75.3%. Therefore, the tailored treatment based on H. pylori susceptibility to antibiotics was added to the recommendation [20,21].
Expert consensus with modified Delphi agreement process
The aim of expert consensus by modified Delphi methods was to determine the extent to which experts agreed about draft recommendation including evidence [22]. The first round of Delphi process was conducted with e-mail voting and 44 experts were invited, with 30 participating in the first agreement. The first round was investigated using the 9-likert scale’s self-reporting questionnaire asking the extent of agreement, along with evidence data from the Development Committee for each recommendation. In the response scale, one point was “completely disagreeable” and nine points were “very agreeable.” Consent was considered when the ratio of points from 7 to 9 (high agreement) was more than two-thirds. Eight recommendations were agreed upon for a total of 12 recommendations. Indication of H. pylori eradication therapy with IDA and atrophy/IM and eradication regimen of H. pylori with standard triple therapy and sequential therapy failed to reach agreement, so the Development Committee revised these 4 recommendations after the first voting and conducted the second round of voting in a face-to-face agreement (December 14, 2019). A second round of voting was conducted anonymously, and recommendations for atrophy gastritis/IM were rejected by 48% of 23 respondents, with the remaining recommendations passed and finally 11 recommendations were adopted.
Internal and external review
In the second round of face-to-face voting for the expert consensus process, various drafts were adopted after the anonymous vote. After the process of expert agreement and external review, the draft was revised with their opinions.
Dissemination of the guidelines and update information
This guideline was provided on the Korean College of Helicobacter and Upper Gastrointestinal Research (http://www.hpylori.or.kr) and the Korean Association of Internal Medicine (http://www.kaim.or.kr) websites. In addition, this guideline will be published in Korean and English as a paper and will be spread throughout the academic symposium.
CLINICAL PRACTICE GUIDELINES
Newly added indication for H. pylori eradication
Iron deficiency anemia
Can H. pylori eradication increase the hemoglobin in patients with idiopathic iron deficiency anemia?
Statement 1: H. pylori eradication can be helpful to improve the anemia in subset of adults with unexplained iron deficiency anemia.
Grade of recommendation: weak
Level of evidence: very low
Experts’ opinions: completely agree (28.0%), mostly agree (48.0%), partially agree (10.0%), mostly disagree (10.0%), completely disagree (5.0%), not sure (0%)
Anemia is a major health problem and mostly caused by iron deficiency [23,24]. The estimated prevalence of anemia is 24.8% (95% CI, 22.9% to 26.7%), affecting 1.62 billion people (95% CI, 1.50 to 1.74 billion) globally [23], and concentrated in preschool children and women. H. pylori infection causes diverse gastrointestinal diseases, including chronic gastritis, peptic ulcer disease and cancer. Furthermore, H. pylori chronic gastritis can induce decreasing gastric acid secretion and gastric ascorbic acid, which are essential for the absorption of dietary iron [25,26].
H. pylori has been associated with IDA. A recent meta-analysis revealed a significantly higher likelihood of IDA in subjects with H. pylori-infection (pooled odds ratio [OR], 1.72; 95% CI, 1.23 to 2.42) [27]. However, this association was strong in children, but subgroup analysis of adult population revealed weaker association with significant heterogeneity (pooled OR, 1.70; 95% CI, 1.01 to 2.85) [23].
The role of H. pylori infection in IDA was shown in studies with H. pylori eradication therapy combined with iron supplementation to treat the IDA. However, these studies were heterogeneous because of confounders including age (children or adolescent vs. adults), sex and different study setting in terms of different definition of IDA or outcomes (quantitative assessment of ferritin or hemoglobin or qualitative assessment, such as recovery from anemia). Meta-analysis including children, adolescent, or adults showed significant increase of ferritin after the eradication, not hemoglobin. Meta-analysis of s even RCTs showed increased ferritin, standardized mean difference (SMD) 0.53 (95% CI, 0.21 to 0.85), but not hemoglobin, SMD 0.36 (95% CI, −0.07 to 0.78) [27]. However, there were limited studies which showed the efficacy of H. pylori eradication in adult population. Non-randomized comparative study in adults with IDA showed the additional effect of H. pylori eradication on iron supplement in adult patients with IDA and H. pylori-positive chronic gastritis [28]. In a prospective observational study, H. pylori infection was correlated with low serum ferritin level and after eradication, serum ferritin increased; however, the sample size was too small [29]. In this study, serum ferritin in premenopausal women was significantly lower than that of post-menopausal women, but not different in men. In other observational studies with adult patients with IDA, IDA was resolved after 38.1% of eradication of H. pylori-eradicated patients (32/84). This was more frequent in men and postmenopausal women compared with premenopausal women (75% vs. 23%, p < 0.01) [30]. Despite the very low level of evidence, it was decided as a “weak recommendation” because short-term treatment of H. pylori infection has the potential for long-term benefits and low risk for serious harm.
After endoscopic resection of gastric adenoma
Is H. pylori eradication helpful to prevent metachronous recurrence after endoscopic resection of gastric adenoma?
Statement 2: H. pylori eradication can be recommended after endoscopic resection for H. pylori-positive gastric adenoma to prevent metachronous recurrence.
Grade of recommendation: weak
Level of evidence: low
Experts’ opinions: completely agree (60.0%), mostly agree (20.0%), partially agree (20.0%), mostly disagree (0%), completely disagree (0%), not sure (0%)
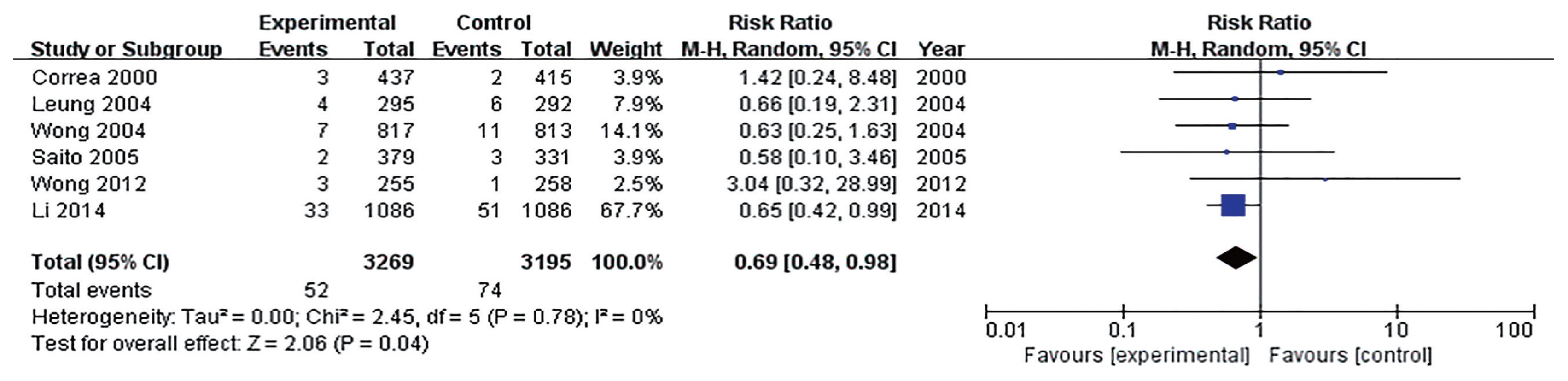
Many studies have reported that the incidence rate of metachronous cancer decreased with H. pylori eradication after ER of early gastric cancer (EGC) [31–33]. Thus, H. pylori should be eradicated to prevent metachronous recurrence after ER of EGC. However, there was no definite guideline about H. pylori eradication after ER of gastric adenoma. Until now, there were two RCTs about H. pylori eradication to prevent metachronous gastric cancer after ER of gastric tumors including EGC and adenoma (Supplementary Table 1) [31,33]. Three retrospective studies about H. pylori eradication after ER of gastric adenoma were reported [34–36]. All of them were conducted in Korea. According to studies, the incidence of metachronous recurrence was lower in H. pylori eradicated group than non-eradicated group (3.24% vs. 4.87% [33]; 7.69% vs. 14.29% [31]; 7.76% vs. 10.80% [34]; 8.20% vs. 19.44% [35]; 4.71% vs. 11.36% [36]). When meta-analysis included five studies, the effect of H. pylori on prevention of metachronous recurrence after ER of gastric adenoma was statistically significant (OR, 0.55; 95% CI, 0.34 to 0.92) (Fig. 2).
