How important is dietary management in chronic kidney disease progression? A role for low protein diets
Article information
Abstract
High dietary protein intake may lead to increased intraglomerular pressure and glomerular hyperfiltration, which in the long-term can lead to de novo or aggravating preexisting chronic kidney disease (CKD). Hence, a low protein diet (LPD, 0.6 to 0.8 g/kg/day) is recommended for the management of CKD. There are evidences that dietary protein restriction mitigate progression of CKD and retard the initiation of dialysis or facilitate incremental dialysis. LPD is also helpful to control metabolic derangements in CKD such as metabolic acidosis and hyperphosphatemia. Recently, a growing body of evidence has emerged on the benefits of plant-dominant low-protein diet (PLADO), which composed of > 50% plant-based sources. PLADO is considered to be helpful for relieving uremic burden and metabolic complications in CKD compared to animal protein dominant consumption. It may also lead to favorable alterations in the gut microbiome, which can modulate uremic toxin generation along with reducing cardiovascular risk. Alleviation of constipation in PLADO may minimize the risk of hyperkalemia. A balanced and individualized dietary approach for good adherence to LPD utilizing various plant-based sources as patients’ preference should be elaborated for the optimal care in CKD. Periodic nutritional assessment under supervision of trained dietitians should be warranted to avoid protein-energy wasting.
INTRODUCTION
There is growing concern worldwide regarding the exponential increase in the prevalence of chronic kidney disease (CKD), particularly in Asia and including Korea, which has the highest incidence rate of end-stage renal disease according to United States Renal Data System (USRDS) data [1]. Balanced nutritional therapy along with appropriate pharmacological interventions are essential aspects of the management of CKD patients for stabilization of kidney function and prevention of other end-organ complications [2]. However, there are still controversies regarding certain dietary strategies, such as controlling the amount and quality of protein intake in CKD patients. Furthermore, recommendations for a low protein diet (LPD) may differ across the stages of CKD due to differential risk-to-benefit ratio profiles. This review is focused on the nephron-protective role of LPD in CKD patients and presents a summary of current clinical practice guidelines and supporting evidence for nutritional management regarding the amount of protein intake in patients with CKD.
CURRENT STATUS OF DIETARY PROTEIN INTAKE AND CONCERNS ABOUT KIDNEY HEALTH
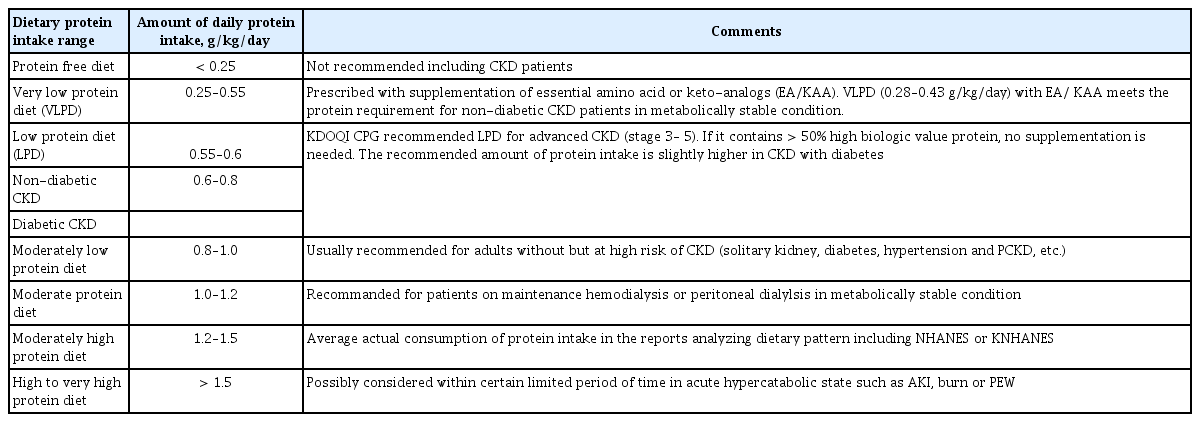
The essential amount of protein intake was originally calculated to compensate for daily obligatory nitrogen loss through protein degradation and nitrogen excretion. The estimated average requirement (EAR) for protein intake in adults was suggested to be 0.46 to 0.66 g/kg/day (grams of protein per kilogram of ideal body weight per day), which corresponds to the amount of dietary protein required to avoid negative nitrogen balance [3], while a dietary protein intake as low as 0.46 g/kg/day would suffice to avoid negative nitrogen balance if all essential amino acids are provided [4]. The recommended daily allowance for protein intake was estimated as 0.8 g/kg/day to meet the requirements of 97% to 98% of the population (two standard deviations above the EAR) [5], which has become the upper threshold for LPDs. Although most nutritional guidelines for CKD patients recommend a LPD, there appears to be a significant gap from the actual protein consumption. According to a study based on the National Health and Nutrition Examination Survey (NHANES) between 2001 and 2008, an average American consumed 1.3 to 1.5 g/kg of protein per day, and protein intake exceeded 1 g/kg/day even in those aged > 75 years or with advanced renal insufficiency, such as CKD stage 4. Dietary patterns in South Korea have also changed toward consuming larger amounts of protein. A report based on the Korean National Health and Nutrition Examination Survey (KNHANES) indicated estimated protein consumption of 250% to 300% of the average required amount in the population between 10 and 64 years old, which is comparable to the NHANES data [6]. The definitions for range of protein intake are summarized in Table 1 [7,8].
