Eosinophilic endotype of chronic obstructive pulmonary disease: similarities and differences from asthma
Article information
Abstract
Approximately 25% to 40% of patients with chronic obstructive pulmonary disease (COPD) have the eosinophilic endotype. It is important to identify this group accurately because they are more symptomatic and are at increased risk for exacerbations and accelerated decline in forced expiratory volume in the 1st second. Importantly, this endotype is a marker of treat ment responsiveness to inhaled corticosteroid (ICS), resulting in decreased mortality risk. In this review, we highlight differences in the biology of eosinophils in COPD compared to asthma and the different definitions of the COPD eosinophilic endotype based on sputum and blood eosinophil count (BEC) with the corresponding limitations. Although BEC is useful as a biomarker for eosinophilic COPD endotype, optimal BEC cut-offs can be combined with clinical characteristics to improve its sensitivity and specificity. A targeted approach comprising airway eosinophilia and appropriate clinical and physiological features may improve identification of subgroups of patients who would benefit from biologic therapy or early use of ICS for disease modification.
INTRODUCTION
The prevalence of chronic obstructive pulmonary disease (COPD) is 11% to 26%, higher than that of asthma [1,2]. This worrisome trend is expected to continue over the next 25 years [2]. COPD is responsible for 2.6% of the global disability adjusted life years [3] and is projected to be the third leading cause of death worldwide in 2030 [4]. Recurrent exacerbations in COPD are associated with an accelerated decline in physical function and forced expiratory volume in the 1st second (FEV1), and a high economic burden [5–7]. The 30-day COPD readmission rate is 15% to 30% [8]. Approximately 30% of all COPD exacerbations are life-threatening, requiring mechanical ventilation. After hospital discharge, the readmission rate stagnates at 20% with a 90-day mortality rate of up to 20% [9].
Because recurrent COPD exacerbation is a poor prognostic indicator, guidelines are increasingly recognizing the importance of accurately identifying risk factors in individual patients to reduce the disease burden [10]. However, risk prediction using clinical characteristics, such as a history of prior exacerbation, cannot identify the underlying pathobiological mechanisms [7]. This often results in empirical treatment and suboptimal treatment outcomes. Hence, in the last 8 years, there has been increased awareness of COPD as a heterogeneous disease with different endotypes, similar to asthma [11,12].
Although COPD is typically characterized by neutrophilic inflammation, the eosinophilic endotype is not rare. Approximately 25% to 40% of patients with COPD have eosinophilic airway [13], and 28% of acute exacerbations in COPD are associated with airway eosinophilia [14]. Uncontrolled airway eosinophilia gives rise to recurrent exacerbations and hospitalizations [15,16]. As such, the COPD eosinophilic endotype is an important subgroup to identify and target because though it is associated with an increased risk of exacerbations, the risk is mitigated by inhaled corticosteroid (ICS) [17]. However, the response to ICS and anti-eosinophilic agents among eosinophilic COPD patients is mixed and different from asthma [18–20]. In this review, we discuss the role of eosinophil in COPD compared to asthma, the definitions of the COPD eosinophilic endotype and the corresponding limitations.
THE BIOLOGY OF EOSINOPHILS IN THE AIRWAYS
Eosinophils are recognized by their distinctive bilobed nuclei and protein-filled granules. Under the influence of cytokines such as granulocyte-monocyte colony-stimulating factor (GM-CSF), interleukin (IL)-3, and IL-5, eosinophils differentiate and mature from hematopoietic stem cells in the bone marrow (Fig. 1). Thereafter, eosinophils infiltrate the lung tissues via the bloodstream [2]. Eosinophil transmigration from the blood into the lungs prolongs the eosinophil half-life from hours to days, especially in the presence of mediators such as GM-CSF, IL-3, IL-25, IL-33, and thymic stromal lymphopoietin during airway inflammation [21,22]. Eosinophils release a wide array of cytokines, chemokines, and proteins that cause mucus hypersecretion from goblet cells, airway remodeling, and airway hyperreactivity [23]. Four major proteins mediate eosinophil’s toxic effects in the airways: major basic protein (MBP), eosinophil cationic protein (ECP), eosinophil peroxidase (EPO), and eosinophil derived neurotoxin [2]. MBP and EPO induce airway hyperreactivity by stimulating histamine secretion from mast cells and basophils [23,24]. MBP and ECP can trigger excessive damage-repair pathway activation in the respiratory epithelium, contributing to airway remodeling [23–25]. This can be augmented by the release of matrix metalloproteinase (MMP)-9 and transforming growth factor-b by eosinophils to recruit and activate neighboring fibroblasts [23,25]. In addition, eosinophils promote goblet cell differentiation and mucus hypersecretion by releasing IL-13 [25,26]. Together, these factors drive the hallmarks of an eosinophilic exacerbation with increased sputum production and bronchoconstriction.