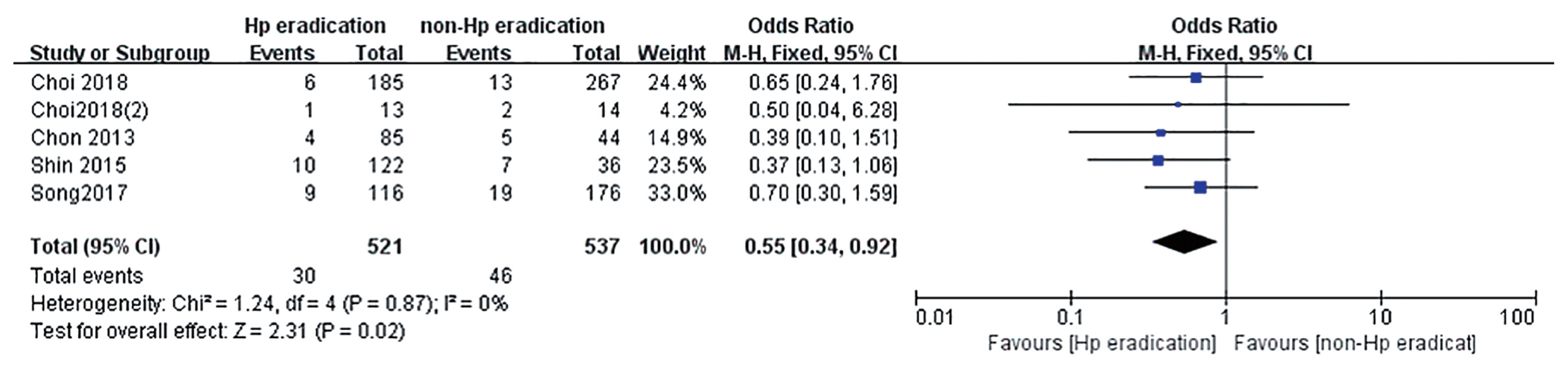
Comparison of occurrence of metachronous gastric cancer after endoscopic resection of gastric adenoma between Helicobacter pylori (Hp) eradication and placebo. M-H, Mantel-Haenszel; CI, confidence interval.
According to studies, H. pylori eradication is helpful to prevent metachronous recurrence after ER of gastric adenoma. Therefore, H. pylori eradication is indicated after ER for H. pylori-positive gastric adenoma. However, RCT focused on gastric adenoma is required to support this recommendation.
Functional dyspepsia
Is H. pylori eradication helpful in symptom relief in patients with functional dyspepsia?
Statement 3: H. pylori eradication can be recommended for long-term improvement of dyspeptic symptoms in patients with functional dyspepsia.
Grade of recommendation: weak
Level of evidence: high
Experts’ opinions: completely agree (43.3%), mostly agree (26.7%), partially agree (23.4%), mostly disagree (3.3%), completely disagree (3.3%), not sure (0%)
In the meta-analysis of RCTs, when H. pylori was eradicated in dyspeptic patients, the symptom improvement was not significant in the short-term (3 months) follow-up, but symptoms were significantly improved in the long-term (6 to 12 months) follow-up [37,38]. Based on these results, the Maastricht V guidelines in Europe and the United States and Canadian guidelines strongly recommend the eradication of H. pylori as the first-line treatment for dyspepsia [11,39].
In the present guideline, 18 RCTs from January 1997 to December 2017 were selected and meta-analysis was conducted to evaluate the long-term effects of H. pylori eradication in patients with dyspepsia (Supplementary Table 2) [40–57]. In a meta-analysis of 4,672 patients from 18 RCTs, the risk ratio (RR) of persistence of dyspeptic symptoms in the control group was 1.18 (95% CI, 1.07 to 1.31) compared with the eradication group. Although statistically significant, the number of patients needed for treatment (number needed to treat [NNT]) was 14, and heterogeneity among studies was moderate (I2 = 34%) (Supplementary Fig. 1) [58].
Because of the heterogeneity among the studies, subgroup analysis was performed by region to analyze five RCTs from the Asian regions and 13 RCTs from outside Asian regions. Meta-analysis from RCTs from outside Asia regions showed significant improvement of dyspeptic symptoms in eradication group (RR, 1.22; 95% CI, 1.08 to 1.38; I2 = 33%). However, because of analysis of RCTs from Asia, the effect of eradication on improvement of dyspeptic symptoms was not significant (RR, 1.10; 95% CI, 0.92 to 1.31; I2 = 32%).
In summary, eradication of H. pylori improved dyspeptic symptoms significantly, however, the clinical effect was not large due to the improvement of symptoms in 1 of 14 treated patients (NNT = 14) and the result of subgroup analysis of RCTs conducted in Asia was not statistically significant. The prevalence of H. pylori in Korea is estimated to be 54% (95% CI, 50.1% to 57.8%) according to a study that estimates the prevalence of H. pylori worldwide [59]. In areas with high prevalence of H. pylori, costs, adverse effects associated with eradication therapy, the risk of emergence of resistance strains, and re-infection are thought to be higher than those of low prevalence regions. Therefore, in the present guideline, it was decided to make weak recommendations despite the high level of evidence for H. pylori eradication in patients with functional dyspepsia. The RCTs, including cost-effectiveness analysis of eradication therapy in patients with functional dyspepsia in areas with high prevalence of H. pylori, including in Korea, are likely to be needed.
Chronic atrophic gastritis and intestinal metaplasia
Is H. pylori eradication effective to prevent gastric cancer in the presence of chronic atrophic gastritis and intestinal metaplasia?
H. pylori eradication can reduce the risk of gastric cancer development. However, it is controversial whether the eradication can be beneficial in individuals with pre-neoplastic lesions including chronic atrophic gastritis (CAG) and IM.
Recently published two meta-analyses showed that individuals with non-atrophic or CAG benefited from eradication in reducing the risk of gastric cancer, whereas individuals with IM and/or dysplasia did not [60,61]. The effect of H. pylori eradication can be affected by the degree of mucosal atrophy. H. pylori eradication can be more beneficial to subjects with mild mucosal atrophy than those with extensive atrophic gastritis [62]. Maastricht V guideline recommended that the risk for gastric cancer can be reduced more effectively by eradicating H. pylori before atrophy and IM develop [11].
However, the two meta-analyses included the studies that evaluated the effect of H. pylori eradication on the occurrence of metachronous gastric cancers after ER of EGC, as well as the studies in the general population. The effect of H. pylori eradication may be different between in the general population and in the high-risk group. Moreover, a population-based cohort study in China, which was not included in the previous meta-analyses, showed that H. pylori eradication can benefit individuals with IM and/or dysplasia at baseline, suggesting H. pylori eradication can benefit an entire population regardless of the baseline gastric histopathology [63].
When the meta-analysis was performed using the RCTs in the general population only, H. pylori eradication significantly reduced the incidence of gastric cancer, as in previous studies (Fig. 3). In a subgroup analysis that included only subjects with CAG or IM, eradication had no effect on the prevention of gastric cancer, and two studies involving subjects without CAG and IM did not demonstrate significant gastric cancer prevention effect (Supplementary Fig. 2). However, in the latter case, the number of incidences of gastric cancer were small, and there were limitations in drawing an accurate conclusion. In expert consensus based on these analyses, only 48.0% agreed in the first e-mail questionnaire, and only 63.3% agreed in the second face-to-face meeting. In other words, there is no firm evidences or expert agreement to recommend eradication of H. pylori in subjects with CAG or IM, so re-discussion is needed after more researches have been accumulated. The indications for H. pylori eradication treatment presented above are summarized in Table 3.
Forest plot of studies reporting impact of Helicobacter pylori eradication on gastric cancer from the studies involving general population. M-H, Mantel-Haenszel; CI, confidence interval.
H. pylori eradication therapy
First line therapy
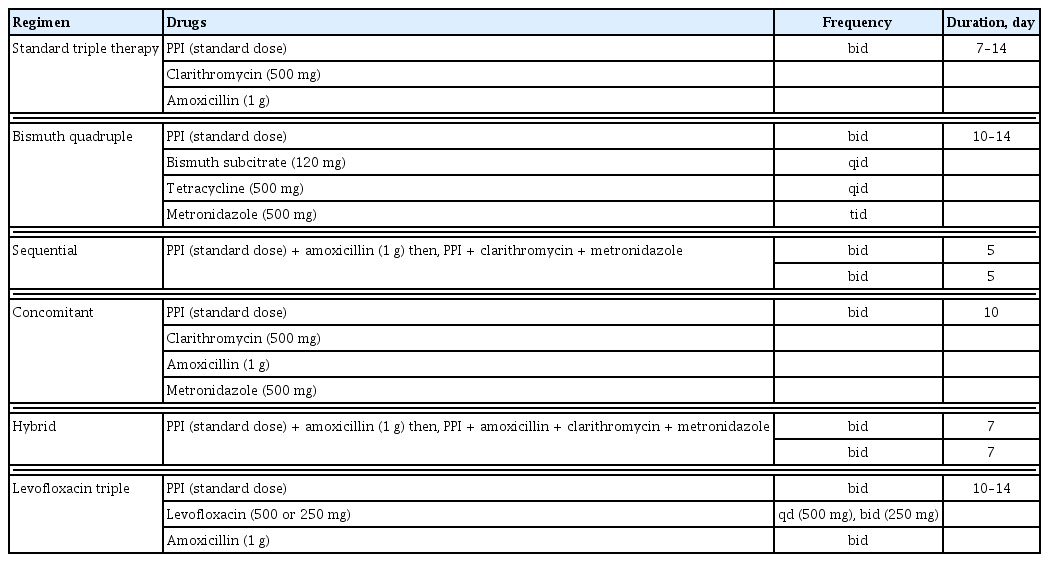
In patients undergoing H. pylori eradication for the first time, one of the following four regimens can be used: (1) 14-day standard triple therapy; (2) non-bismuth quadruple therapy; (3) 7-day standard triple therapy after clarithromycin resistance test; and (4) bismuth quadruple therapy.