Trends in increasing protein intake have raised concerns about kidney health. There have been several studies regarding the impact of high protein consumption on the kidneys. This issue was first studied in animal experiments, which showed that high-protein meals resulted in dose-dependent increases in glomerular filtration rate (GFR) [9], with estimated maximal GFR increase of nearly 80%. Similar results were demonstrated in human studies showing that a high-protein diet (protein comprising 25% of calories) increased estimated GFR (eGFR) by 3.8 mL/min/1.73 m2 compared to a lower protein diet (protein comprising 15% of calories) after 6 weeks of treatment [10]. Glomerular hyperfiltration ultimately stimulates mesangial cell signaling to increase the level of transforming growth factor-β (TGF-β), which subsequently contributes to the progression of kidney fibrosis [11]. Long-standing glomerular hyperfiltration induced by high protein intake may cause kidney damage and decline of kidney function, especially in those with pre-existing CKD. In the 11-year observational Nurses’ Health Study, every 10-g increase in protein intake was significantly associated with a worsening in eGFR of −1.69 mL/min/1.73 m2 (95% CI, −2.93 to −0.45) among women with mild renal insufficiency (defined as eGFR of 55 to 80 mL/min/1.73 m2) [12].
High dietary protein intake may accelerate apoptosis of podocytes. Protein-rich foods, such as meat cooked at high heat, contain high levels of advanced glycation end products (AGEs) [13], which impairs protein degradation leading to basement membrane thickening and mesangial expansion in glomerulus of diabetic kidney disease [14]. Amino acid and high glucose treatment can lead to podocyte and mesangial cell cycle arrest and apoptosis in vitro experiment. Moreover, the podocyte count was reduced in diabetic mice fed high protein diet [15]. These pathogenic response of AGEs could be mediated with a proinflammatory receptor for AGE (RAGE) presented on glomerular cells [14]. RAGE activated signals culminating in cellular inflammation and death, and inhibition of RAGE attenuated apoptosis and inflammatory cytokine production caused by amino acid and high glucose treatment in podocyte and mesangial cells [15]. The vulnerability of podocytes to AGEs is accentuated in glomerular hypertrophy because a single podocyte must cover a larger surface area [16]. Possible mechanisms of high dietary protein diet on kidney injury was summarized in Fig. 1.

Possible mechanisms of high dietary protein intake on kidney health. High protein diet leads to the dilation of the afferent arteriole and increased glomerular filtration rate, which may lead to damage to kidney structure over time due to glomerular hyperfiltration. RAAS, renin-angiotensin-aldosterone system; TGF-β, transforming growth factor-beta; RAGE, receptor for advanced glycation end products; DKD, diabetic kidney disease.
BENEFITS OF LOW PROTEIN DIET
A LPD has many advantages in the management of CKD patients by reducing nitrogen waste products and decreasing kidney workload through lowering intraglomerular pressure, which has a kidney protective effect especially in those with a decreased reservoir in functioning nephrons. LPD also leads to favorable metabolic effects that can preserve kidney function and help to control uremic symptoms.
