Rectal NSAIDs-based combination modalities are superior to single modalities for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a network meta-analysis
Article information
Abstract
Background/Aims
Different modalities have been employed to reduce the risk and severity of post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP). However, there has been a paucity of studies comparing the efficacy of various prophylactic modalities for preventing PEP. This network meta-analysis (NMA) aimed to determine the relative efficacy of pancreatic duct stents and pharmacological modalities for preventing PEP.
Methods
We performed a systematic and comprehensive search to identify and analyze all randomized controlled studies published until June 2020 that examined the effectiveness of pancreatic duct stents, rectal non-steroidal anti-inflammatory drugs (NSAIDs) based regimens, hydration, and their combinations for the prevention of PEP. The primary outcome was the frequency of PEP. An NMA was performed to combine direct and indirect comparisons of different prophylactic modalities.
Results
The NMA included 46 studies evaluating 18 regimens in 16,241 patients. Based on integral analysis of predictive interval plots, and expected mean ranking and surface under the cumulative ranking curve values, combination prophylaxis with indomethacin + lactated Ringer’s solution (LR), followed by diclofenac + nitrate and indomethacin + normal saline, was found to be the most efficacious modality for the overall prevention of PEP. Indomethacin + LR, followed by diclofenac and pancreatic duct stents, was the most efficacious modality for high-risk groups.
Conclusions
Rectal NSAIDs-based combination regimens with aggressive hydration or nitrate are superior to single modalities for the prevention of PEP.
INTRODUCTION
Acute pancreatitis is the most common and arguably most feared adverse event related to endoscopic retrograde cholangiopancreatography (ERCP) [1]. The frequency of post-ERCP pancreatitis (PEP) has been reported to be 3.5% to 6.0% in average-risk populations [2,3] and 8% to 13.1% in high-risk patients [3,4]. PEP results in substantial morbidity, occasional mortality, and increased health-care costs. Therefore, considerable efforts using different modalities have been employed to reduce the risk and severity of PEP including patient selection, technical maneuvers, and pharmacological prophylaxis [5]. However, the prophylaxis for the prevention of PEP has yet to be conclusively determined.
More than 35 pharmacological agents with different mechanisms of action have been evaluated for the prevention of PEP [6–8]. Rectally administered non-steroidal anti-inflammatory drugs (NSAIDs) have proven to be effective in preventing PEP and reducing its severity [2–4,9,10]. However, the prophylactic effects of rectal NSAIDs are suboptimal and remain controversial according to cohort risks and combinations of other pharmacological agents or endoscopic interventions [11,12].
Pancreatic duct (PD) stent placement can be performed prophylactically in patients with high-risk of PEP or in those facing difficulties in selective bile duct cannulation during ERCP [13,14]. PD stents can prevent mechanical pancreatic outflow obstructions due to swelling of the pancreatic orifice, and can diminish the risk of PEP by retaining pancreatic drainage. To date, several randomized clinical trials (RCTs) have indicated that the protective effects of prophylactic pancreatic stent placement against PEP are suboptimal based on the patient risk, type of PD stents and study design [15,16]. A meta-analysis of eight RCTs revealed that prophylactic pancreatic stents decreased the risk of PEP in high-risk groups [17]. In clinical practice, PD stent placement is not always successful, even when performed by experienced hand. In this regard, failure or immoderate attempts for PD stent placement can induces PEP. Therefore, routine prophylactic PD stent placement for preventing PEP remains a topic of debate.
Fluid therapy with lactated Ringer’s solution (LR) has been established as main treatment for acute pancreatitis, and aggressive hydration is recommended in early management of acute pancreatitis in clinical practice guidelines [18–20]. Recent RCTs have demonstrated that aggressive hydrations with LR solution reduces the frequency and severity of PEP [21–23]. Moreover, a meta-analysis revealed a reduction in the frequency of overall and moderate-to-severe PEP cases upon aggressive hydration with LR solution [24].
Most recently, several RCTs have been performed to assess the effect of rectal NSAIDs based combination modalities including prophylactic pancreatic stent placement, aggressive hydration, papillary epinephrine spray, and sublingual nitrate for PEP prevention. In a RCT, combination of rectal diclofenac and sublingual nitrates is superior to rectal diclofenac alone for PEP prevention [25]. In another RCT, combination regimen of rectal indomethacin and sublingual nitrate before ERCP significantly reduced the frequency of PEP compared with single rectal indomethacin regimen [26].
To date, there has been a paucity of studies comparing the efficacy of various prophylactic modalities for preventing PEP. Network meta-analysis (NMA) is a meta-analysis in which multiple treatments are being compared using both direct and indirect comparisons across trials based on a common comparator. NMA improves the precision of estimate by using more information, compared with only using direct comparison, and provides a relative ranking of estimate according to study outcomes. This study aimed to compare the efficacy of PD stents, pharmacological agents, and aggressive hydration for preventing PEP using a systematic review and NMA [27].
METHODS
Protocol and registration
We developed the protocol for this systematic review and NMA according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses Protocol (PRISMA-P) statement [28]. We registered the review protocol at the International Prospective Register of Systematic Reviews (PROSPERO) (https://www.crd.york.ac.uk/PROSPERO/CRD42020189210).
This systematic review and NMA of PD stents, rectal NSAIDs-based regimens and aggressive hydration for the prevention of PEP was performed according to the protocol recommended by the Cochrane Collaboration [29], and reported according to the PRISMA extension for NMA guidelines [30].
Eligibility criteria
Inclusion criteria
We included only RCTs that compared PD stents, rectal NSAIDs, aggressive hydration, and the combination of these three modalities for the prevention of PEP.