1) Standard triple therapy
Can standard triple therapy be one of the first line therapy for H. pylori eradication?
Statement 4: Standard triple therapy (standard dose PPI, amoxicillin 1 g, and clarithromycin 500 mg twice daily) for 14 days is recommended for first-line regimen.
Grade of recommendation: strong
Level of evidence: moderate
Experts’ opinions: completely agree (27.0%), mostly agree (50.0%), partially agree (14.0%), mostly disagree (9.0%), completely disagree (0%), not sure (0%)
To make appropriate choice of first-line regimen, we need to consider the regional resistance pattern and eradication rate. Many factors, such as compliance, gastric acidity, and bacterial loads are related to the efficacy of triple therapy. However, the eradication rate of standard triple therapy is mainly influenced by clarithromycin resistance. Clarithromycin resistance has increased during the last 10 years in Korea, and the resistance rate of clarithromycin in Korea is reported to be 17.8% to 31.0% [2,14]. The geographic distribution of clarithromycin resistance is highly variable. According to the recently published nationwide antibiotics resistance mapping study in Korea, the resistance rate of clarithromycin was less than 15% in the Seoul and Chungcheong areas and over 15% in other parts of Korea [2]. These results suggest that clarithromycin triple therapy is still acceptable as a first-line treatment in some part of Korea.
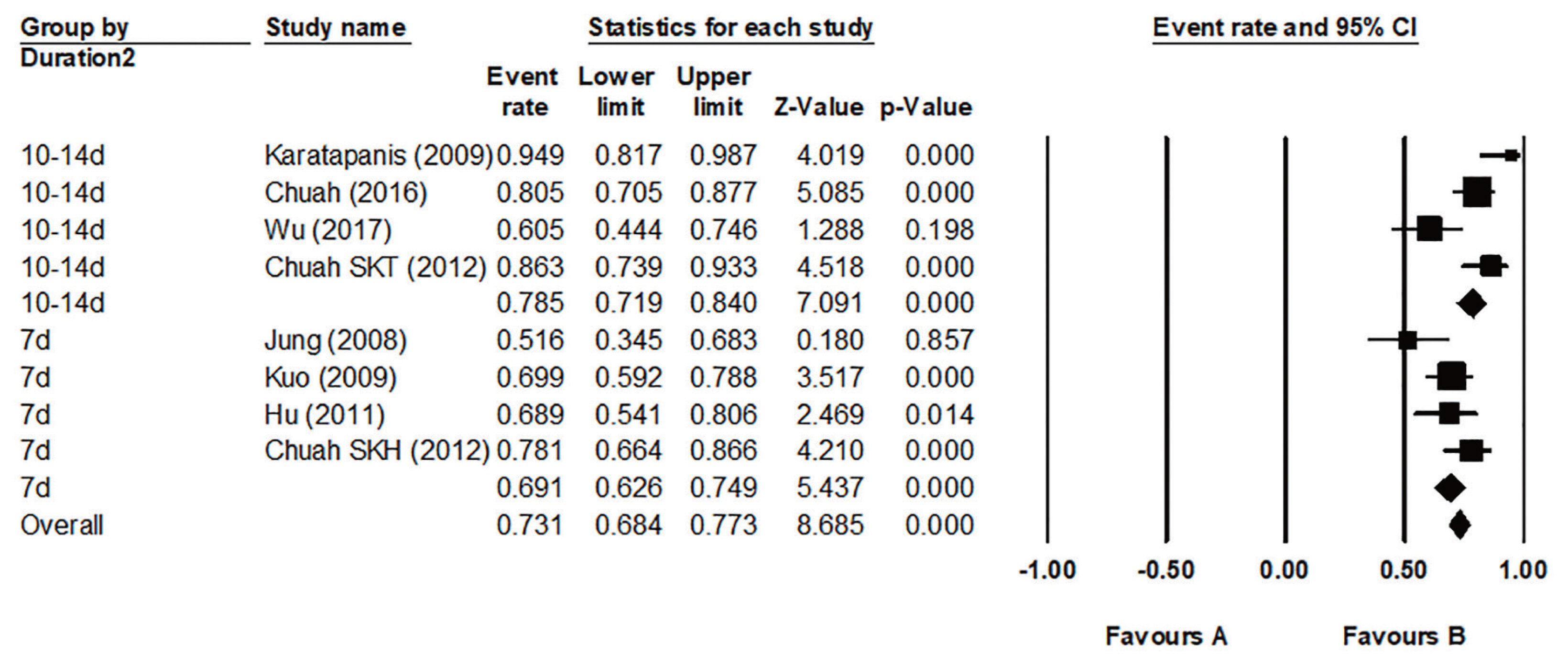
To be eligible for a first-line treatment of H. pylori eradication therapy, the regimen must show at least 80% to 85% of the eradication rate [8,64]. To find an eradication rate for standard triple therapy in Korea, we searched and selected all RCTs conducted in Korea which have used clarithromycin triple therapy since 2007. Twenty-six studies were included in meta-analysis (Supplementary Table 3) [65–90]. Overall pooled eradication rates of standard triple therapy derived from these studies were 71.6% (95% CI, 69.9% to 73.3%) in intention-to-treat (ITT) analysis and 79.6% (95% CI, 76.6% to 82.2%) in per protocol (PP) analysis (Fig. 4). Studies were divided into 2007–2011 and 2012–2016. Pooled eradication rates of 2007–2011 and 2012–2016 were 72.3% (95% CI, 71.2% to 74.4%) and 70.3% (95% CI, 68.4% to 72.1%) in ITT analysis, respectively. The pooled eradication rate of standard triple therapy in 2012–2016 was on the decline compared with 2007–2011. The pooled eradication rate of standard triple therapy was inadequate to be used as a first-line treatment. This result was similar to that of prospective RCT conducted in 2018 by Korean College of Helicobacter and Upper Gastrointestinal Research [91]. Therefore, to use the standard triple therapy as a first-line treatment, it is necessary to consider introducing a clarithromycin resistance test or extending the treatment duration.

Time trends of pooled Helicobacter pylori eradication rates of standard triple therapy from randomized controlled trials performed in Korea by years. Overall eradication rates of standard triple therapy were 71.6% (95% confidence interval [CI], 69.9% to 73.3%) in intention-to-treat (ITT) analysis and 79.6% (95% CI, 76.6% to 82.2%) in per protocol (PP) analysis. Eradication rates from 2007 to 2011 years were 72.3% (95% CI, 71.2% to 74.4%) in ITT analysis and 81.5% (95% CI, 79.9% to 82.9%) in PP analysis. Eradication rates from 2012 to 2016 years were 70.3% (95% CI, 68.4% to 72.1%) in ITT analysis and 77.4% (95% CI, 75.6% to 79.2%) in PP analysis. ap < 0.01.
Regarding duration of standard triple therapy, we analyzed the pooled eradication rate of 7-, 10-, and 14-day therapy. The pooled eradication rate of 7-day standard triple therapy was 70.0% (95% CI, 68.5% to 71.4%) and that of 10-day therapy was 73.7% (95% CI, 69.8% to 77.2%) in ITT analysis. The pooled eradication rate (ITT) of 14-day therapy was 78.1% (95% CI, 75.2% to 80.7%) which was significantly higher than those of 7- and 10-day therapy (p < 0.01 for both duration) (Supplementary Fig. 3). Eradication rates between the 7- and 10-day therapy were not significantly different. A network meta-analysis published in 2017 that analyzed 34 RCTs since 2005 also showed similar results [92]. The pooled eradication rates (ITT) of 7-, 10-, and 14-day standard triple therapy were 71.1% (95% CI, 68.3% to 73.7%), 67.0% (95% CI, 60.0% to 73.4%), and 76.4% (95% CI, 73.3% to 79.2%), respectively. In addition, according to a national multicenter study published in 2019, the eradication rates of 7-day standard triple therapy were 63.9% in ITT analysis and 71.4% in PP analysis [91].
Based on the above analysis and the available evidences, a 14-day therapy is recommended when considering standard triple therapy as a first-line treatment without clarithromycin resistance test.
2) Non-bismuth quadruple therapy
(1) Sequential therapy
Can sequential therapy be one of the first line therapy of H. pylori eradication?
Statement 5: Sequential therapy (standard dose PPI, amoxicillin 1 g twice daily for 5 days followed by standard dose PPI, clarithromycin 500 mg, and metronidazole 500 mg twice daily for 5 days) can be one of first line therapies for H. pylori eradication.