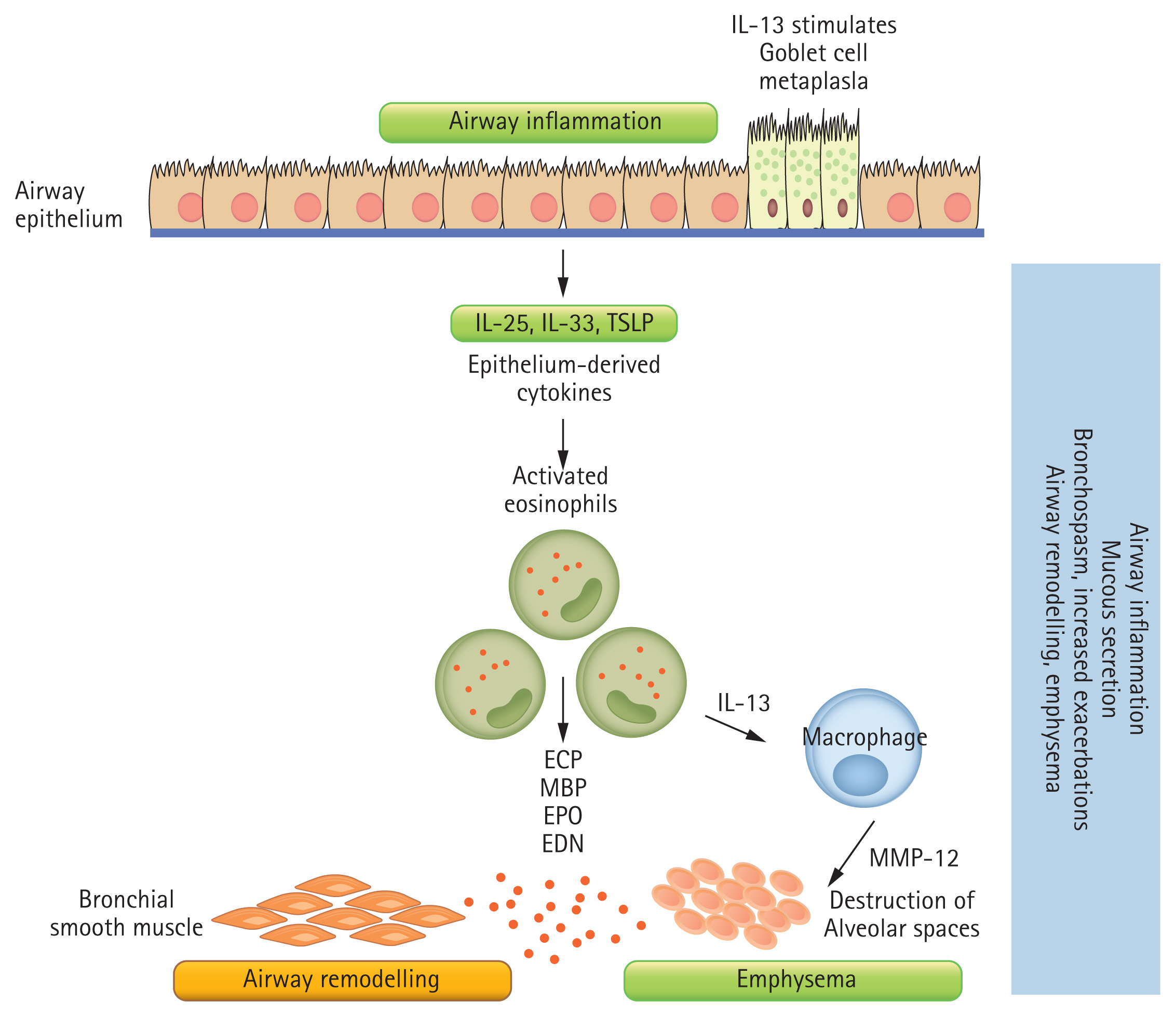
The biology of eosinophilic airway inflammation. T2-high inflammation (eosinophilic) can be allergic or non-allergic. Allergens trigger the production of interleukin 4 (IL-4) and a cascade of T2-high inflammation, leading to specific immunoglobulin E (sIgE) production by activated B cells and mast cell degranulation. Non-allergic T2-high inflammation relies on IL-5 and IL-13 by type 2 innate lymphoid cell (ILC2) and T helper type 2 cell (Th2) cells. Both allergic and non-allergic T2-high inflammation lead to bronchial smooth muscle hypertrophy and goblet cell metaplasia, which cause bronchospasm, mucous hypersecretion and airway remodelling in chronic asthma. TSLP, thymic stromal lymphopoietin; ECP, eosinophil cationic protein; MBP, major basic protein; EPO, eosinophil peroxidase; EDN, eosinophil derived neurotoxin; MMP, matrix metalloproteinase.