In our review, we organized study data by PICO-SD information: patients (P), intervention (I), comparison (C), outcome measurement (O), and study design (SD). Patients included all patients receiving ERCP. Interventions considered were PD stents, rectal NSAIDs, aggressive hydration (with normal saline and LR) and their combination with other pharmacological agents (intravenous somatostatin, sublingual nitrate, intraduodenal epinephrine, or double dose of indomethacin). Comparisons were performed among PD stents, rectal NSAIDs, aggressive hydration, their combination with other pharmacological agents, and placebo or no treatment. Outcome measurements included the overall reduction in the frequency of PEP in patients who received these prophylactic modalities. The study design was RCTs.
Exclusion criteria
Review articles, case reports, case-series, letters to the editor, commentaries, proceedings, laboratory science studies, and all other irrelevant studies were excluded. In addition, studies that failed to report the outcomes of interest were excluded.
Information sources and search strategy
We searched MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), and Google Scholar using the search terms “pancreatic duct stent,” “indomethacin,” “diclofenac,” “naproxen,” “NSAIDs,” “hydration,” and “post-ERCP pancreatitis” from database establishment to June 29 2020. Two authors screened the titles and abstracts of the retrieved articles. Reference lists were imported to Endnote software 8.1 (Thomson Reuters, Culver City, CA, USA), and duplicate articles were removed. Additional but relevant articles were identified by scanning the reference lists of articles obtained from the original search.
Study selection and data collection
The titles and abstracts identified through the search strategy described above were reviewed independently by two investigators (H.C.O. and T.Y.P.). To minimize data duplication due to multiple reporting, papers from the same authors, organization, or country were compared. For articles determined to be eligible based on the title and/or abstract, the full paper was retrieved. Potentially relevant studies chosen by at least one author were retrieved and the full text was evaluated.
Full papers that were selected based on the title and/or abstract, were assessed separately by both the investigators. Any disagreements were resolved through discussion. In cases where an agreement could not be reached, disputes were resolved with the help of a third investigator (H.K.).
Data extraction
Using a standardized data extraction form, the following data were extracted independently by two investigators (H.C.O. and T.Y.P.): (1) title; (2) name of first author; (3) name of journal; (4) year of publication; (5) study design; (6) type of intervention to prevent PEP; (7) type of PD stent, dose of pharmacological agents or dose of hydration; (8) country; (9) risk of bias; (10) inclusion criteria; (11) exclusion criteria; (12) age; (13) number of subjects; (14) frequency of overall PEP; (15) frequency of mild PEP; (16) frequency of moderate-to-severe PEP; (17) frequency of PEP in average-risk groups; and (18) frequency of PEP in high-risk groups
If the provided information was inadequate or missing, attempts were made to contact the study authors and additional information was requested. If unsuccessful, missing information was calculated from the available data when possible or was extracted from figures using the open source software Plot Digitizer version 2.6.8 (http://plotdigitizer.sourceforge.net). Only data of peer-reviewed works were used, and unpublished data were not requested from authors. Any disagreements were resolved with the help of a third investigator (H.K.).
Risk of bias assessment
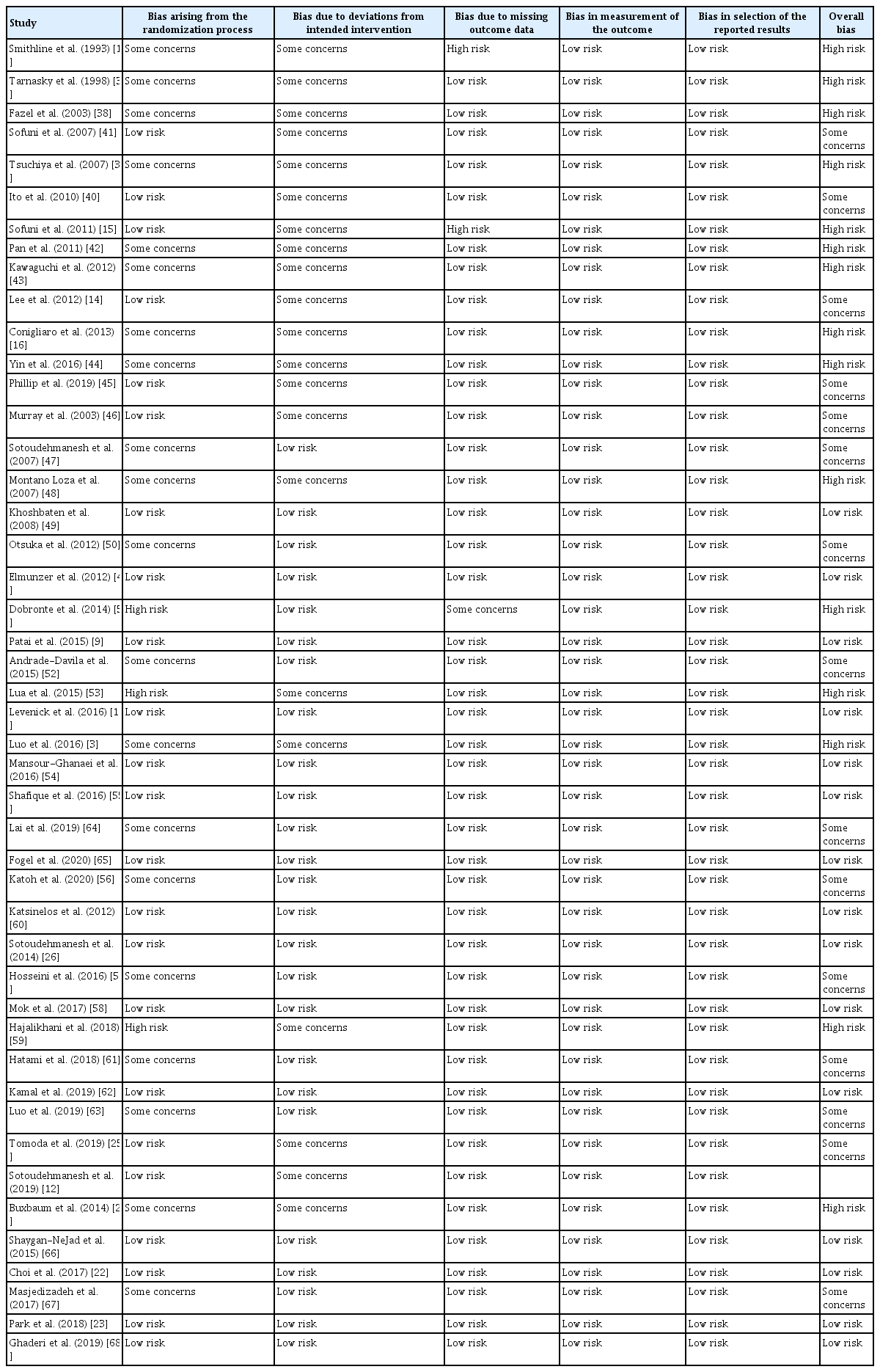
The quality of the studies was independently assessed by two of the study’s authors (H.C.O. and T.Y.P.) using the Revised Cochrane risk of bias tool for randomized trial (RoB 2.0). Risk of bias judgement was assessed in the following domains: bias arising from the randomization process, bias due to deviations from intended intervention, bias due to missing outcome data, bias in measurement of the outcomes, and bias in selection of the reported results. Based on the results of risk of bias judgement, formal overall risk of bias judgement was characterized as “low-risk of bias,” “some concern,” and “high-risk of bias” [31].