Grade of recommendation: strong
Level of evidence: high
Experts’ opinions: completely agree (31.0%), mostly agree (39.0%), partially agree (9.0%), mostly disagree (21.0%), completely disagree (0%), not sure (0%)
In recent guidelines, non-bismuth quadruple therapy, sequential or concomitant treatment, or bismuth quadruple therapy is recommended as the first-line treatment in regions where clarithromycin resistance is more than 15% [11,93]. Non-bismuth quadruple therapy uses amoxicillin, clarithromycin, and metronidazole simultaneously with PPI, but each method has different duration of use for individual antibiotic. Sequential therapy is a method of using PPI and amoxicillin for the first 5 days, and then administering PPI, clarithromycin and metronidazole for 5 days from 6 to 10 days.
A meta-analysis of 24 RCTs (n = 5,070) was conducted to confirm the effect on sequential therapy as the first-line treatment (Supplementary Table 4) [75,77–80,90,94–111]. Twenty RCTs compared comparing standard triple therapy, two RCTs comparing bismuth quadruple therapy, and two RCTs comparing hybrid therapy were included. RCTs comparing sequential therapy with concomitant therapy (CT) are described in the CT section.
① Sequential therapy vs. standard triple therapy
Twenty studies compared the eradication rate between sequential therapy and conventional therapy. In all, 3,224 and 3,152 patients were treated with sequential and standard triple therapies, respectively. The eradication rates of sequential therapy and overall standard triple therapy were 84.1% and 74.9% in the ITT analysis, respectively. The rates of sequential therapy and overall standard triple therapy were 85.9% and 77.3% in the PP analysis, respectively. The pooled RR of the ITT eradication rates (sequential therapy vs. standard triple therapy) was 1.37 with the fixed effects model (95% CI, 1.21 to 1.54) (Fig. 5), while the pooled RR of the PP eradication rates was 1.60 with the fixed effects model (95% CI, 1.40 to 1.83) (Supplementary Fig. 4).
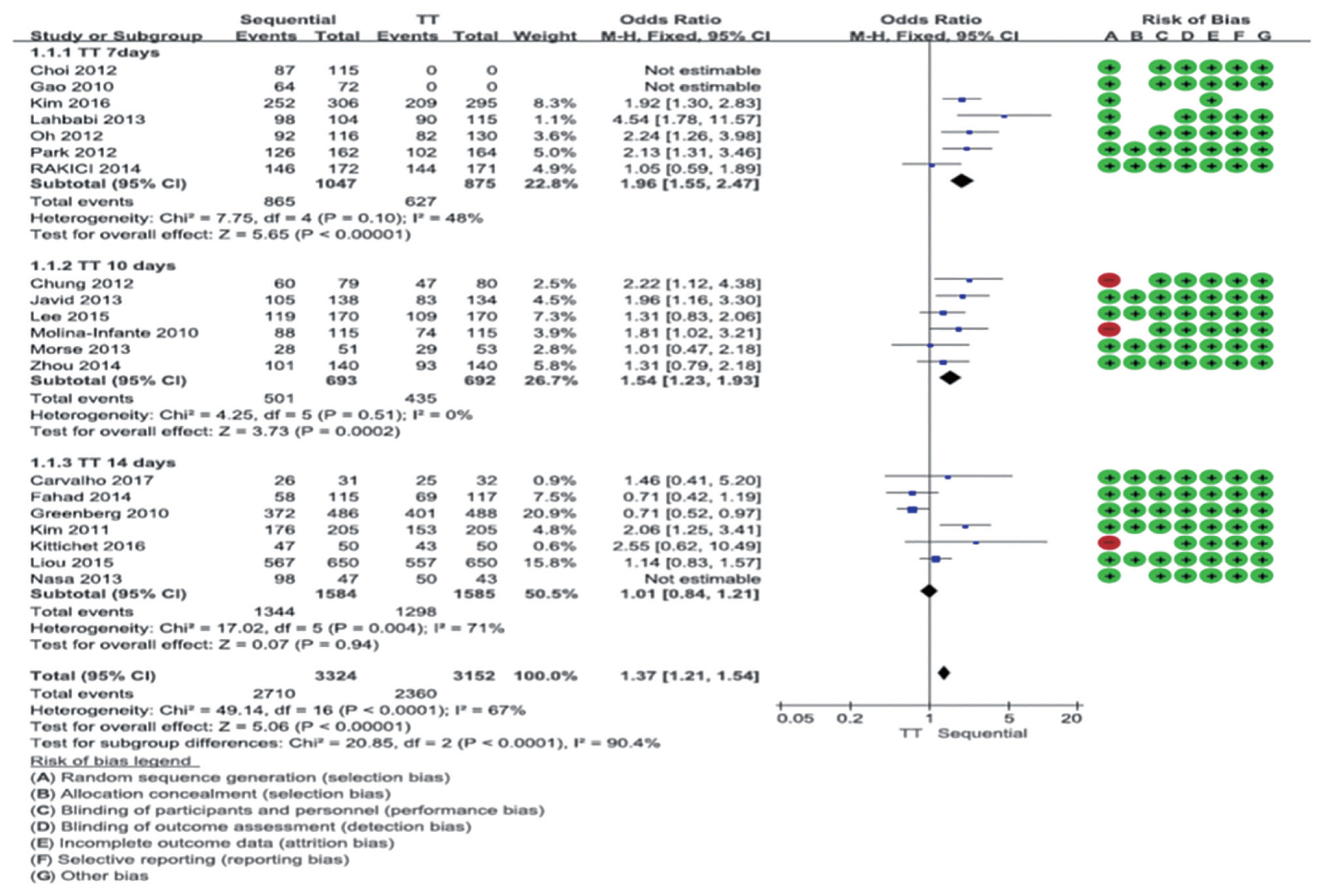
Comparison between 10-day sequential therapy and standard triple therapy according to treatment duration of standard triple therapy in intention-to-treat analysis. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); G, other bias. TT, standard triple therapy; M-H, Mantel-Haenszel; CI, confidence interval.
② Sequential therapy vs. bismuth quadruple therapy
In the meta-analysis of two RCTs comparing 10-day sequential therapy with bismuth quadruple therapy, the eradication rates of the two therapies were not significantly different (RR, 0.79; 95% CI, 0.47 to 1.32 in ITT analysis; RR, 0.63; 95% CI, 0.27 to 1.49 in PP analysis). However, there was a limitation that the number of patients included was small and local studies were not included (Supplementary Fig. 5).
③ Sequential therapy vs. hybrid therapy
In a meta-analysis of two RCT studies comparing 10-day sequential therapy with hybrid therapy, the 10-day sequential therapy showed a lower eradication rate than hybrid therapy (RR, 0.36; 95% CI, 0.23 to 0.58 in ITT analysis; RR, 0.18; 95% CI, 0.10 to 0.35 in PP analysis) (Supplementary Fig. 6). However, there was a limitation that the number of patients included was small and no local studies were included.
In summary, the eradication rate of 10-day sequential therapy as a first-line treatment was higher than that of standard triple therapy, and in the subgroup analysis, sequential therapy showed a higher eradication rate than the 7- and 10-day standard triple therapy but a comparable eradication rate with the 14-day standard triple therapy. The comparative analysis of bismuth quadruple therapy and hybrid therapy was difficult to conclude due to the small number of RCTs. Therefore, as clarithromycin resistance increases in Korea, 10-day sequential therapy is recommended when first-line treatment is considered without clarithromycin resistance testing.
(2) Concomitant therapy
Can concomitant therapy be one of the first line therapy of H. pylori eradication?
Statement 6: Concomitant therapy (standard dose PPI, clarithromycin 500 mg, amoxicillin 1 g, and metronidazole 500 mg twice daily for 10 days) is recommended as a first-line treatment.
Grade of recommendation: strong
Level of evidence: high
Experts’ opinions: completely agree (66.7%), mostly agree (13.3%), partially agree (13.4%), mostly disagree (3.3%), completely disagree (3.3%), not sure (0%)
CT, in which amoxicillin, clarithromycin, and metronidazole are administered simultaneously for 10 days is one of the non-bismuth quadruple therapy (concomitant, sequential, and hybrid therapy) used to overcome the decreased eradication rates of standard triple therapy. To investigate the efficacy of CT as first-line treatment of H. pylori eradication, we selected RCTs including CT. Short term treatment of CT (5-day CT or 7-day CT) were excluded. A total of 26 RCTs were finally eligible in this analysis and the characteristics of each study are shown in Supplementary Table 5 [84,89,109,112–132]. A total of 21 studies of 10-day CT, six studies of 14-day CT and one RCT of both 10-day CT and 14-day CT were included. The eradication rate for 10-day CT was 85% for ITT analysis and 91% for PP analysis, and the rate for 14-day CT was 86% for ITT analysis and 94% for PP analysis, which was higher than that of standard triple therapy. There was no difference in eradication rate according to the duration in the subgroup analysis. From the analysis including studies conducted in Korea only, the eradication rates of 10-day CT were 84% in ITT analysis and 92% in PP analysis, and those of 14-day CT were 79% in ITT analysis and 94% in PP analysis; there was no difference in eradication rate according to the administration duration (Supplementary Table 6).