Eosinophil functions are highly regulated by its cell surface receptors, including IL-5 receptor (IL-5R), sialic acid-binding immunoglobulin-like lectin 8 (Siglec-8), and CC-chemokine receptor 3 (CCR3), which controls its differentiation, migration, and survival [23,24]. Other receptors include toll-like receptor (TLR) 3, 7, 8, and 9, which promote viral asthma exacerbations, whereas TLR 2/6 heterodimer facilitates clearance of respiratory syncytial virus [27,28]. Among these receptors, IL-5R is the major player in eosinophil maturation, activation, and survival [29,30].
This has led to the introduction of anti-IL-5 biologic therapies specifically targeted at reducing eosinophilic asthma exacerbations [31–36]. However, blockade of the IL-5 pathway alone is insufficient for controlling asthma exacerbations [29,37], suggesting that eosinophil activity is dependent on multiple receptor networks, and highlighting the complexity of eosinophil biology. Furthermore, preferential expression of major histocompatibility complex class II (MHC-II) on activated eosinophils in the presence of GM-CSF and IL-4 suggests that eosinophils play a role in antigen presentation and B-cell activation [27,38].
MECHANISTIC EFFECT OF EOSINOPHIL IN COPD VERSUS ASTHMA
The mechanism behind eosinophilic COPD is distinct from eosinophilic asthma. The activation of eosinophils by IL-33, rather than IL-5, correlates with the increase in IL-13 levels in COPD, promoting emphysematous MMP-12 release by macrophages [39]. This suggests an indirect mechanism in which eosinophils induce airway remodeling in COPD. Moreover, although the release of MBP is highly associated with eosinophilic asthma, COPD exacerbation has been linked to higher ECP levels [23,24,40].
In parallel with their destructive role, eosinophils could also promote resolution of inflammation by secreting pro-resolving lipid mediators such as resolvin E3 and protectin D1 and recruiting alternatively activated macrophages [41]. The overall action of eosinophils in inflammation involves the fine coordination of a complex network of mediators and surface receptors. It has been postulated that eosinophil functions in asthma are regulated by dendritic cells and T helper 2 (Th2) cells, whereas eosinophil functions in COPD are coordinated by type 2 innate lymphoid cells (ILC2) [2].
BIOMARKERS OF THE EOSINOPHILIC ENDOTYPE OF COPD
Sputum cell count quantification
Sputum eosinophilia > 3% is the Global Initiative for Chronic Obstructive Lung Disease (GOLD) standard of airway eosinophilia in asthma and COPD [42–44]. In asthma, sputum eosinophil counts can more reliably guide physicians to tailor ICS treatment to prevent exacerbations, compared to other markers [45]. It has also been used as a biomarker of severe eosinophilic asthma patents in the phase 2 mepolizumab studies [31].
The application of airway endotyping among COPD patients has gained traction only in the last few years, despite its introduction in the 1990s [43,46,47]. An analysis of the SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study) cohort of 2,499 COPD patients further validated the clinical utility of sputum eosinophilia identification in this population, and greater sputum eosinophilia highlighted a subgroup of patients with more severe disease (i.e., lower predicted FEV1), more frequent exacerbations, and increased emphysema on quantitative computed tomography scan when compared to blood eosinophilia [48].