Statistical analysis
Ad hoc tables were designed to summarize data from the included studies by showing their key characteristics and important questions related to the review objectives. After extracting the data, reviewers determined the feasibility of a meta-analysis.
A multiple treatment comparison NMA is a meta-analysis generalization method that includes both direct and indirect comparison of treatments in RCTs. A random-effects NMA based on a frequentist framework was performed using STATA software version 15 (StataCorp LP, College Station, TX, USA) based on mvmeta with NMA graphical tools developed by Chaimani et al. [32].
Before conducting the NMA, we evaluated the transitivity assumption by examining demographics and types of pharmacological agents as potential treatment-effect modifier across comparisons, and the comparability of the risk of bias (all vs. removing high risks of bias for randomization, allocation concealment, and blinding of outcome assessor),
A network plot linking the included rectal NSAIDs and their combination with other pharmacological agents was formed to indicate the types of agents, number of patients on different agents, and the level of pair-wise comparisons. The nodes show comparisons of pharmacological agents that were selected as interventions in the study, and the edges show the available direct comparisons among the pharmacological agents from the RCTs. The nodes and edges were weighed on the basis of the weights applied in NMA and inverse of standard error of effect.
We evaluated the consistency assumption for the entire network using the design-by-treatment interaction model. We also evaluated each closed loop in the network in order to evaluate local inconsistencies between the direct and indirect effect estimates for the same comparison. For each loop, we estimated the inconsistency factor (IF) as the absolute difference between the direct and indirect estimates and 95% credible intervals for each paired comparison in the loop [33]. When the IF value with 95% confidence intervals (CIs) started at 0, it indicated the direct evidence and the indirect evidence were very consistent.
Mean summary effects with CIs were presented with their predictive intervals (PrIs) to facilitate interpretation of the results considering the magnitude of heterogeneity. PrIs provide an interval that was expected to encompass the estimate of a future study.
A rankogram and cumulative ranking curve were drawn for each pharmacological agent. Rankogram plots indicate the probabilities for treatments to assume a possible rank. We used the surface under the cumulative ranking curve (SUCRA) values to present the hierarchy of pharmacological agents for the incidence of PEP. The SUCRA is a relative ranking measure that accounts for uncertainty in the treatment order; i.e., it accounts for both the location and variance of all relative treatment effects. A higher SUCRA value is regarded as a more positive result when comparing individual interventions [34].
A comparison-adjusted funnel plot was used to assess the presence of small-study effects [35]. If all studies were symmetrically distributed around the X = 0 vertical line, and the funnel plot was symmetrical, we regard that there was no evidence of small sample effects in the study network.
Quality of the evidence
The evidence grade was determined using the guidelines of the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system which uses sequential assessment of the evidence quality that is followed by an assessment of the risk-benefit balance and a subsequent appraisal of the strength of the recommendations [36].
RESULTS
Study selection
We initially retrieved 5,615 articles from the MEDLINE, EMBASE, and the CENTRAL databases, in addition to the manual search. The flowchart depicting study selection in provided as Fig. 1.