EFFECTS OF LOW PROTEIN DIET ON PROTEINURIA
Glomerular hyperfiltration caused by a high protein diet is associated with glomerular structural damage and increased pressure on the remaining glomeruli, which may lead to increased risk of proteinuria. Although the precise pathophysiology has not been elucidated, high protein intake is involved in renal solute excretion workload and tubular amino acid overload, resulting in vasodilation of the afferent glomerular arterioles. The paracrine factors and mediators associated with glomerular hemodynamics, such as insulin-like growth factor 1, prostanoids, nitric oxide, and the renin-angiotensin-aldosterone system (RAAS), have been suggested to be involved in this response [17]. Moreover, increased proinflammatory cytokine gene expression by a high-protein diet may be associated with structural damage and hyperfiltration by the remaining glomeruli [18]. This consequence of a high-protein diet has been shown clinically to be associated with increased risk of albuminuria, compared to a standard protein intake in several observational studies, even after accounting for the influence of sociodemographic factors, comorbidities, anthropometric factors, health behaviors (e.g., physical activity, energy intake, smoking status), and medication history, although other studies yielded inconsistent data indicating no association or associations only in patients with hypertension and diabetes. In a study with a crossover design among patients with nephrotic syndrome comparing standard versus LPD periods, protein restriction was shown to lead to a up to 20% reduction in proteinuria in all patients, which supported the beneficial role of LPD on proteinuria even after considering that it was conducted in a small group with a short intervention period (2 weeks) [19]. LPD was also associated with better control of blood pressure, which is closely related to kidney outcome [20]. In a study among patients with CKD IV and V, a very-LPD supplemented with ketoanaloguess (SVLPD) resulted in significant reductions in blood pressure of about 10% (143 ± 19/83 ± 10 to 128 ± 16/78 ± 7 mmHg; p < 0.001) [21]. The effects of LPD on kidney physiology showed many similarities to those of RAAS inhibition, and an experimental study showed that it had an additive anti-proteinuric effect with RAAS inhibition [17]. A recent review by Koppe and Fouque [17], the authors outlined potential mechanisms of action and additive efficacy of an LPD and RAAS inhibitors in CKD, with a particular emphasis on phosphate levels, uremic toxin production, acid load, and salt intake. Therefore, combined treatment with LPD and the RAAS blockade may be warranted to achieve lower urinary protein levels and to further reduce the risk of CKD progression. Recently, sodium glucose cotransporter-2 (SGLT2) inhibitors have also been shown reno-protection including reduction of albuminuria and attenuation of renal function decline. It is suggested that reno-protective effects of SGLT2 are mediated possibly through glomerular hyperfiltration via the improvement of the tubulo-glomerular feedback, which share the protective mechanisms in LPD and RAAS blockade [22,23]. Future studies are needed to examine whether a similarly synergistic effects would exits if SGLT2 inhibitors are given in combination with LPD and plant-dominant diets [24].
EFFECTS OF LOW PROTEIN DIET ON RETARDATION OF CKD PROGRESSION AND DELAY OF DIALYSIS
As the kidneys are responsible for the excretion of most protein degradation products, there will be accumulation of these byproducts, such as p-cresyl sulfate, indoxyl sulfate, and trimethylamine oxide, in CKD patients [25], which will result in further progressive impairment of kidney function [26]. However, the Modification of Diet in Renal Disease (MDRD) study, the largest controlled trial in CKD patients to date, failed to demonstrate the effectiveness of LPD in retarding CKD progression [27], Instead, it suggested that LPD may have negative effects in the management of CKD. Nevertheless, secondary analysis of the MDRD study with a longer observation period showed that each 0.2 g/kg/day decrease in protein intake was associated with a slower decline of GFR by 1.15 mL/min/1.73 m2 per year, and with a halved risk of kidney failure or death [28]. Moreover, subsequent meta-analyses and systemic reviews also reported similar results. A systemic review analyzing data of non-diabetic CKD patients demonstrated that restricted protein intake significantly reduced the number of patients initiating dialysis treatment by about 32% [26]. A more recent meta-analysis including seven randomized controlled trials (RCTs) reported significant protective effects of LPD and SVLPD on decrease in eGFR, compared to a normal protein diet [29]. Reduced uremic milieu with SVLPD was also helpful for maintaining urine volume and residual renal function, which enabled implementation of a weekly incremental dialysis program. After 24 months of the program, 40% of patients were still on weekly dialysis treatment, without deterioration of nutritional status, aggravated anemia, or metabolic derangement [30].