CT for 10 days showed a slightly higher eradication rate compared to sequential therapy and a 17% higher eradication rate compared to 10-day/14-day standard triple therapy, and the evidence level was high. There was no difference in eradication rate between bismuth quadruple therapy and hybrid therapy, and the levels of evidence were evaluated as moderate and high, respectively.
Through the systematic search, 21 RCTs comparing 10-day CT with other regimens were selected. Eight RCTs compared 10-day CT with 10-day/14-day standard triple therapy. The eradication rate of 10-day CT was significantly higher than that of 10-day/14-day standard triple therapy (RR, 1.17; 95% CI, 1.05 to 1.30 in ITT analysis; RR, 1.15; 95% CI, 1.06 to 1.25 in PP analysis) (Fig. 6).

Comparison between 10-day concomitant therapy and 10-/14-day standard triple therapy in intention-to-treat analysis. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); G, other bias. CT, concomitant therapy; ST, sequential therapy; M-H, Mantel-Haenszel; CI, confidence interval.
In 13 RCTs, 10-day CT and 10-day sequential therapy were compared. The eradication rate of 10-day CT was significantly higher than that of the 10-day sequential therapy, but the difference seems to be small (RR, 1.04; 95% CI, 1.00 to 1.08 in ITT analysis; RR, 1.04; 95% CI, 1.01 to 1.07 in PP analysis) (Supplementary Fig. 7). The eradication rate of CT was slightly higher than that of sequential treatment, which is thought to be because CT is more effective than sequential treatment if it is resistant only to either clarithromycin or metronidazole. In fact, in case of clarithromycin resistance, CT had a higher eradication rate compared to sequential therapy [133,134]. In the case of metronidazole-resistant but, not clarithromycin-resistant, CT also showed higher eradication rate than sequential therapy [133,135].
In six RCTs, 10-day CT and 10-day/14-day of bismuth quadruple therapy were compared. The eradication rate of 10-day CT was not significantly different from that of bismuth quadruple therapy (RR, 1.05; 95% CI, 0.96 to 1.15 in ITT analysis; RR, 1.01; 95% CI, 0.97 to 1.06 in PP analysis) (Supplementary Fig. 8). In two RCTs, 10-day CT and hybrid therapy were compared, and there was no difference between the two groups (RR, 0.99; 95% CI, 0.93 to 1.05 in ITT analysis). The eradication rates of 14-day CT and 10-day/14-day sequential treatment were compared in three RCTs. The eradication rate of 14-day CT did not differ from that of 10-day/14-day sequential therapy in the ITT analysis (76% vs. 79%), but in the PP analysis, the eradication rate of 14-day CT was slightly higher than that of sequential therapy (89% vs. 82%). In two RCTs, 14-day CT and 14-day standard triple therapy were compared, and 14-day CT showed significantly higher eradication rate than 14-day standard triple therapy (88% vs. 79% in ITT analysis; 94% vs. 82% PP analysis). In two RCTs, 14-day CT and hybrid therapy were compared, and 14-day CT showed slightly higher eradication rate than hybrid therapy (91% vs. 85%, p = 0.05 in ITT analysis; 96% vs. 92%, p = 0.07 in PP analysis).
In summary, the eradication rate of 10-day CT as first-line treatment was significantly higher than that of 10-day/14-day standard triple therapy but slightly higher than that of sequential therapy and similar to those of bismuth quadruple therapy and hybrid therapy. The eradication rate of 14-day CT was significantly higher compared to 14-day standard triple therapy in both PP and ITT analysis, and 10-day/14-day sequential therapy in the PP analysis. The eradication rates of 10-day CT and 14-day CT were similar. Therefore, if first-line treatment is considered without resistance test, 10-day CT is recommended.
3) Standard triple therapy based on clarithromycin resistance test
Does clarithromycin resistance test improve the eradication rate of standard triple therapy?
Statement 7: Clarithromycin resistance test by PCR or sequencing is recommended when a 7-day standard triple therapy is considered as a first-line treatment.
Grade of recommendation: strong
Level of evidence: low
Experts’ opinions: completely agree (76.7%), mostly agree (6.6%), partially agree (16.7%), mostly disagree (0%), completely disagree (0%), not sure (0%)
The eradication rate of empirical standard triple therapy (PPI + amoxicillin + clarithromycin) has been declined to about 70% through the last decades in Korea [136,137]. The 2013 revised-Korean guideline recommended this therapy as one of primary regimens for H. pylori eradication, even though experts’ complete agreement rate of this strategy was only 53.6% [8]. Maastricht V guideline recommended that clarithromycin-based triple therapy without prior susceptibility testing should be abandoned when the regional clarithromycin resistance rate is more than 15% [11]. Considering the decreasing and sub-optimal eradication rate of this empirical therapy and high resistance rate of clarithromycin in Korea, new strategies are desperate for improving eradication rate of H. pylori.
Although clarithromycin susceptibility test by H. pylori culture is the best method for appropriate selection of H. pylori eradication regimens [138,139], it is very difficult to apply culture-based results to clinical practice because of slow growth of H. pylori and demanding culture conditions. On the contrary, tailored therapy after molecular testing using polymerase chain reaction (PCR)-kits or sequencing methods detecting 23S ribosomal RNA point mutations related to clarithromycin resistance is one of easily applicable methods.
In the large case-control study (n = 1,232), the patients who had the A2142G and A2143G point mutations associated with clarithromycin resistance based on dual priming oligonucleotide-based multiplex PCR were treated with PPI + amoxicillin + metronidazole (PAM) for 7 days, and the patients without clarithromycin resistance were treated with standard triple therapy for 7 days (tailored therapy). The eradication rate of H. pylori in the tailored therapy group was 80.7% (176/218), which was significantly higher than that in the empirical 7-day standard triple therapy (69.5% [214/308], p < 0.01) or PAM (71.1% [219/308], p = 0.01) control groups in ITT analysis [82]. In addition, the recent two case-control studies reported that 7-day tailored therapy with bismuth quadruple therapy, PAM, or standard triple therapy had higher eradication rates than those of empirical 7-day standard triple therapy (91.8 vs. 72.1%; 94.3 vs. 76.5%, respectively) in PP analysis [20,21]. Importantly, the costs for a successful eradication with tailored therapy could be similar or superior to those of empirical 14-day standard triple therapy [21].
4) Bismuth quadruple therapy
Can bismuth quadruple therapy be one of the first line therapy of H. pylori eradication?
Statement 8: Eradication rates of bismuth quadruple therapy (standard dose PPI twice daily, metronidazole 500 mg three times daily, bismuth 120 mg and tetracycline 500 mg four times daily for 10 to 14 days) are similar to 14 days standard triple therapy, 10 days concomitant therapy, and 10 days sequential therapy. However, because of its high adverse effects and potential use as second-line therapy, it can be recommended to be used as first-line therapy if other first-line therapy options are not available.
Grade of recommendation: weak
Level of evidence: moderate
Experts’ opinions: completely agree (50.0%), mostly agree (33.4%), partially agree (10.0%), mostly disagree (3.3%), completely disagree (3.3%), not sure (0%)
Established guidelines recommended that bismuth quadruple or non-bismuth quadruple therapies are suitable for the first-line H. pylori eradication therapy in high clarithromycin resistance areas [11,140].
In two network meta-analysis studies of the RCTs, the efficacy of bismuth-containing quadruple therapy varied depending on the type of eradication regimens and duration of therapies [141,142]. We performed meta-analysis including nine RCTs from January 2008 to July 2018 investigating the efficacy and safety of bismuth quadruple therapy for the first-line H. pylori eradication (Supplementary Table 7) [109,116,121,143,144]. Pooled eradication rates of bismuth quadruple therapy by ITT analysis and PP analysis were 84.5% (95% CI, 74.9% to 90.9%) and 90.6% (95% CI, 82.8% to 95.1%), respectively. However, there was no statistically significant difference of ITT eradication rates in 10 to 14 days bismuth quadruple therapy compared to 14 days standard triple therapy (RR, 1.28; 95% CI, 0.97 to 1.70) (Fig. 7A), 10 days sequential therapy (RR, 0.96; 95% CI, 0.83 to 1.12) (Fig. 7B), and 10 days CT (RR, 1.01; 95% CI, 0.93 to 1.10) (Fig. 7C). In addition, there was no statistically significant difference of PP eradication rates in 10 to 14 days bismuth quadruple therapy compared to 14 days standard triple therapy (RR, 1.37; 95% CI, 0.95 to 1.99), 10 days sequential therapy (RR, 0.99; 95% CI, 0.93 to 1.05), and 10 days CT (RR, 1.01; 95% CI, 0.95 to 1.07). Heterogeneity among studies was generally moderate, and we should interpret these results with caution due to small numbers of the current meta-analysis.