Study characteristics
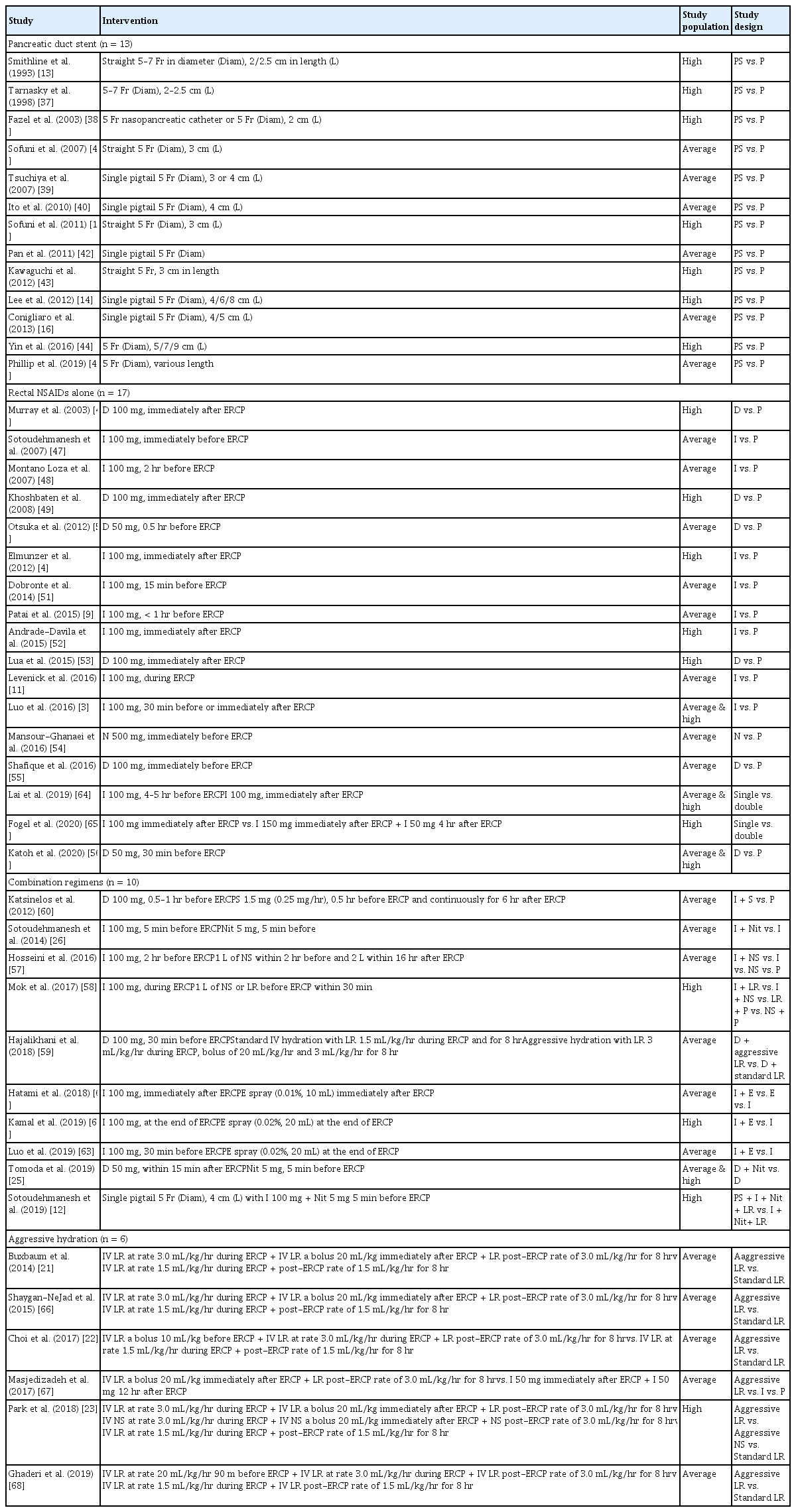
Baseline characteristics are summarized in Table 1. A total of 16,241 patients were included from 46 trials. The preventive efficacy of PEP was evaluated by comparing PD stents with no stent in 13 studies [13–16,37–45], rectal NSAIDs with placebo in 15 studies [3,4,9,11,46–56], combination of rectal NSAIDs and intravenous fluids in three studies [57–59], combination of rectal NSAIDs and somatostatin in one study [60], combination of rectal NSAIDs and intraduodenal epinephrine spray in three studies [61–63], combination of rectal NSAIDs and isosorbide dinitrate in two studies [25,26], double dose of rectal NSAIDs in two study [64,65], combination of rectal NSAIDs, isosorbide dinitrate and PD stents in one study [12], and aggressive hydration with standard hydration in six studies [21–23,66–68]. Based on the inclusion criteria stated in the manuscript, 17 studies were conducted in groups at high-risk for developing PEP [4,12–15,23,37,38,43,44,46,49,52,53,58,62,65].
Study quality assessment
The risk of bias assessment performed using the Cochrane tool for the included studies is presented in Table 2. Among 13 studies that evaluated the efficacy of PD stents, bias due to deviations from intended intervention were assessed as “some concerns” as difficult or failed PD stent placement can potentially increase the risk of pancreatitis.