However, sputum cell count quantification is mainly available in airway research centers [49]. Several obstacles prevent its use in mainstream airway assessment: sputum induction yields an adequate yield only 70% of the time and can result in significant bronchospasm in patients with baseline low FEV1 [50,51], the sputum samples must be processed within 2 hours [49], and the sputum processing and cell-count quantification methods are manual and comparatively labor-intensive [49]. Although the repeatability of sputum eosinophilia is moderate (intraclass coefficient [ICC] 0.63) in the short-term (i.e., 2 weeks) and weak (ICC 0.49) in the medium- to long-term (i.e., 12 weeks) [52,53], this might reflect ongoing changes in the airway immune responses as a result of environmental triggers.
Blood eosinophil count
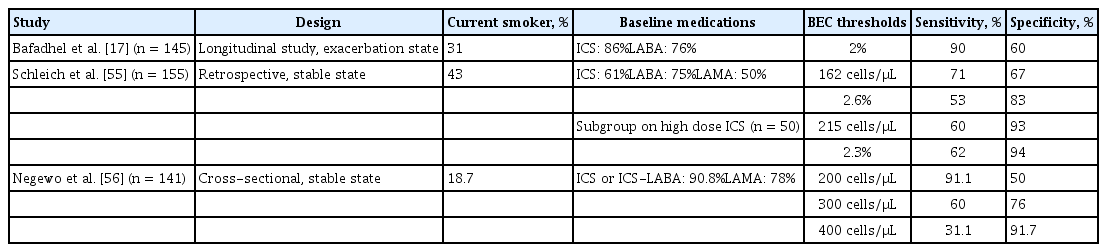
Blood eosinophil count (BEC) is an easily measured surrogate of airway eosinophilia. A high BEC is associated with higher sputum and BAL eosinophil counts and IL-5 level, and greater tissue remodelling [54]. BEC has reasonable specificity and sensitivity for predicting airway eosinophilia (Table 1) [17,55,56]. However, there are several limitations to using BEC as a predictor of airway eosinophilia and a biomarker of corticosteroid and biologic therapy responsiveness.
BEC cut-offs in the various studies have been arbitrary, with no over-arching consensus. Some studies have defined airway eosinophilia based on the population of eosinophils as a percentage of the total leukocyte count [57,58] whereas others have focused on an absolute BEC of 150 to 300 cells/μL [59–64]. Cut-off values relying on percentages are less reliable [65], giving rise to interpretation ambiguity. As such, the ideal cut-off is unknown and depends on individual patient characteristics and the clinical context [55,56,66]. A higher specificity is preferred to rule-in a patient with a true eosinophilic endotype, particularly among patients with COPD already on maintenance ICS who are ex-smokers [67], but such cut-offs require further validation.
Although a high BEC is associated with increased risk for exacerbations, up to 50% of patients with a single high BEC are not frequent exacerbators [13]. The timing of the BEC matters. BEC levels do fluctuate throughout the COPD course, which is also affected by age, gender, and illness phase (at baseline or during exacerbation) [68,69]. However, most studies have relied on baseline BEC levels to determine the status of airway eosinophilia, which may not be a true reflection of the actual airway inflammatory status [57–64]. This may lead to occasional contrary results on the influence of ICS among patients with higher BEC levels [59].
In fact, BEC levels poorly correlate with sputum eosinophilia [48]. Although a ≥ 3% sputum eosinophil count at baseline is associated with an increased risk for eosinophilic-driven acute COPD exacerbation (odds ratio, 2.7; p = 0.01) [14], the converse is not true of a high BEC in a stable state. A 2% BEC cut-off, used to identify sputum eosinophilia ≥ 3%, has a high sensitivity of 90% and a low specificity of 60%, indicating that the BEC cut-off is more useful as a rule-in rather than a confirmatory biomarker [14]. These limitations are unsurprising; the correlation between sputum eosinophilia and BEC levels is low to moderate, ranging from 0.24 to 0.53 [13,56], with sputum cell counts varying widely at specific blood eosinophil cut-offs. Additionally, the repeatability of BEC decreases over time: the intraclass-coefficient is high at 0.8 within 4 weeks [56] but is only 0.49 over a 12-week period [53]. In one study, only 37% of patients had a BEC that remained persistently above 2% over 12 months [13]. These findings further highlight the limitations of BEC for identifying patients with eosinophilic COPD.