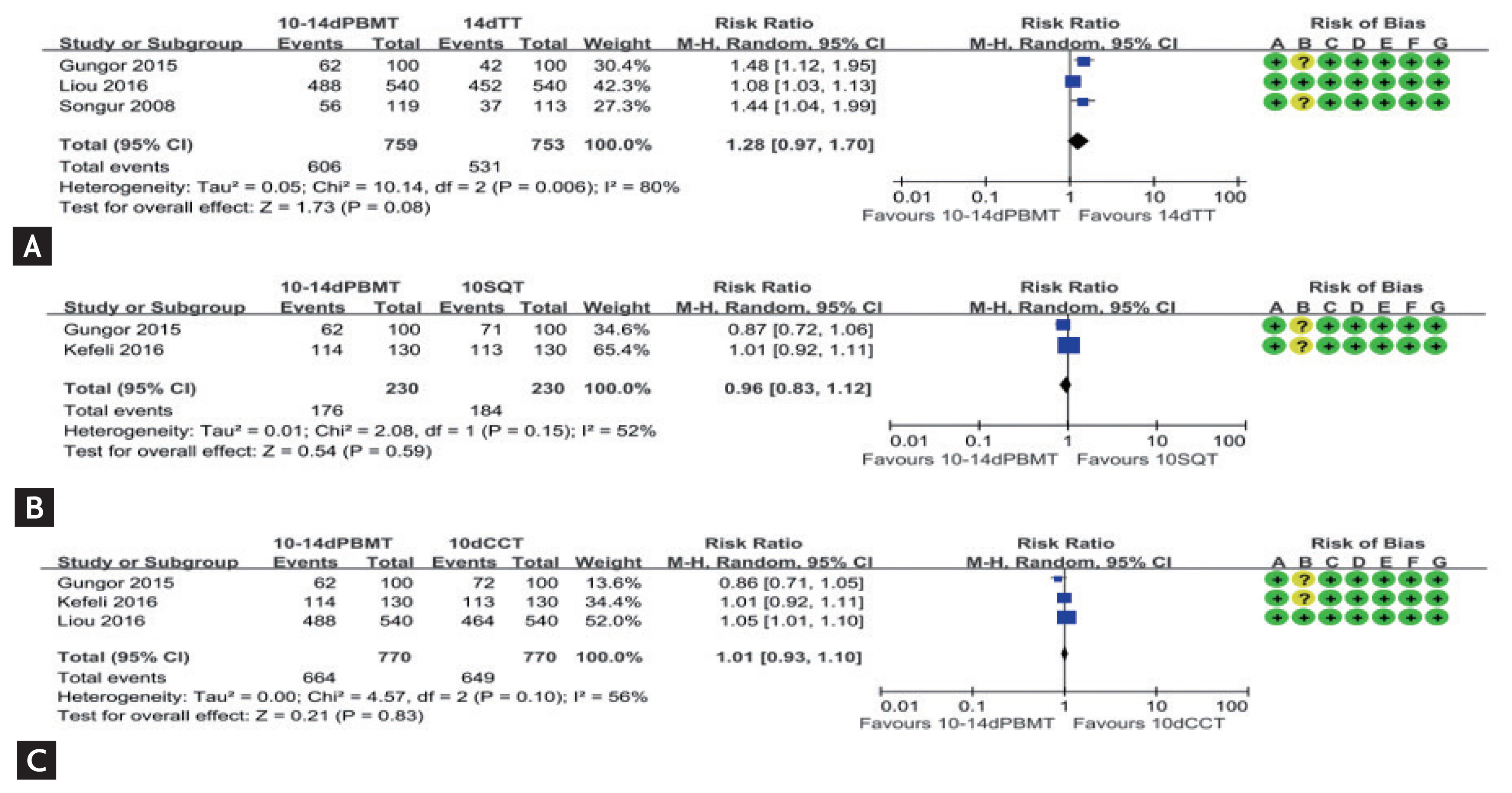
Comparison of eradication rate of bismuth quadruple therapy in intention-to-treat analysis as a first line therapy. (A) 10-/14-day bismuth quadruple therapy vs. 14-day standard triple therapy; (B) 10-/14-day bismuth quadruple therapy vs. 10-day sequential therapy; (C) 10-/14-day bismuth quadruple therapy vs. 10-day concomitant therapy. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); G, other bias. PBMT, bismuth quadruple therapy; TT, standard triple therapy; M-H, Mantel-Haenszel; CI, confidence interval; SQT, sequential therapy; CCT, concomitant therapy.
In terms of adverse events, bismuth quadruple therapy was significantly higher than other eradication therapies (RR, 1.72; 95% CI, 1.23 to 2.40) (Supplementary Fig. 9). However, considerable heterogeneity was shown among studies (I2 = 92%).
Bismuth quadruple therapy is regarded as a promising treatment for patients with allergy to penicillin as well as H. pylori with dual resistance [140]. Therefore, it may be one of the attractive options for H. pylori eradication. Unfortunately, there are few rescue therapies for H. pylori eradication, and high adverse events of bismuth quadruple therapy cannot be ignored in clinical practice. Thus, bismuth quadruple therapy can be first-line treatment for H. pylori eradication when other first-line options are unavailable, due to its high adverse events rate and bismuth quadruple therapy is a widely used rescue therapy for other regimens.
Further well-designed studies are required to confirm the efficacy of bismuth quadruple therapy for fist-line H. pylori eradication in Korea. Additionally, regarding the bismuth dosage, bismuth subcitrate (De-Nol, Astellas Pharma Europe B.V., Leiderdorp, The Netherlands) 300 mg contains elemental bismuth 120 mg. Therefore, physicians can prescribe bismuth subcitrate 300 mg four times daily in clinical practice.
Salvage therapy
What is the recommended salvage regimen after failure of previous H. pylori eradication therapy?
In the last decade, the efficacy of PPI, clarithromycin, and amoxicillin triple therapy has decreased mainly due to clarithromycin resistance [145,146]. As a result, it has become a common situation in clinical practice to choose a salvage regimen after failure of one or more eradication attempts. Moreover, the selection of a rescue regimen may be more complicated with the emergence of alternative first-line treatments such as sequential or CT.
In the systematic literature review, there were 36 RCTs that compared different combinations of antibiotics, different durations of a regimen, or different PPIs as salvage therapy after one or more eradication failures between 2008 and 2017 (Supplementary Table 8) [147–182]: 24 RCTs evaluated second line regimens, five evaluated third line regimens, five compared different durations of a regimen, and two compared different doses or kinds of PPIs. Most studies for second-line regimens were conducted after failure of clarithromycin-based triple therapy; there was no RCT that evaluated second-line regimens after failure of non-bismuth or bismuth quadruple therapy, and there was no RCT that evaluated third-line regimen after failure of first-line clarithromycin-based triple therapy followed by second-line bismuth quadruple therapy. Meta-analyses were conducted and there were more than three RCTs. The recommendations on the salvage regimen were primarily based on those RCTs and their meta-analyses. However, when no suitable trial was found, the most relevant cohort studies, published systematic reviews of cohort studies, and RCTs published before 2008 were referenced for the recommendations. All eradication rates presented below are from ITT analyses. The evidences which were included in the meta-analysis of regimens for salvage therapy are summarized in the Supplementary Table 9.
Statement 9: After failure of standard triple therapy, a bismuths quadruple therapy (PPI, bismuth, tetracycline, and metronidazole) for 14 days is recommended as a second-line therapy.
Grade of recommendation: strong
Level of evidence: high
Experts’ opinions: completely agree (90.0%), mostly agree (6.7%), partially agree (3.3%), mostly disagree (0%), completely disagree (0%), not sure (0%)
In the 2013 revised Korean guidelines, bismuth quadruple therapy (PPI, bismuth, tetracycline, and metronidazole) for 7 to 14 days was recommended. The systematic review conducted for the present guidelines identified 15 RCTs which compared 31 treatment arms as 2nd line treatment after failure of 1st line PPI-clarithromycin-amoxicillin triple therapy [147,152,156,161–163,165–168,174–176,179,182]. Of those, nine studies adopted bismuth quadruple therapy [156,161,162,165,167,175, 176,179,182], and eight studies adopted levofloxacin triple therapy (PPI, amoxicillin, and levofloxacin) [147,152,165,166,168,175,176,182]; of those, four studies compared bismuth quadruple therapy and levofloxacin triple therapy directly [165,175,176,182].