Synthesis of results
We present the network plot for all outcomes of each datum (Fig. 2 and Supplementary Fig. 1), inconsistency plot (Supplementary Fig. 2), PrI plot compared with placebo (Fig. 3 and Supplementary Fig. 3), rankogram (Supplementary Fig. 4), cumulative ranking curve (Supplementary Fig. 5), expected mean ranking and SUCRA values of each pharmacological agent for the outcomes (Fig. 4 and Supplementary Fig. 6), and comparison-adjusted funnel plot (Supplementary Fig. 7). The summary of the results is presented in Fig. 2 through Fig. 4
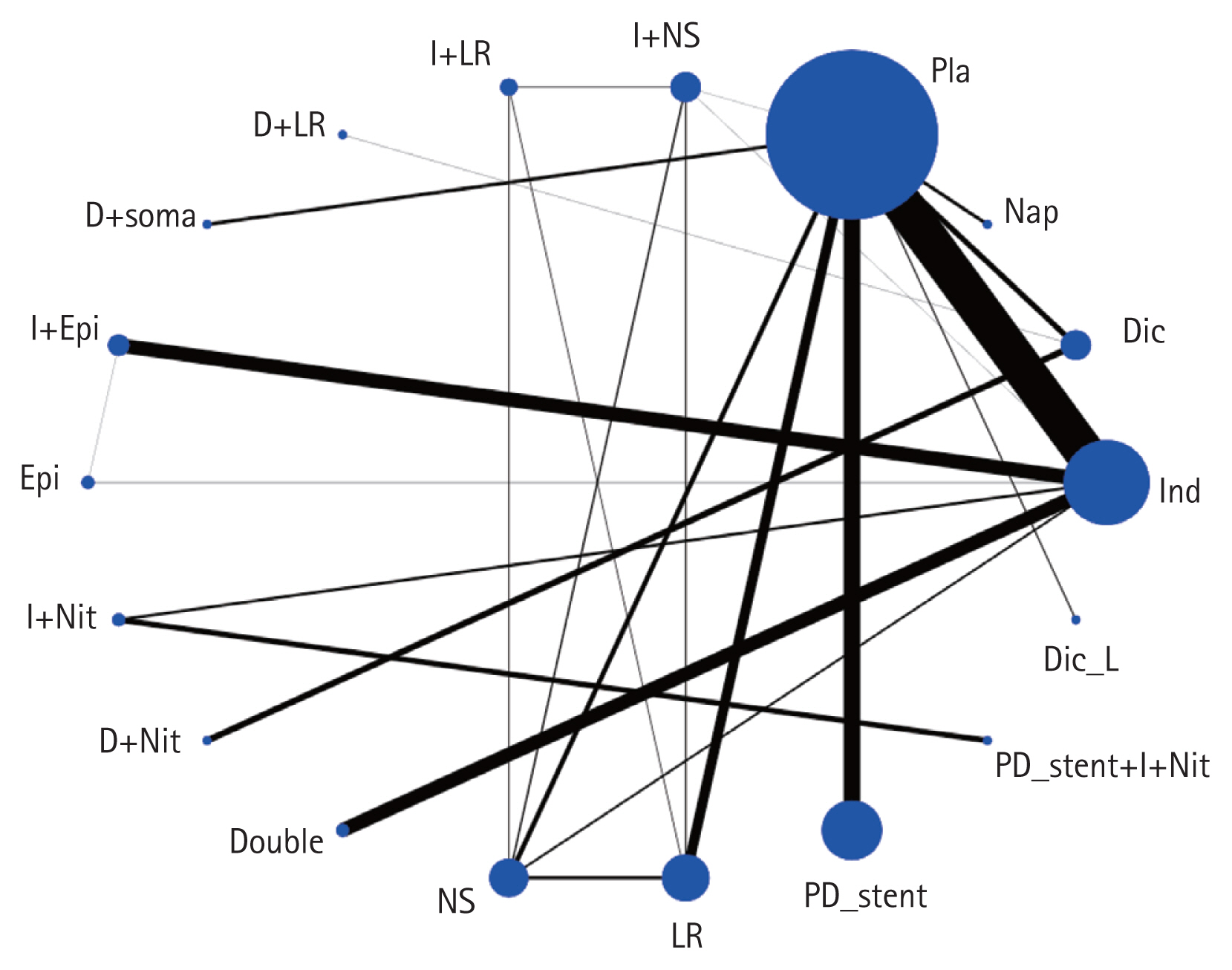
Network plot of included studies comparing different prophylactic modalities for their efficacy to prevent post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP): overall PEP. The nodes show a comparison of prophylactic modalities to prevent post-ERCP pancreatitis, and the edges show the available direct comparisons among the prophylactic modalities. The nodes and edges are weighed on the basis of the weights applied in network meta-analysis and inverse of standard error of effect. D or Dic, diclofenac; Dic_L, diclofenac low dose; Double, double dose of indomethacin; Epi, epinephrine; I or Ind, indomethacin; LR, lactated Ringer’s solution; Nap, naproxen; Nit, nitrate; NS, normal saline; PD, pancreatic duct; Pla, placebo; soma, somatostatin.

Predictive interval (PrI) plots between each management modality and placebo group: overall post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP). Diamond shape represents the mean summary effects. Black line represents the 95% confidence interval (CI), and red line represent the PrI. PrIs provide an interval that is expected to encompass the estimate of a future study. D or Dic, diclofenac; Dic_L, diclofenac low dose; Double, double dose of indomethacin; Epi, epinephrine; I or Ind, indomethacin; LR, lactated Ringer’s solution; Nap, naproxen; Nit, nitrate; NS, normal saline; PD, pancreatic duct; Pla, placebo; soma, somatostatin.

Expected mean ranking and surface of under cumulative ranking curve (SUCRA) values: overall post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis. X-axis corresponds to expected mean ranking based on SUCRA value, and y-axis corresponds to SUCRA value. D or Dic, diclofenac; Dic_L, diclofenac low dose; Double, double dose of indomethacin; Epi, epinephrine; I or Ind, indomethacin; LR, lactated Ringer’s solution; Nap, naproxen; Nit, nitrate; NS, normal saline; PD, pancreatic duct stent; Pla, placebo; soma, somatostatin.
Overall post-ERCP pancreatitis
A total of 46 studies (16,241 patients) were included to measure the frequencies of PEP. The network plot of all the eligible comparisons for this endpoint is depicted in Fig. 2. Although all of the 18 management modalities (nodes) were connected to the network, two comparisons (placebo and indomethacin) were more directly comparable than the other 16 nodes.
Evaluation of network inconsistency using a design-by-treatment interaction model suggested no significant inconsistency (χ2 (6) = 6.12, p = 0.410). Ten closed loops in the network were identified from the comparison of the overall frequency of PEP. One loop [indomethacin (I) + normal saline (NS) – I + LR – LR] was formed only by the multi-arm trial. The lower limit of the IF 95% CI for the 10 loops contained 0 after the consistency test, which indicating that no obvious local inconsistencies between the direct and indirect point estimates were found (Supplementary Fig. 2A).