Fractional exhaled nitric oxide
Although fractional exhaled nitric oxide (FeNO) has been proposed to guide ICS use among asthma patients, it is not a reliable surrogate marker of airway eosinophilia [45]. FeNO is mediated by IL-13 and IL-4 rather than IL-5, which may explain the minimal impact of mepolizumab, an anti-IL-5 biologic agent, on FeNO levels [31]. Several small studies have demonstrated a positive correlation among FeNO, sputum eosinophils [70], and BEC levels [71] during COPD exacerbations. However, the diagnosis of the COPD eosinophilic endotype should not be made during an exacerbation, but rather when the patient is stable. Schleich et al. [55] showed that, compared to BEC levels, FeNO had a weaker correlation with sputum eosinophils at steady state. As such, FeNO is not used to define the COPD eosinophilic endotype.
EOSINOPHILIC COPD: CLINICAL IMPLICATIONS
Higher BEC among COPD patients is associated with a lower FEV1, greater bronchodilator reversibility, and larger differences between baseline and post-bronchodilator FEV1 [15,48]. The ECLIPSE (Evaluation of COPD to longitudinally identify predictive surrogate endpoints) study had contrasting findings but focused on neutrophilic rather than eosinophilic airway inflammation [13]. These significant differences in FEV1 are unlikely to be clinically significant. More importantly, the rate of FEV1 decline is accelerated among those with airway eosinophilia [72], necessitating early intervention. COPD patients with higher BEC tend to be more symptomatic, with quicker progression of emphysema and air trapping [48].
These patients are generally at increased risk of recurrent exacerbations [15,16]. Up to 28% of acute COPD exacerbations are associated with airway eosinophilia [14]. This has been observed in many prospective studies, of which the largest was the Copenhagen General Population study, where 7,225 COPD patients were followed up for a median of 3.3 