Bismuth quadruple therapy showed pooled eradication rate of 75.5% (95% CI, 71.6% to 79.1%) in the meta-analysis of the nine studies (Fig. 8). Regarding the treatment duration, four studies treated for 7 days, two for 10 days, and three for 14 days. Because 10- and 14-day regimens showed similar efficacy, subgroup analysis was conducted to compare 7 day versus 10- to 14-day bismuth quadruple therapy. In result, 10- to 14-day therapy showed significantly higher eradication rates (pooled eradication rate, 81.6%; 95% CI, 76.9% to 85.6%; I2 = 29.6%) than 7-day therapy (pooled eradication rate, 68.4%; 95% CI, 53.0% to 73.5%; I2 = 73.8%) (p < 0.01). The systematic review also identified three RCTs comparing 14-day versus 7-day bismuth quadruple therapies. Meta-analysis of these RCTs also showed a significant eradication rate with 14-day regimen than 7-day regimen (risk difference [RD], 0.09; 95% CI, 0.02 to 0.15; p < 0.01).

Meta-analysis of nine studies comparing bismuth quadruple therapy with other regimens after failure of first-line standard triple therapy. Pooled eradication rate of bismuth quadruple therapy as second-line therapy was 75.5% (95% confidence interval [CI], 71.6% to 79.1%).
Levofloxacin triple therapy showed pooled eradication rate of 73.1% (95% CI, 68.4% to 77.3%) in the meta-analysis of the eight studies (Fig. 9). In the subgroup analysis comparing treatment duration, the 10- to 14-day regimen in four studies showed a significantly higher eradication rate (78.5%; 95% CI, 71.9% to 84.0%) than 7-day regimen in four studies (69.1%; 95% CI, 61.6% to 74.9%; p = 0.04). One factorial RCT reported 10-day therapy showed significantly higher eradication rate with 7-day therapy (87.5% vs. 67.5%, p < 0.01) [177]. Meanwhile, there was no significant difference in the dose of levofloxacin between 500 mg daily and 1,000 mg daily in this study (p = 1.00).
Meta-analysis of eight studies which compared levofloxacin triple therapy with other regimens after failure of first-line line clarithromycin triple therapy. Pooled eradication rate of levofloxacin triple therapy as second-line therapy was 73.1% (95% confidence interval [CI], 68.4% to 77.3%).
There was no significant difference in the eradication rates between bismuth quadruple therapy and levofloxacin triple therapy in the meta-analysis of four RCTs. There were non-significant contradictory trends favoring levofloxacin triple therapy in ITT analysis (bismuth quadruple vs. levofloxacin triple: RD, −0.06; 95% CI, −0.14 to 0.02; p = 0.16) but favoring bismuth quadruple therapy in PP analysis (RD, 0.02; 95% CI, −0.05 to 0.10; p = 0.58) (Supplementary Fig. 10). These results may be because of low tolerability of bismuth quadruple therapy. In two systematic reviews published in 2006, 10-day levofloxacin triple therapy showed superior efficacy compared to 7-day bismuth quadruple therapy [183,184]. In our meta-analysis, two regimens were treated for the same duration (7 days vs. 7 days, 10 days vs. 10 days, or 14 days for 14 days). Thus, it can be suggested that bismuth quadruple therapy and levofloxacin triple therapy may have similar efficacy when the two treatments are administered for the same duration.
A major limitation of levofloxacin triple therapy is that efficacy of the regimen is substantially reduced in the presence of levofloxacin resistance [165]. In Korea, the resistance rate for levofloxacin in H. pylori strains has been increasing rapidly as high as 28.1% [14,185,186]. Very recently, the nationwide antibiotic resistance profile of H. pylori in Korean population was reported [2]. According to this report, resistance rate against levofloxacin was 37.0%. Thus, bismuth quadruple therapy would be favored over levofloxacin triple therapy in Korea. However, it should also be noted that resistance rate against metronidazole was also as high as 29.5% in the same study. Because resistance to metronidazole can be overcome with increased duration and dose, 14-day course would be preferred to 10- to 14-day course for bismuth quadruple therapy as a salvage regimen in Korea [187]. Therefore, bismuth quadruple therapy for 14 days is recommended as a second-line therapy after failure of standard triple therapy.
Statement 10: After failure of non-bismuth quadruple therapy (sequential or concomitant therapy), a bismuth quadruple therapy is recommended as a second-line therapy.
Grade of recommendation: strong
Level of evidence: very low
Experts’ opinions: completely agree (60.0%), mostly agree (30.0%), partially agree (6.7%), mostly disagree (3.3%), completely disagree (0%), not sure (0%)
There was no RCT comparing salvage regimens after failure of first-line non-bismuth quadruple therapy. There was a meta-analysis of cohort studies in which most studies included were a levofloxacin triple regimen [188]. In this study, the pooled eradication rate of levofloxacin triple therapy from five studies including 86 patients was 81% (95% CI, 71% to 91%; I2 = 28%). Another meta-analysis conducted in Maastricht V guidelines showed 81% (six studies; 95% CI, 73% to 90%; I2 = 19%) after failure of sequential therapy and 78% (three studies; 95% CI, 58% to 97%; I2 = 67%) after failure of CT [11]. However, these results may not be directly applicable to Korean population because of high levofloxacin resistance rates as previously discussed [2,14,185,186].
Bismuth quadruple therapy showed eradication rate of 84% (95% CI, 63% to 106%; I2 = 56%) in the meta-analysis conducted in Maastricht V [11]. However, the analysis included only two cohort studies of similar sample sizes with each other. One of them was a study conducted in Korea where 14-day bismuth quadruple therapy achieved successful eradication in 10 of 14 patients.
Therefore, bismuth quadruple therapy is recommended as a second-line therapy after failure of first-line non-bismuth quadruple therapy based on currently available evidences. However, more data is required to support this recommendation.
Statement 11: After failure of bismuth quadruple therapy as 1st-line or 2nd-line therapy (after failed standard triple or non-bismuth quadruple therapy), a levofloxacin triple therapy is suggested as a salvage therapy.
Grade of recommendation: weak
Level of evidence: very low
Experts’ opinions: completely agree (40.0%), mostly agree (30.0%), partially agree (13.3%), mostly disagree (16.7%), completely disagree (0%), not sure (0%)
The most common situation in which bismuth quadruple therapy fails in Korea is failures as a second-line regimen after failure of first-line standard triple therapy. This approach was recommended by previous Korean guidelines 2013 and Maastricht IV guidelines [8,189]. The scenario in which second-line bismuth quadruple therapy fails after failure of first-line non-bismuth quadruple therapy is also expected to be a more common situation. In these cases, it is not recommended to use clarithromycin again in the third-line regimen [187]. It would be also inappropriate to use clarithromycin after failure of first-line bismuth quadruple therapy because this regimen had been chosen as first-line when clarithromycin resistance was suspected [8]. Treatment regimen may be decided based on antibiotics susceptibility tests, either by culture, PCR, or sequencing analysis. Generally, it is recommended not to use clarithromycin, fluoroquinolone, and rifabutin again in the presence of resistance to respective drugs, while amoxicillin and metronidazole may be re-used [187]. However, it is noteworthy that benefits of susceptibility-guided therapy over empirical regimen was evident only in the first-line treatment, not in the second-line setting in a recent systematic review [139]. Neither was it in the third-line treatment in a recent RCT [190]. The first-line and salvage treatment regimens and algorithms for H. pylori treatment combined with the regimens are summarized in Table 4 and Fig. 10, respectively.

Proposed algorithm for Helicobacter pylori treatment in Korea. Bismuth quadruple therapy of first-line therapy is dotted because it is less preferred than other regimens. CM, clarithromycin; PAC, pantoprazole, amoxicillin, clarithromycin; PPI, proton pump inhibitor; PAM, PPI + amoxicillin + metronidazole.
Levofloxacin triple therapy
There was no RCT comparing rescue options after failure of bismuth quadruple therapy either as first-line or second-line regimen. In 2012, Gisbert et al. [191] reported on the efficacy of third-line PPI-amoxicillin-levofloxacin triple therapy in a systematic review of six cohort studies including 350 patients and a cohort study including 200 of their own patients. This was updated in Maastricht V as 501 patients in five studies resulting in pooled eradication rate of 70.0% (95% CI, 62.4% to 76.6%; I2 = 58.5%) [11]. A Korean study reported retrospective data from 14 medical centers in 2017, in which 110 patients received levofloxacin third-line therapy, 88 adhered to the treatment protocol, and 63 achieved successful eradication (62 after PPI-amoxicillin-levofloxacin and one after PPI-amoxicillin-clarithromycin-levofloxacin). The estimated eradication rate with third-line levofloxacin triple therapy was 56.9% (62/109) [192]. Although previously failed first- and second-line regimens were not specified in this study, this low eradication rate may have been due to the high levofloxacin resistance rate in Korea [2,14,185,186]. Therefore, a levofloxacin triple therapy is suggested as a salvage therapy after failure of first-line or second-line bismuth quadruple therapy. However, the efficacy of this regimen may be lower than observed in the systematic review as indicated in the Korean retrospective study.