The frequency of PEP was lower for indomethacin + LR, diclofenac + nitrate, PD stent + indomethacin + nitrate, indomethacin + NS, indomethacin + nitrate, diclofenac, LR, PD stent than for placebo based on both 95% CI and 95% PrI. The frequency of PEP was lower for naproxen, NS, indomethacin alone than for placebo based on 95% CI only (Fig. 3). Lack of significance observed in the 95% PrIs may be clarified by future RCTs.
The rankogram revealed that the frequency of PEP was lowest for diclofenac + LR and indomethacin + LR (Supplementary Fig. 4A). A cumulative ranking plot was generated, and the SUCRA probabilities of different pharmacological agents for PEP were calculated (Supplementary Fig. 5A). The expected mean rankings and SUCRA values of each pharmacological agent are presented in Fig. 4. According to the SUCRA value, the frequency of PEP was lowest in indomethacin + LR (86.4 %), followed by diclofenac + LR (80.7%), diclofenac + nitrate (79.6%), indomethacin + NS (76.1%), and PD stent + indomethacin + nitrate (73.0%).
The league table for the overall PEP was also produced (Supplementary Table 1). Estimates are presented by the log odds ratio with 95% CI in parentheses and 95% PrI in bracket. In the league table, log odds ratio above 0 suggest that treatment in the left column is superior, and log odds ratio below 0 suggest that treatment in the below column is superior.
The comparison-adjusted funnel plots revealed that the funnel plots were symmetrical at the zero line, suggesting a low likelihood of publication bias (Supplementary Fig. 7A).
Mild post-ERCP pancreatitis
A total of 35 studies (14,474 patients) were included to measure the frequency of mild PEP. A network plot of all the eligible comparisons for this endpoint is depicted in Supplementary Fig. 1A. Although all of the 18 management modalities (nodes) were connected to the network, two comparisons (placebo and indomethacin) were more directly comparable than the 16 other nodes.
Indomethacin and placebo were more directly comparable to other pharmacological agents. No network inconsistency was noted (χ2 (3) = 6.75, p = 0.08).
Six closed loops in the network were identified from the comparison of the overall frequency of mild PEP. Two loops (indomethacin + NS – indomethacin + LR – LR, indomethacin + NS – indomethacin + LR – NS) were formed only by multi-arm trial. The lower limit of the IF 95% CI for the six loops contained 0 after the consistency test, which indicating that no obvious local inconsistencies between the direct and indirect point estimates were found (Supplementary Fig. 2B).
PEP frequency was lower for PD stent than for placebo based on 95% CI (Supplementary Fig. 3A). The rankogram and cumulative ranking plot showed that PEP frequency was lowest for diclofenac + LR (Supplementary Figs. 4B and 5B). The order expected mean rankings and SUCRA values of each pharmacological agent was diclofenac + LR (80.1%), followed by PD stent (73.2%), diclofenac + nitrate (72.4%), epinephrine (71.0%) and indomethacin + NS (69.4%) (Supplementary Fig. 6A). The likelihood of publication bias was lower in the comparison-adjusted funnel plot (Supplementary Fig. 7B).
Moderate-to-severe post-ERCP pancreatitis
A total of 35 studies (14,474 patients) were included to measure the frequency of moderate-to-severe PEP. A network plot of all eligible comparisons for this endpoint is depicted in Supplementary Fig. 1B.
Although all 18 management modalities (nodes) were connected to the network, two comparisons (placebo, and indomethacin) were more directly comparable than the 16 other nodes. No evidence of network inconsistency was identified (χ2 (3) = 2.92, p = 0.4039). Six closed loops in the network were identified from the comparison of the overall frequency of mild PEP. Two loops (indomethacin + NS – indomethacin + LR – LR, indomethacin + NS – indomethacin + LR – NS) were formed only by multi-arm trial. The lower limit of the IF 95% CI for the six loops contained 0 after the consistency test, which indicating that no obvious local inconsistencies between the direct and indirect point estimates were found (Supplementary Fig. 2C).
A lower frequency of moderate-to-severe PEP was noted for PD stent and indomethacin than for placebo based on both 95% CI and 95% PrIs (Supplementary Fig. 3B). The rankogram and cumulative ranking plot disclosed that the frequency of PEP was lowest for diclofenac + LR and epinephrine (Supplementary Figs. 4C and 5C). The order of expected mean rankings and SUCRA values of each pharmacological agent was diclofenac + nitrate (84.6%), followed by diclofenac + somatostatin (72.7%), diclofenac (66.9%), PD stent (65.1%), diclofenac + LR (63.0%) (Supplementary Fig. 6B). The likelihood of publication bias was lower in the comparison-adjusted funnel plot (Supplementary Fig. 7C).
Post-ERCP pancreatitis in average-risk group
In total, 16 pharmacological agents were compared in 29 studies (10,734 patients) for measuring the frequencies of PEP in average-risk groups (Supplementary Fig. 1C). Although all 16 management modalities (nodes) were connected to the network, two comparisons (placebo and indomethacin) were more directly comparable than the 14 other nodes. Indomethacin and placebo were more directly comparable than other pharmacological agents. No network inconsistency was noted (χ2 (2) = 3.71, p = 0.157). Five closed loops in the network were identified from the comparison of the overall frequency of mild PEP. Two loops (indomethacin – indomethacin + NS – NS, placebo – indomethacin + NS – NS) were formed only by the multi-arm trial [57]. The lower limit of the IF 95% CI for the five loops contained 0 after the consistency test, which indicating that no obvious local inconsistencies between the direct and indirect point estimates were found (Supplementary Fig. 2D).
