Glomerular filtration rate as a kidney outcome of diabetic kidney disease: a focus on new antidiabetic drugs
Article information
Abstract
Diabetes has reached epidemic proportions, both in Korea and worldwide and is associated with an increased risk of chronic kidney disease and kidney failure (KF). The natural course of kidney function among people with diabetes (especially type 2 diabetes) may be complex in real-world situations. Strong evidence from observational data and clinical trials has demonstrated a consistent association between decreased estimated glomerular filtration rate (eGFR) and subsequent development of hard renal endpoints (such as KF or renal death). The disadvantage of hard renal endpoints is that they require a long follow-up duration. In addition, there are many patients with diabetes whose renal function declines without the appearance of albuminuria, measurement of the eGFR is emphasized. Many studies have used GFR-related parameters, such as its change, decline, or slope, as clinical endpoints for kidney disease progression. In this respect, understanding the trends in GFR changes could be crucial for developing clinical management strategies for the prevention of diabetic complications. This review focuses on the clinical implication of the eGFR-related parameters that have been used so far in diabetic kidney disease. We also discuss the use of recently developed new antidiabetic drugs for kidney protection, with a focus on the GFR as clinical endpoints.
INTRODUCTION
Diabetic kidney disease (DKD) is a major cause of kidney failure (KF), and both its incidence and prevalence are increasing worldwide [1–6]. In particular, in the Korean population, the prevalence rate of patients with diabetes undergoing hemodialysis treatment has rapidly increased, from 23.8% in 2002 to 47.8% in 2017 [2]. Moreover, chronic kidney disease (CKD) is closely related to premature death [7], and the age-adjusted mortality rate of individuals with CKD is more than twice that of individuals without CKD (96.0 vs. 41.0 per 1,000 patients/year, respectively), according to the United States Renal Data System 2020 annual data report [3]. Additionally, in a nationwide study in Korea, the hazard ratio (HR) for mortality in patients with CKD who were not undergoing dialysis was 4.88 (95% confidence interval [CI], 4.36 to 5.47; p < 0.001) compared with that in age- and sex-matched controls [8]. Moreover, patients with CKD showed a higher mortality rate (HR, 4.36; 95% CI, 3.92 to 4.85; p < 0.001) than patients with hypertension or diabetes without CKD. These findings suggest that kidney function is intimately related to patient mortality, irrespective of the underlying disease [8]. Consequently, the management and monitoring of kidney function in patients with diabetes are crucial.
To date, in clinical fields, the kidney function has been evaluated using an estimated glomerular filtration rate (eGFR), including serum creatinine or cystatin C levels. Recently, several drugs have been reported to help preserve kidney function in patients with diabetes [9–11]. In addition, standardized prognostic assessment indicators are required to evaluate the effectiveness of treatments and judge the prognosis of DKD. This requirement has previously been overlooked, and kidney outcome indicators (as endpoints) have been heterogeneous in the realm of the kidney research. Unlike the lack of those for kidney outcomes, there are evaluation indices, such as the three-component major adverse cardiac events (MACE) endpoint, that are relatively consistently used in the research on cardiovascular (CV) diseases. Among many indicators, long-term changes in kidney function are the most important in the management and treatment of patients with DKD. Thus, understanding the progressive course of the decline of kidney function is a must to help prevent DKD progression to KF and reduce patient mortality. Physicians should also be aware of the eGFR as an indicator of kidney function and the importance of evaluating and utilizing it for proper disease management [12].
CHANGES OF THE GLOMERULAR FILTRATION RATE IN DKD
The Kidney Disease Improving Global Outcomes (KDIGO) organization has suggested that DKD should be managed using a comprehensive strategy to reduce kidney disease progression by monitoring both albuminuria and the glomerular filtration rate (GFR) [13]. Despite recent exciting developments in medical science, to date, there is no conclusive marker, except for albuminuria and the GFR to monitor the progression of kidney disease. Recently, albuminuria has been found to be controlled more effectively than the GFR by a renin-angiotensin system blocker, and the proportion of patients with DKD with a reduced GFR showed an increase, irrespective of albuminuria [14,15]. Moreover, although the prevalence of diabetic complications decreased from 1990 to 2010, the decrease in the rate of KF was the smallest compared with those in the rates of myocardial infarction, stroke, and amputation (KF, −28.3%; myocardial infarction, −67.8%; stroke, −52.7%; and amputation, −51.4%) [16]. Although from 2006 to 2015, the incidence rate of KF decreased by 35.2% (from 168.2 to 109.0 per 100,000 people) in patients with diabetes in Korea [17], KF, as a complication of diabetes, has remained a significant burden. In the U.K. Prospective Diabetes Study 74, 28% (1,132/4,006) of the population with type 2 diabetes developed kidney dysfunction, 51% (575/1,132) did not have albuminuria prior to decreases in the GFR [18]. This is a new phenotype of DKD that is different from the traditional clinical courses of diabetic nephropathy. Recent studies have frequently reported this phenotype, and its incidence is increasing. In this respect, understanding the trends in GFR changes could be crucial for developing clinical management strategies for the prevention of diabetic complications.