The renoprotective effect of LPD may be reinforced in proportion to the extent of protein restriction. A recent RCT showed that SVLPD (0.3 g/kg/day) mitigated kidney function decline and reduced the number of patients requiring renal replacement therapy in comparison with conventional LPD (0.6 g/kg/day) [31]. Supplementation with ketoanaloguess, which can be converted and utilized as essential amino acids, help to maintain protein-energy status without increases in levels of nitrogen waste products with reduced phosphorus and acid load in VLPD along with decreased protein degradation and enhanced protein synthesis [32]. SVLPD was demonstrated to reduce the levels of major uremic toxins, such as indoxyl sulfate, in predialysis CKD patients [33]. In another RCT among patients with GFR 5 to 7 mL/min, SVLPD even delayed the initiation of dialysis treatment effectively by a mean period of 10.7 months without negative consequences, which provided economic benefits estimated at €21,180 per patient in the first year [34].
BENEFICIAL EFFECTS OF LOW PROTEIN DIET ON METABOLIC CONSEQUENCES
Regarding metabolic acidosis
Metabolic acidosis, a common metabolic consequence of advanced CKD, should be controlled to retard CKD progression and prevent other complications, such as insulin resistance and cardiovascular diseases [35]. As acid is generated during the process of protein degradation, including sulfur-containing amino acids, higher intake of proteins and elevated dietary acid load are associated with a rapid decrease in kidney function [36], and it is independently associated with increased risk of end-stage renal disease in CKD patients [37]. Therefore, LPD, especially if it is predominantly from plant sources (see below), is believed to have favorable effects on metabolic acidosis in CKD patients. Indeed, LPD was reported to ameliorate metabolic acidosis in patients with advanced CKD. In an RCT investigating the effects of diet on serum bicarbonate for 1 year, mean serum bicarbonate level remained < 19 mmol/L after 1 year of LPD, whereas it increased to the normal level in the SVLPD group [31]. In another study, the amount of oral bicarbonate replacement to maintain a similar level of serum bicarbonate (or comparable serum total carbon dioxide [38]) was lower in the SVLPD group compared to participants who consumed higher levels of protein [39]. Correcting metabolic acidosis either by dietary modification or by sodium bicarbonate replacement was helpful to retard the decline of kidney function in patients with stage 4 CKD [40].
Regarding hyperphosphatemia
As protein-rich foods are the main natural source of phosphorus intake (1 g of protein contains about 13 mg of phosphorus) [41], and there is a good correlation between dietary protein and phosphorous intake, LPD could be helpful for controlling hyperphosphatemia in CKD patients, which is a well-known risk factor for cardiovascular disease and bone strength abnormalities [42]. In an RCT in patients with CKD IV and V, dietary protein restriction was shown to lead to declines in serum phosphate levels and calcium-phosphorus product [43]. The efficacy of LPD for lowering serum phosphorus levels also led to reductions in serum levels of parathyroid hormone and fibroblast growth factor 23 [44,45], and better control of markers of mineral bone disorder through dietary protein restriction may be associated with slowing the progression of vascular calcification and improving cardiovascular outcome [46].
Regarding nephrolithiasis
High-protein diets, especially non-dairy animal protein (poultry, meat, fish, eggs), with low-alkali food is considered to be associated with stone formation in the urinary tract. It is thought to cause negative calcium balance, low urinary pH, and low urinary excretion of citrate, potassium, and magnesium [47]. Especially, animal protein intake increases purine metabolism causing hyperuricosuria, which contributes to both uric acid and calcium nephrolithiasis [48,49]. Dietary protein restriction leads to significant decreases in urinary calcium, uric acid, oxalate, and hydroxyproline levels. On the other hand, urinary citrate was reported to increase after adoption of LPD along with decreased urinary excretion of urea [50]. It is possible that there are individual variations that result in differences in the effects of dietary factors on stone formation. High animal protein intake was shown to be associated with urinary oxalate excretion in patients with idiopathic calcium nephrolithiasis, whereas no such effect was observed in healthy subjects [51].