years. Those who had elevated BEC (i.e., > 345 cells/μL) were at increased risk for moderate and severe exacerbations [15]. Nonetheless, these patients respond favorably to ICS with fewer treatment failures [67], have a shorter median hospital stay [73], and are associated with decreased mortality [74,75]. Recent reports on ICS addition and withdrawal reaffirmed these findings among the subset of COPD patients with airway eosinophilia (Tables 2 and 3) [57–64]. Importantly, higher BEC attenuates the pneumonia risk [76,77]. This is biologically plausible because eosinophils have antibacterial properties, and is further supported by the inverse relationship between bacterial infection and BEC in patients with COPD exacerbations [78].
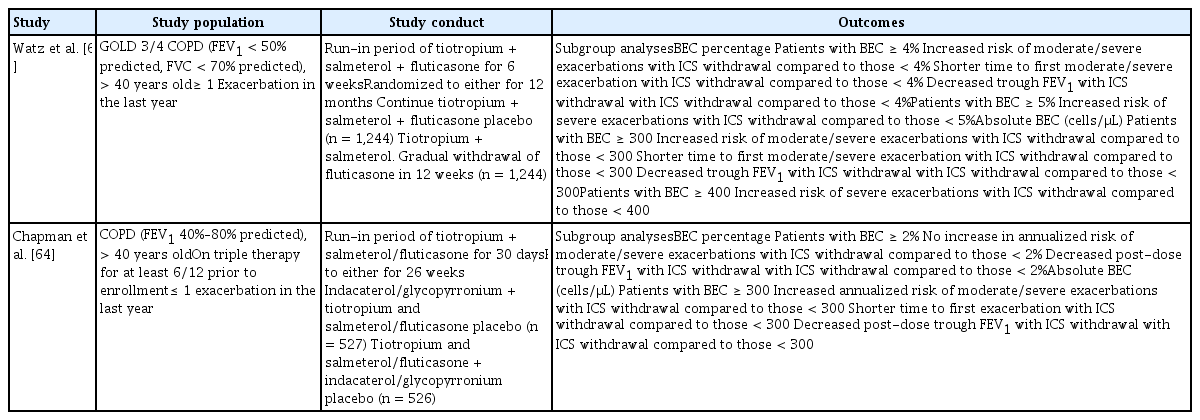
Prospective studies of the effects of withdrawing inhaled corticosteroids among patients with COPD and eosinophilia
RESPONSES TO ANTI-EOSINOPHILIC TREATMENT IN EOSINOPHILIC COPD VERSUS ASTHMA
Inhaled corticosteroids
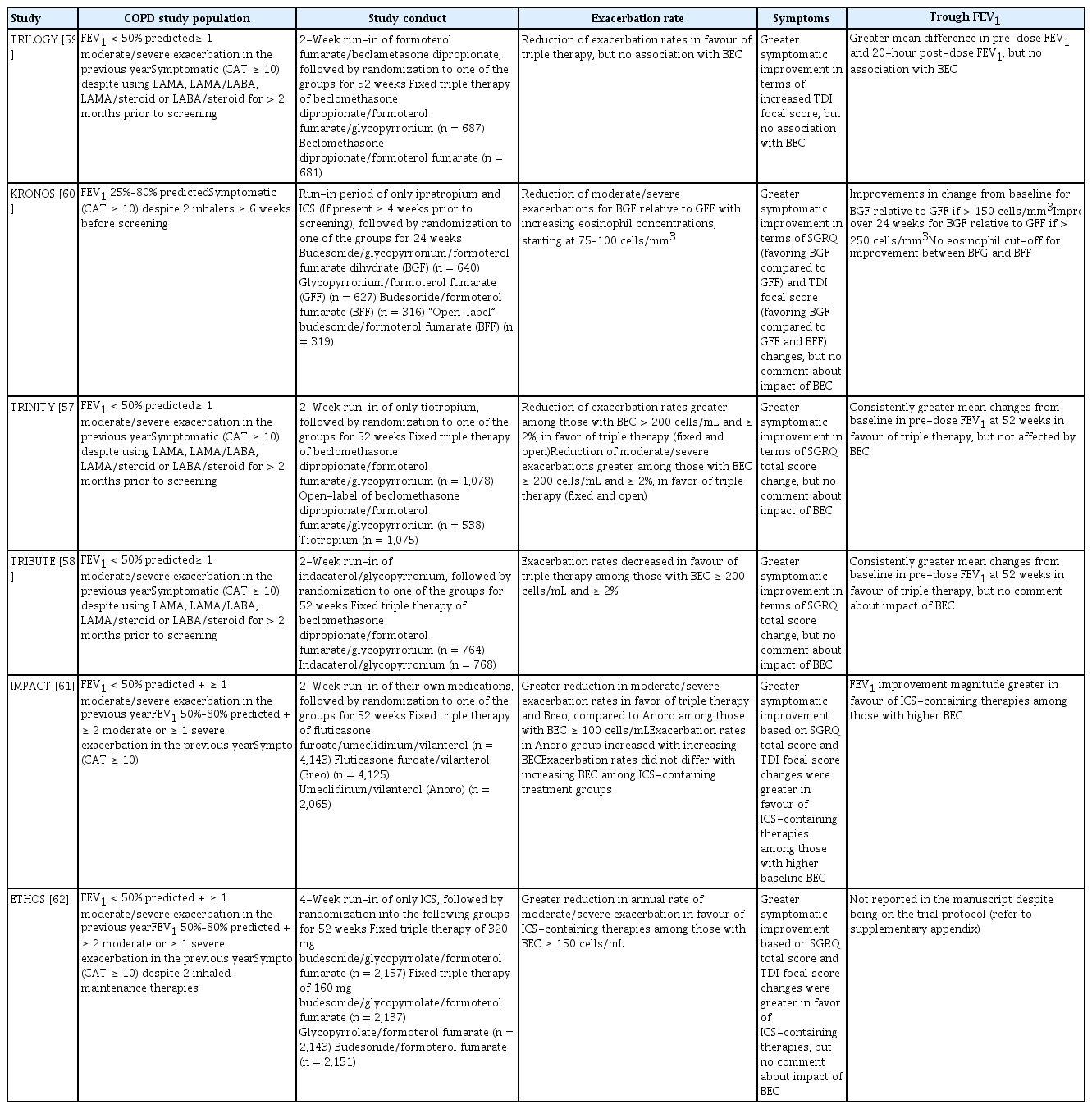
Unlike asthma management where ICS has been the mainstay inhaler [42], the GOLD guidelines do not advocate the initiation of ICS as the first-line treatment [79]. Long-acting bronchodilators (LABs) remain the initial inhaler of choice as a first-line therapy given their superior clinical efficacy for COPD patients of lower severity. The post hoc analysis of the FLAME (Effect of Indacaterol/Glycopyronium versus Salmeterol/Fluticasone on COPD exacerbations) study, which compared the efficacy of indacaterol-glycopyrronium and salmeterol-fluticasone among LAB-naïve COPD patients, suggested that dual LABs are more effective, regardless of the BEC [80]. ICS would be initiated only for patients with COPD who remain symptomatic, and are frequent exacerbators with BEC > 300 cells/μL, despite being compliant with dual LAB. This is in line with data from the recent studies of the addition of ICS to dual LAB [57–62].