The frequency of PEP was lower for indomethacin + NS, diclofenac + nitrate, indomethacin + nitrate, PD stent, diclofenac and LR than for placebo based on both 95% CI and 95% PrIs, PEP frequency was lower for indomethacin than for placebo based on 95% CI only (Supplementary Fig. 3C). The rankogram and cumulative ranking plot reveaked that PEP frequency was lowest for indomethacin + NS (Supplementary Figs. 4D and 5D). The order of expected mean rankings and SUCRA values of each pharmacological agent was indomethacin + NS (93.8%), followed by diclofenac + LR (79.0%), diclofenac + nitrate (77.1%), epinephrine (68.4%) and indomethacin + nitrate (66.4%) (Supplementary Fig. 6C). The likelihood of publication bias was lower in the comparison-adjusted funnel plot (Supplementary Fig. 7D).
Post-ERCP pancreatitis in high-risk group
In total, 18 studies (5,507 patients) measured the frequency of PEP in high-risk groups. Of these studies, one was separated from the loops [12], which we therefore excluded from NMA. A total of 17 studies (5,093 patients) comparing four pharmacological agents were included in the NMA (Supplementary Fig. 1D). Although all 11 management modalities (nodes) were connected to the network, two comparisons (placebo, and PD stent) were more directly comparable than the nine other nodes. No network inconsistency was observed (χ2 (1) = 0.42, p = 0.516).
Five closed loops in the network were identified from the comparison of the overall frequency of mild PEP. Two loops (indomethacin + NS – indomethacin + LR – NS, indomethacin + NS – indomethacin + LR – LR) [58] were formed only by the multi-arm trial. The lower limit of the IF 95% CI for the five loops contained 0 after the consistency test, which indicating that no obvious local inconsistencies between the direct and indirect point estimates were found (Supplementary Fig. 2E). PEP frequency was lower for indomethacin + LR, diclofenac and PD stent than for placebo based on 95% CI only (Supplementary Fig. 3D).
The rankogram and cumulative ranking plot revealed that PEP frequency was the lowest for indomethacin + LR (Supplementary Figs. 4E and 5E). The order of expected mean rankings and SUCRA values of each pharmacological agent was indomethacin + LR (91.6%), followed by indomethacin + NS (72.3%), diclofenac (72.0%), LR (63.5%), and PD stent (59.3%) (Supplementary Fig. 6D). The likelihood of publication bias was lower in the comparison-adjusted funnel plot (Supplementary Fig. 7E).
Summary of NMA
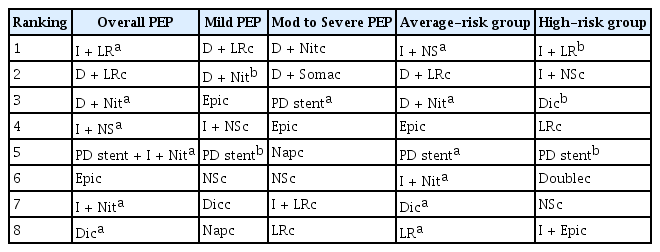
The overall results of this NMA are summarized in Table 3. Based on integral analysis of 95% CIs and PrIs (Fig. 3), and expected mean ranking (Fig. 4), Prophylactic modalities indicated in footnote ‘a’ (i.e. for overall PEP, indomethacin + LR is followed by diclofenac + nitrate and indomethacin, etc.) presented with statistically significant efficacy in terms of both 95% CIs and PrIs, and a high expected mean ranking for preventing PEP. Prophylactic modalities in footnote ‘b’ (among high-risk group, indoemthacin + LR followed by diclofenac and pancreatic duct [PD] stent) exhibited statistically significant efficacy only in terms of 95% CIs. Although a modality may be placed in a high expected mean ranking, it may not be efficacious for preventing PEP in the absence of statistical significance (e.g., modalities footnote ‘c’). The modalities indicated footnote ‘c’ did not exhibit statistical significance upon individual analysis, regardless of ranking. Thus, the rankings should not be misinterpreted as a guide for the selection of modalities to prevent PEP.
Quality of the evidence
Three outcomes were evaluated using the GRADE system. The evidence quality for each outcome was moderate or low (Supplementary Table 2).
DISCUSSION
The current NMA revealed that indomethacin + LR, followed by diclofenac + nitrate and indomethacin + normal saline, are the most efficacious combinations of pharmacological agents for the overall prevention of PEP, based on PrI plots and SUCRA values (presented in Figs. 3 and 4). Combination regimens or modalities are superior to rectal NSAIDs, PD stent, and aggressive hydration in isolation.
According to a recent clinical guideline [69], routine rectal NSAIDs administration is recommended immediately before ERCP in all patients without contraindications of rectal NSAIDs. In patients with contraindications of rectal NSAIDs, aggressive hydration with LR is recommended if the patients are not at risk of fluid overload. Sublingual nitrate is recommended in case of contraindication of rectal NSAIDs and aggressive fluid hydration. In high risk patients for PEP, the additional prophylactic pancreatic stent placement in strongly recommended. In current NMA, combination of rectal NSAIDs with aggressive hydration or sublingual nitrate is the most efficacious modality for overall PEP prevention. PD stent placement in addition to pharmacological prevention is considered to be beneficial if PD stent placement is easily performed. Recently, there have been performed several NMAs regarding PEP prevention. The conclusions of previous and current NMA is summarized in Table 4 [70–73].
The pathogenesis of PEP is multifactorial. Therefore, multiple modalities with different hypothetical mechanisms have been evaluated for therapeutic efficacy. PD stent placement, rectal NSAIDs, aggressive hydration, sublingual nitrate, somatostatin and their combinations have demonstrated preventive effects on PEP. As reported in the current NMA, combination modalities with rectal NSAIDs and aggressive hydration or rectal NSAIDs and sublingual nitrate ranked on a higher position and were superior to single modalities for preventing overall PEP. Synergistic effects from combination modalities with different mechanism of action for the prevention of PEP are supported by this NMA.