IMPORTANCE OF SOURCE OF PROTEIN INTAKE (PLANT VS. ANIMAL PROTEIN)
In addition to the amount of protein intake, the possibility of different effects according to the source of dietary protein has attracted interest. Consumption of animal protein, especially processed red meat, is highly associated with the incidence and progression of CKD. The Atherosclerosis Risk in Communities Study (ARIC), a prospective cohort study as an observation of United States community population without diabetes and cardiovascular disease in eGFR > 60 mL/min/1.73 m2 with a median follow-up of 23 years, showed an increased risk of the incidence of CKD among those who consumed the highest quintile of red/processed meat compared to those who consumed the least [52]. Beneficial association of plant-based protein intake with CKD incidence was evident in healthy plant-based diet consuming whole grain, fruit, vegetable, nuts and legumes rather than less-healthy plant-based diet with refined grain, potatoes, fruit juice and sugar-sweetened beverages [53]. Similar results were demonstrated in a study performed in Asia; the Singapore Chinese Health Study, which showed a strong association of red meat consumption with ESRD risk in a dose-dependent manner [54]. Renoprotective effect of plant-based protein consumption was associated lower mortality in CKD patients [55].
Although detailed mechanism for the different effect regarding the sources dietary protein, it was demonstrated in a clinical study that higher glomerular hyperfiltration was observed in population with more meat and less vegetable protein intake [56]. This result was supported with another longitudinal observational study, the Nurses’ Health Study with 11-year follow-up periods upon 3,328 population, which demonstrated that the highest quartile of animal fat and two or more servings of red meat per week were directly associated with microalbuminuria [57]. Animal protein diet is also believed to cause an imbalance in the composition of the gut microbiome [58] resulting in the production of greater amounts of ammonia and sulfur-based materials with a proinflammatory profile, and thus lead to increases in inflammatory cytokines and oxidative stress [59,60]. A longitudinal controlled trial among patients with diabetic nephropathy demonstrated that replacing half of animal protein intake with soy protein consumption for 4 years decreased the degree of proteinuria and urinary creatinine [61] along with improvement of markers of metabolic syndrome, which is related to increased risk of CKD [62].
Moreover, many studies have suggested that plant protein is more helpful for controlling metabolic acidosis. Plant-based protein contains higher levels of glutamate, an anionic amino acid that consumes hydrogen ions in metabolism, resulting in maintenance of neutral pH. Plant foods also contain higher levels of anionic potassium salts, which also result in a decrease in levels of hydrogen ions [63]. The Chronic Renal Insufficiency Cohort Study demonstrated that consumption of a greater proportion of plant protein was associated with higher bicarbonate levels [45]. As oral bioavailability of phosphorus is higher in animal protein, compared to plantbased protein, whose phosphorus in the form of phytate is less bioavailable in human gut, more animal protein consumption may also be harmful for controlling hyperphosphatemia in CKD patients [64].
Based on the results of many studies on the favorable effect of plant-based protein intake, there is a movement to recommend a plant-dominant low-protein diet (PLADO) in CKD patients [7,60,65]. It is defined as a diet of dietary protein intake of 0.6 to 0.8 g/kg/day with at least 50% plant-based sources, which should be whole, unrefined, and unprocessed foods. Other features of PLADO include low sodium intake less than 3 to 4 g/day and high dietary fiber of at least 25 to 30 g/day under ensuring adequate energy intake (30 to 35 Cal/kg/day) [60]. Although introduction of PLADO regimen in CKD patients is supposed to yield various beneficial effect as stated above, there are also several concerns. Most of animal protein are complete proteins, which provide all essential amino acid supplied necessarily from foods.
It is important to note that plant proteins are often considered be lack some of essential amino acids, and therefore are usually categorized as having a low biological value as compared to the so-called “high biologic value” (HBV) proteins that are mostly of animal origin, in which more essential amino acids are bioavailable and absorbable via human gut. However, despite the traditionally lower HBV ranking of plant-based protein, they do not have significantly lower “protein digestibility-corrected amino-acid score” (PDCAAS), which is the preferred method for measuring protein quality, compared to animal-based proteins. Indeed, clinical studies using near total plant-based diet or exclusively plant-based diet in CKD patients did not show any nutritional deficits [66-68]. Hence, careful monitoring about the adequacy for quantity and quality through utilizing a variety of foods may enable to use plant-based diet in CKD patients without adverse effect. A recent United States national study showed that higher HBV protein was associated with poor outcomes in those with CKD [69].