These recommendations are based on data from post hoc analyses showing that high BEC cut-offs are indicative of the eosinophilic endotype that benefits from ICS treatment, with a modest exacerbation reduction of 30% [17,81]. They are also intended to leverage the risk-benefit relationship between ICS efficacy and pneumonia incidence, given that unnecessary ICS may portend increased risk for severe pneumonia [82]. However, clinicians should be aware that the recommendations are based on the assumption that these patients have recurrent exacerbations due to persistently raised sputum eosinophilia and so the improvement with ICS is secondary to eosinophil reduction [83].
This assumption has been challenged. In one study, a 4-week course of ICS reduced sputum total cell counts but did not improve lung function or reduce the differential eosinophil count or exhaled nitrous oxide [84]. In a meta-analysis, ICS reduced lymphocyte levels in the bronchial wall, and lymphocyte and neutrophil levels in the bronchoalveolar lavage [85]. Interestingly, in another study, high-dose inhaled fluticasone reduced sputum leukocyte density and neutrophilic inflammatory indices (IL-1B, IL-8, and leukotriene B4 [LTB4]) in bronchiectasis patients, suggesting an immunomodulatory effect unrelated to eosinophilia [86]. These studies highlight that ICS might have anti-inflammatory benefit in COPD beyond eosinophil depletion, because it reduces the counts of total cells and neutrophils in sputum. However, Bafadhel et al. [87] demonstrated that utilizing BEC to direct oral corticosteroid or antibiotic therapy was as safe as conventional therapy. The benefit was seen even among those with severe exacerbations requiring hospitalization, irrespective of the inflammatory state [88]. Thus, the benefit of corticosteroids is likely pleiotropic in COPD patients and the mechanisms underlying the association between higher BEC levels and the ICS effect are unclear.
The optimal BEC cut-off is unknown. Early studies used sputum eosinophil counts instead of BEC [43]. In those studies, systemic corticosteroids and ICS reduced airway eosinophilia and improved post-bronchodilator FEV1 and exercise tolerance. Subsequent studies defined eosinophilic COPD based on BEC cut-offs of 2%–4% to 100–300 cells/μL [48,57–64,67,80]. In one study, budesonide-formoterol, compared to formoterol, was associated with an exacerbation reduction (rate ratio, 0.75; 95% confidence interval, 0.57 to 0.99; interaction = 0.015) in patients with COPD and a BEC ≥ 100 cells/μL [17]. In another study, adding fluticasone to vilanterol in eosinophilic patients with COPD with a BEC ≥ 2% resulted in an exacerbation reduction of 29% [81]. However, results have occasionally been contradictory, with BEC levels failing to predict ICS treatment response at cut-offs of 2% and 200 cells/μL in the TRIBUTE (Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease) study [59]. Lower BEC cut-offs tend to be more sensitive rather than specific, thus the population studied may have included milder and even perhaps non-eosinophilic COPD patients. For the general COPD population, this may also lead to over- and under-treatment with ICS. Thus, several experts prefer a targeted BERN—on bronchiolitis, eosinophilia, responsiveness to bronchodilator and non-smoking status—approach, based on bronchiolitis, eosinophilia, responsiveness to bronchodilators, and non-smoking status, to select COPD patients for whom ICS might have greater benefit [89]. In this select group, ICS might be a disease modifier and reduce the rate of decline in lung function.