According to RCT among high-risk patients [4] and NMA [74], rectal NSAIDs were reported to be superior to PD stents for preventing PEP. In a recent NMA of high-risk groups for PEP prevention [70], PD stent placement exhibited the highest SUCRA probability (0.81; 95% CI, 0.83 to 0.80) as a preventive strategy of PEP. However, in the current NMA, rectal diclofenac alone was superior to PD stents for preventing overall PEP and PEP among high-risk groups, and rectal indomethacin was inferior to PD stents (Figs. 3 and 4). This discrepancy may be due to the separation of individual rectal NSAIDs (indomethacin vs. diclofenac vs. naproxen) in the analysis. Direct comparisons in large-scale RCTs will help to clarify the difference in efficacy of these modalities [75].
A recent clinical trials that compared pharmacological prophylaxis with PD stent placement + pharmacological prophylaxis in high-risk patients reported non-inferiority of pharmacological prophylaxis [12]. In this trial, triple combination prophylaxis including rectal indomethacin, sublingual nitrate, and aggressive hydration was administered to both groups, and an additional PD stent was placed to the intervention group. The non-inferiority of pharmacological prophylaxis alone may result from the synergistic preventive effects provided by triple combination prophylaxis, and those receiving additional PD stent presented with a trend for outcomes, but this was not significantly superior to pharmacological prophylaxis. Considering the increased risk of PEP (34.7%) due to failed PD stent placement [76], pancreatic dust stents in addition to pharmacological prophylaxis are recommended when unintended or easy cannulation of PD is obtained.
A single dose of 100 mg of rectal indomethacin or diclofenac was administered in all included studies except four (two with 50 mg of diclofenac and two with 200 mg of indomethacin) [25,50,56,64]. The preventive effects of low dose (50 mg) of rectal diclofenac for PEP were determined to be inefficacious in the current NMA. Diclofenac undergoes first-pass metabolism with only 50% to 60% of the drug reaching the systemic circulation as intact diclofenac. In contrast, indomethacin is not subject to substantial first-pass metabolism [6]. With low dose (50%) diclofenac, effective blood concentrations exceeding therapeutic levels could not be stained. Two studies reported that double dose of rectal indomethacin (200 mg) was not superior to a single dose, suggesting that maximal dosing (at least of indomethacin) is not required for PEP prevention [64,65].
The current NMA is ultimately different from the previous NMA [77] in study design. In the current NMA, four effective prophylactic modalities (PD stent, rectal NSAIDs alone, aggressive hydration, and combination therapy) were compared by detailed direct and indirect methods. In a result, the prophylactic effect of rectal NSAIDs-based combination therapy rather than single therapy is indicated. In terms of clinical impact, the role of aggressive hydration and PD stent in high-risk group for PEP prevention has been introduced, especially when rectal NSAIDs is commercially unavailable.
The current NMA has several strengths relative to previous NMAs. First, a rigorous literature search regarding PEP was performed using a self-developed review protocol for systematic reviews and NMA. Therefore, most publications regarding PEP prophylaxis were identified, and most RCTs regarding PEP were enrolled in the final analysis. Second, inconsistencies among the enrolled studies were not significant, and publication bias of the enrolled studies was minimal. Third, most enrolled studies exhibited a low-risk of bias, with the exception of bias from randomization process and bias due to deviations from intended intervention domains. Fourth, in addition to overall PEP, subgroup analysis according to the severity or stratified risk of PEP were performed in this NMA. Fifth, 10 RCTs of rectal NSAIDs with additional modalities (somatostatin, nitrate, epinephrine spray, aggressive hydration, or PD stent placement) were categorized into combination regimens, and thoroughly evaluated for direct and indirect comparative efficacy for PEP compared with PD stent, rectal NSAIDs, and aggressive hydration in isolation.
Several limitations of this NMA should be noted. First, the three most efficacious modalities determined in the current NMA were documented to be effective in a limited number of clinical trials. These studies may have the risk of lack of power to discriminate the effectiveness of pharmacological interventions clearly, and over- or under-estimation of true treatment effects. Second, as with all meta-analysis, there were heterogeneities among the enrolled studies, regarding study population with variable risk for developing PEP and methods of prophylactic intervention. Third, quality of evidence assessments conducted by GRADE system was moderate to low. Fourth, there was inherent limitation of NMA, indirect comparison of RCTs. Not head-to-head comparison limits the definite effect of various modalities for preventing PEP. Therefore, large scale RCTs with the qualified protocol are needed to confirm the result of current NMA and to determine an optimal modality for preventing PEP or attenuating the severity of PEP.
In routine practice, the use of rectal NSAIDs is recommended for PEP prevention. However, rectal NSAIDs are not always available. For example, rectal NSIADs suppository for PEP prevention is unavailable in South Korea because it is commercially unavailable. If rectal NSAIDs is unavailable in high-risk patient, PD stent placement is recommended. However, selective cannulation of PD is not always successful, even by expert endoscopist. In this regard, immoderate attempts for PD stent placement should be avoided because it can rather induce PEP.
In conclusion, combination prophylaxis with indomethacin + LR, followed by diclofenac + nitrate and indomethacin + normal saline, is the most efficacious modality for the overall prevention of PEP. PD stent placement in addition to pharmacological/hydration modalities may be beneficial if selective cannulation of PD is easily obtained.
KEY MESSAGE
1. Combination prophylaxis with indomethacin + lactated Ringer’s solution (LR), followed by diclofenac + nitrate and indomethacin + normal saline, is the most efficacious modality for the overall prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis.
2. Pancreatic duct (PD) stent placement in addition to pharmacological/hydration modalities may be beneficial if selective cannulation of PD is easily obtained.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This research was supported by the Chung-Ang University Research Grants in 2021 (Tae Young Park).