Triple therapy containing other fluoroquinolones
Three Japanese RCTs evaluated fluoroquinolone based triple therapy after first-line PPI-amoxicillin-clarithromycin and second-line PPI-amoxicillin-metronidazole failure [150,160,181]: the sitafloxacin-PPI-amoxicillin regimen showed an eradication rate of 70.0% (49/70; 95% CI, 59.0% to 81.0%) after 7-day therapy and 81.0% (51/63; 95% CI, 71.0% to 90.9%) after 10-day therapy [150,160], while gatifloxacin-PPI-amoxicillin 7-day therapy was administered to only eight patients with six eradication successes (75%) [181]. In European prospective cohort study, moxifloxacin-PPI-amoxicillin 14-day therapy showed 82.4% eradication rate (206/250; 95% CI, 77.0% to 87.0%) after failure of first-line clarithromycin triple or non-bismuth quadruple therapy [193]. However, in a Korean retrospective cohort study, the same regimen with 7 to 14 days showed successful eradiation in only 67.9% (95% CI, 51.5% to 84.9%) after failure of first-line bismuth quadruple therapy [194]. Thus, evidences are limited for triple therapy containing other fluoroquinolones after failure of bismuth quadruple therapy because the above-mentioned studies are highly heterogeneous regarding the design and previous regimens. The potential cross-resistance among fluoroquinolones may further limit the usage of other fluoroquinolones as alterative to levofloxacin in rescue therapy [186].
Fluoroquinolone quadruple therapy
There was no RCT evaluating fluoroquinolone quadruple therapy after failure of bismuth quadruple therapy. However, eight RCTs compared 10 arms of various fluoroquinolone quadruple regimens with other regimens after failure of first-line triple therapy [147,151,152,157,162,163,169,171].
The levofloxacin-bismuth quadruple regimen (levofloxacin, bismuth, PPI, and amoxicillin) was suggested as an ‘encouraging salvage strategy’ in patients failing previous bismuth quadruple therapy in Maastricht V guidelines because of synergistic effects between bismuth and levofloxacin to overcome antibiotics resistance [11]. This regimen was evaluated in two RCTs with successful eradication rates of 84.8% (28/33; 95% CI, 72.6% to 97.1%) after 10-day therapy and 88.1% (126/143; 95% CI, 81.6% to 92.9%) after 14-day therapy after failure of first-line PPI-amoxicillin-clarithromycin/metronidazole [147,157]. Two prospective cohort studies reported that levofloxacin-bismuth quadruple therapy showed 83.8% (31/37; 95% CI, 71.3% to 96.2%) eradication rate after failure of first-line clarithromycin triple and second-line bismuth quadruple therapy and 90.0% (180/200; 95% CI, 85.8% to 94.2%) after failure of first-line standard triple or non-bismuth quadruple therapy [195,196]. However, in a RCT reported from Hong Kong in 2007, levofloxacin-bismuth quadruple therapy achieved successful eradication in 73% (37/51; 95% CI, 60.0% to 85.2%) subjects after ≥ 1 eradication failures, which was inferior to bismuth quadruple therapy [197]. Therefore, although being expected to be effective, this regimen needs to be validated in Korean population before use.
The levofloxacin sequential regimen (levofloxacin, PPI, amoxicillin, and metronidazole followed by PPI and amoxicillin) also showed promising results in patients failing various first-line triple therapy, with successful eradication rates ranging between 82.2% and 90.2% in three RCTs [151,152,163]. This regimen also needs verification for its efficacy after failure of bismuth quadruple therapy.
Rifabutin containing regimen
The major limitations of using rifabutin for the eradication of H. pylori are high cost, myelotoxicity, and concerns for inducing resistance to Mycobacterium tuberculosis [198]. Three RCTs comparing rifabutin triple therapy (PPI-amoxicillin-rifabutin) with other regimens as salvage treatment were reported before 2008 [199–201]. A systematic review reported in 2012, which included those three RCTs, showed second-, third-, and forth-line eradication rates of rifabutin triple therapy were 79% (95% CI, 67% to 92%), 66% (95% CI, 55% to 77%), and 70% (95% CI, 60% to 79%), respectively [198]. It is recommended that rifabutin daily dose of 300 mg and treatment duration of 10 days are appropriate for this regimen [140]. There were another three RCTs including rifabutin containing regimens in the systematic review for the current guidelines [149,158,159]. However, no study compared rifabutin triple therapy with other regimen after failure of bismuth quadruple therapy. They had highly heterogeneous designs among the studies with eradication rates ranging 77.8% to 96.3%. Therefore, rifabutin triple therapy may be suggested as one of rescue options after previous failure of multiple attempts including bismuth quadruple therapy. Nevertheless, relative lack of evidences and known risks should be carefully considered.
High dose dual therapy
High dose dual therapy includes amoxicillin ≥ 3 g administered ≥ 3 times daily to maintain high trough levels [140]. Only one RCT found in the systematic review, in which 14-day rabeprazole 20 mg plus amoxicillin 750 mg four times daily achieved eradication rate of 89.3% (50/56; 95% CI, 80.9% to 97.6%) in patients with ≥ 1 eradication failures of unspecified regimens [154]. Two RCTs conducted before 2008 reported eradication rates of 70% (95% CI, 57.5% to 79.7%) and 75.6% (95% CI, 59.7% to 87.6%) as salvage therapy [200,202]. This regimen may also be considered salvage therapy after failure of bismuth quadruple therapy, but more data is required to support this decision.
Concomitant therapy
Although it is inappropriate to use clarithromycin again after failing clarithromycin containing regimen, CT (PPI-amoxicillin-clarithromycin-metronidazole) may be selected as a salvage treatment because combination of clarithromycin and metronidazole may overcome clarithromycin resistance [187]. However, there was no RCT which evaluated CT after failure of bismuth quadruple therapy in the systematic review. There was only one RCT which showed that 7-day CT achieved 86.5% (45/51; 95% CI, 76.9% to 96.1%) after failure of first-line PPI-amoxicillin-clarithromycin [174]. One prospective cohort study nested in a RCT reported that 10-day CT showed 84.6% (11/13; 95% CI, 57.8% to 95.7%) eradication rate after failure of first-line bismuth quadruple therapy [203].
CONCLUSIONS
H. pylori is associated with socioeconomic burdens as it causes various gastrointestinal diseases and has a high prevalence rate of about 50% in Korea. It is clinically effective to establish therapeutic indications for H. pylori and to present effective primary and secondary treatment regimens; this is important and necessary for the efficient use of national medical resources. In recent years, as the resistance rate of H. pylori to clarithromycin has increased, the eradication rate of the existing standard triple therapy has tended to decrease. To overcome this, the treatment period has been extended or non-bismuth quadruple therapy such as sequential therapy or CT has been introduced. In the case of salvage therapy, it was difficult to select the right RCTs for each situation due to the diversity of first-line therapy regimens. As a result of meta-analyses of the latest RCTs published, bismuth quadruple therapy is recommended after standard triple therapy, sequential therapy, or CT has failed. If bismuth quadruple therapy is used as the first-line or salvage therapy, levofloxacin triple therapy is recommended. However, its effectiveness may be reduced in areas with high resistance to levofloxacin, such as Korea.
Although recommendations were made according to the resistance rate of Korea, implementing evidence-based medicine and using de novo meta-analysis, but it can also be applied in Far East Asia, which has a similar antibiotic resistance rate as Korea. In addition, recommendation on clarithromycin resistance testing based on PCR or sequencing have been made based on the latest literatures. We believe that the statement regarding tailored therapy will be useful for the use of resistance tests based on PCR or sequencing, which are widely used in clinical practice recently due to the convenience of use.
In this guideline, expert consensus was not reached on indications for eradication therapy for CAG/IM; therefore, further studies are needed to determine whether eradication therapy may lower the incidence of gastric cancer in CAG/IM. In addition, family history of gastric cancer is also a known risk factor of gastric cancer, and further research is needed to establish therapeutic indications for this. Studies on cost-effectiveness according to various combinations of first-line and salvage therapy regimens are also needed in future.
Notes
These guidelines are co-published by Gut and Liver and Korean Journal of Internal Medicine for facilitated distribution.
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This guideline was published in “Gut and Liver 2021;15(2):168–195.” This was co-published on “The Korean Journal of Internal Medicine” with permission from “The Korean Association of Internal Medicine” and “Korean College of Helicobacter and Upper Gastrointestinal Research.”
We would like to express our deep gratitude to Miyoung Choi, PhD of National Evidence based Healthcare Collaborating Agency who performed initial literature search for systematic review, Ein Soon Shin, PhD & MPH, Research Agency for Clinical Practice Guidelines, Research Center, Korean Academy of Medical Sciences, Seoul, and Su Young Kim, a professor from the Department of Family Medicine, Hallym University College of Medicine for conducting the Workshop on Expert Consensus Method. We also thank Nayoung Kim, a professor from the Department of Internal Medicine, Seoul National University College of Medicine and Kwang Ha Kim, a professor from the Department of Internal Medicine, Pusan University College of Medicine who reviewed the draft of the guidelines by peer review. Finally, we thank the Internet community ‘Bokanyi’ for helping us with the patient preference survey.