Anti-interleukin-5 monoclonal antibodies
BEC levels also failed to predict treatment response to biologic therapy in eosinophilic COPD. This is unexpected given the similarities in clinical and inflammatory features with eosinophilic asthmatics [31–36]. Several factors could explain these surprising findings. First, the inability of anti-IL-5 agents to control asthma exacerbations suggests greater eosinophil biology complexity [29,37]. Given that we know less about eosinophilic pathobiology in patients with COPD, other mediators could influence the efficacy of biologic therapy. Second, the population studied may not actually truly reflect the target population, namely, patients with eosinophilic COPD. This is best illustrated by the phase 3 studies of mepolizumab and benralizumab, in which BEC cut-offs of 150 and 220 cells/μL, respectively, were used to identify a severe eosinophilic subgroup characterized by frequent exacerbations [19,20]. In a subsequent post hoc analysis, Criner et al. [66] found that the subgroup that responded to benralizumab had an elevated baseline BEC > 300 cells/μL and experienced ≥ 3 exacerbations in the prior 12 months despite triple therapy. These treatment responders also had lower FEV1 and significant bronchodilator reversibility. This further illustrates the need for a definition of airway eosinophilia.
ASTHMA-COPD OVERLAP VERSUS COPD EOSINOPHILIC ENDOTYPE
Asthma-COPD overlap (ACO) remains a controversial disease entity and was removed from the Global Initiative for Asthma reports in 2019. Currently, there is no universally accepted definition of ACO, and there is no international consensus on whether ACO represents a distinct airway entity or is merely a continuum of overlapping airway disease phenotypes and endotypes [90,91]. Given their heterogeneity, ACO patients exhibit multiple permutations of asthma and COPD clinical features, making them difficult to differentiate and characterize on a regular basis. Moreover, the wide spectrum of phenotypes denotes overlapping pathophysiological processes of asthma and COPD to varying degrees [92–94]. Airway eosinophilia features strongly among ACO studies [93–95], contrary to the prior belief that neutrophilic airway inflammation predominates in this subpopulation [90]. In fact, Hile et al. [95] demonstrated that the proportion of ACO patients with airway eosinophilia is higher than that of asthma and COPD patients in a cross-sectional observational study in Australia (55% vs. 44% vs. 29%, respectively).
The eosinophilic COPD endotype had previously been described as an extension of the ACO phenotype spectrum [96], but given the ambiguity of the ACO definition, most studies on eosinophilic COPD have attempted to create a homogenous population by excluding patients with a history of asthma and atopy [57–60,63,64]. However, ACO patients experience worse outcomes than those with asthma and COPD, in terms of a steeper FEV1 decline, are more symptomatic, and sustain more exacerbations and hospitalizations [96–98]. They are at higher risk for pneumonia, respiratory mortality, and all-cause mortality. This is particularly so among ACO patients with late-onset asthma [98]. It is plausible this could be a result of the high incidence of airway eosinophilia [72]. Hiles et al. [95] showed that the higher incidence of airway eosinophilia among ACO patients is correlated with an increased exacerbation risk compared to patients with eosinophilic COPD. Further studies are required to validate this observation and the lack of a proper ACO definition has impeded progress thus far.
ACO studies have advocated aggressive management of the asthma and COPD components. Long-acting beta agonist/ICS combination therapy has been the first-line treatment, but this recommendation is based on expert opinion rather than actual studies [96]. Although most ICS studies that have focused on eosinophilic COPD did not include patients with ACO [57–60,63,64], the ETHOS (Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD) study included patients with a prior asthma diagnosis [62] and reported a greater efficacy of ICS among patients with airway eosinophilia (Table 3). This can potentially be extrapolated to patients with ACO and reinforces the need for ICS as the initial therapy of choice.
CONCLUSIONS
Eosinophilic COPD is an airway inflammatory endotype associated with an increased risk for exacerbations and treatment responsiveness to ICS. Although BEC is useful as a biomarker to identify this group, its low concordance with sputum eosinophilia and relatively low specificity can result in over- or under-treatment with ICS. Clinicians should note that the optimal BEC cut-off is dependent on the clinical context (to rule in or rule out an eosinophilic endotype), and must be interpreted together with other clinical characteristics. Importantly, the biological mechanisms of eosinophils might differ between COPD and asthma. ICS might have anti-inflammatory benefit in COPD beyond eosinophil depletion, because it reduces the numbers of total cells and neutrophils in sputum. A targeted approach combining the presence of eosinophilia with other relevant features might promote identification of a subgroup in which ICS functions as a disease modifier.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was partly supported by a grant NUHSRO/2020/044/T1/1 to W.S. Fred Wong and Hui-Fang Lim.