Another concern with plant-based diet is about the risk of hyperkalemia. However, dietary potassium explained only about 2% of the variance in quarterly mean pre-dialysis serum potassium [70]. Moreover, the top five sources of potassium were beef, chicken, Mexican food, hamburgers and legumes [71]. Indeed, a high-fiber diet may enhance bowel motility and likely prevent more absorption of potassium from foods. Alkali supply with plant-based dietary sources may also lower the risk of hyperkalemia [72,73]. Indeed a recent study suggested that lowering dietary potassium was associated with worse mortality in patients with CKD undergoing dialysis [74]. Benefits and challenges of PLADO are summarized in Table 2. Close monitoring for the adherence to intake of proper amount of calori, protein, salt and fiber, and flexible application of protein sources and food types according to the preference of patients may be helpful to convey the better beneficial impact of PLADO regimen on kidney health.
RISK OF PROTEIN ENERGY WASTING & MONITORING
The hypercatabolic state induced by uremia, anorexia due to accumulation of anorectics, inflammation from systemic conditions, and underlying autoimmune conditions as etiologies for CKD have been suggested to be related to a high prevalence of protein energy wasting (PEW) [75] in CKD patients, associated with low muscle mass and increased mortality [76]. As malnutrition is the main risk factor for PEW, restriction of protein intake raised concerns about the possibility of aggravating PEW in CKD patients, and decreased body mass index was shown to be associated with higher mortality of ESRD patients treated with dialysis [77,78]. Analysis of body composition measurements conducted in the MDRD study showed decreases in body weight, arm muscle area, and urine creatinine excretion in the lowand very-low protein intake groups, compared to the control diet. However, caloric intake was also decreased in the protein restriction group, and the averages of most anthropometric and biochemical indices for nutritional status remained within the normal ranges [79].
Another study investigating the effects of SVLPD on body composition among CKD V patients showed slight decreases in lean body mass during the first 3 months after the diet intervention. However, it gradually increased thereafter, and remained stable at 12 and 24 months, suggesting that SVLPD coupled with adequate caloric intake is nutritionally safe for long periods [80]. A meta-analysis of 14 studies evaluated the effects of LPD on body composition in CKD patients, and it demonstrated that there were no major changes in body composition over time in CKD patients with LPD [81]. However, as protein and energy requirements should be varied according to clinical conditions and across disease severities, it is essential to monitor actual dietary intake regularly and to assess nutritional status carefully in the implementation of LPD [82,83]. Dietary intake can be evaluated with dietary recalls, interviews, and food frequency questionnaires, and the amount of actual protein intake can be verified with urinary nitrogen appearance (UNA). However, protein intake can be overestimated by UNA-based calculation if patients are in a hypercatabolic state, including malnutrition, inflammatory conditions, postoperative period, and burn injury. So, other tools for dietary assessment should be adopted in those conditions. [84]. In addition, dialysis treatment stimulates protein catabolism [85]. Whole-body skeletal muscle metabolism appears to increase, which can lead to the net loss of muscle protein during hemodialysis treatment [86]. The guidelines recommend higher dietary protein intake in ESRD patients undergoing dialysis treatment (1.2 to 1.4 g/kg/day), compared to the pre-dialysis period, to avoid aggravation of PEW [87]. Education for close self-monitoring to avoid malnutrition and consistent counselling by CKD-specialized dietitian, who provides pragmatic tools and comprehensible dietary information and skills are necessary to exert the role of LPD on kidney health without risk of PEW.
CONCLUSIONS
In the management of patients with CKD, the beneficial effects of plant-dominant LPD to avoid glomerular hyperfiltration and mitigate accumulation of protein waste products are thought to be useful for better control of uremic symptoms and metabolic complications, facilitating delay in the initiation of dialysis treatment (Table 3) [15,17-19,21,26-29,31,35,36,41,44,45,47,49,63,88-92]. However, concerns regarding PEW have hindered the widespread adoption of these strategies by clinicians. Implementation of LPD as a dietary regimen should be recommended along with close monitoring and precise regular assessment of nutritional status. Multidirectional approaches, including dietary approaches, should be considered to ensure the best outcome for CKD patients.
Notes
No potential conflict of interest relevant to this article was reported.