In a prospective observational cohort study of 1,682 patients with type 2 diabetes, a rapid eGFRcr (eGFR calculated using serum creatinine levels) decline, which was defined as exceeding 4% per year, was observed in 15.6% (263/1,682) of the patients with relatively preserved eGFRcr levels (≥ 60 mL/min/1.73 m2). Albuminuria is a strong predictor of the annual eGFRcr decline, as are other related factors, including an older age, hypertension, insulin treatment, and a lower baseline eGFRcr [19]. Recently, the Hong Kong Diabetes Register has proposed the following four trajectories of the eGFRcr decline in patients with type 2 diabetes and CKD greater than stage 2 or without severely increased albuminuria (A3) over a median follow-up period of 11.8 years: slow (84.3%), curvilinear (6.5%), progressive (6.1%), and accelerated (3.1%) eGFRcr decline [20]. Moderately increased albuminuria (A2) and retinopathy were strongly associated with the accelerated eGFRcr decline, and all-cause mortality was higher in the other three groups than in the slow decline group [20]. These results were consistent with previous data showing that proteinuria was intimately related to the deterioration of kidney function and DKD progression [21]. Notably, the rapid decline group, which was defined based on an age-adjusted eGFRcr decline of > 4%/year using traditional linear mixed-effects models, was further classified into three subgroups in the Hong Kong study. This shows that the clinical course of DKD is heterogeneous, even in rapid decline groups, defined using the standard classification criteria. Additionally, the eGFRcr values in all four main groups were found to be closely related to patient mortality. Taken together, although albuminuria is still an important predictor of kidney dysfunction or KF, the precise prediction of GFR decreases could be another important point to consider for the prevention of a negative kidney outcome in type 2 diabetes. Thus, further relevant clinical studies that aim to predict kidney outcomes in patients with DKD using the eGFRcr trajectory may need to be revised to improve the clinical outcomes of patients with type 2 diabetes.
Unlike that for type 2 diabetes, the natural clinical course can be easily predicted for type 1 diabetes. In 1976, Kussman et al. [22] reported that proteinuria occurred 17.3 ± 6.0 years after the diagnosis of type 1 diabetes, and kidney dysfunction developed after an additional 2 years. Consistent with these findings, in 1983, Mogensen et al. [23] proposed five stages for DKD and observed that albuminuria preceded a decrease in the GFR in type 1 diabetes. Recent observational data from the Joslin clinic have been significant in that they reflect the entire clinical course of disease progression from an eGFRcr decline to KF in 364 patients with type 1 diabetes [24]. That study was regarded as a valuable study in that it reflected the whole clinical course of kidney dysfunction to KF in type 1 diabetes. Notably, the development of kidney dysfunction showed a linear decline pattern in most patients (87%) and occurred non-linearly in the minority (13%) [24]. Consequently, the authors suggested that clinically strategic interventions could be possible by predicting the rate of deterioration of kidney function in patients with type 1 diabetes who showed a linear decline pattern.
Albuminuria is currently a powerful predictor for future CKD occurrence, defined as an eGFRcr of < 60 mL/min/1.73 m2 in the Diabetes Control and Complications Trial (DCCT) and Epidemiology of Diabetes Interventions and Complications (EDIC) cohort, a large follow-up observational study with type 1 diabetes [25]. However, even in type 1 diabetes, albuminuria is not regarded as an essential precursor to the decrease in the GFR. Additionally, the monitoring of albuminuria alone in patients with diabetes may not allow the detection of kidney dysfunction that is manifested by a decrease in the eGFRcr of approximately 24% [25], and therefore, serial monitoring of the eGFRcr is crucial in combination with monitoring albuminuria. In the Joslin kidney study cohort [26], the annual decline of the eGFRcr-cys (eGFR calculated using serum creatinine and cystatin C levels) was more rapid in subjects with microalbuminuria than in those with normoalbuminuria. A rapid decline of the eGFRcr-cys (defined as a loss rate of ≥ 3.3% per year) occurred in 10% of the patients with normoalbuminuria and in 35% of the patients with microalbuminuria. Notably, in studies of populations with eGFRcr values below 60 mL/min/1.73 m2, two distinct patterns were detected by analyzing the eGFRcr decline using spline mixed-effects models, as exemplified in 397 patients with type 1 diabetes without albuminuria [27]. In this study, class I (86%) was characterized by an initial eGFRcr increase followed by a linear decline, whereas class II (14%) showed a rapid eGFRcr decline, despite the absence of albuminuria [27]. Meanwhile, in the presence of albuminuria, the rates of the eGFRcr decline were faster, and the 10 year-averages were of 2.2 and 3.3 mL/min/1.73 m2/year in patients with diabetes with microalbuminuria and macroalbuminuria, respectively, and 1.9 mL/min/1.73 m2/year in patients with diabetes with normoalbuminuria. The reference rate of the eGFRcr decline in healthy people of European descent was found to be 0.4 mL/min/1.73 m2/year [28], while the rate was almost fourfold greater in patients with diabetes without albuminuria.
Collectively, a precise understanding of the clinical course may be imperative to predict and prevent the risk of KF and negative kidney outcomes in patients with both type 1 and type 2 diabetes mellitus (DM), irrespective of the degree of albuminuria. However, as many factors are related to kidney dysfunction in patients with diabetes, the identification of future management targets is challenging. Nevertheless, kidney specialists should continue their efforts to assess and understand the disease using the latest research techniques. It is expected that improved management indicators will be identified in the future using tools such as artificial intelligence and big data in combination with gene-related research.
GFR AS AN ENDPOINT FOR DKD OUTCOMES IN CLINICAL TRIALS
Changes in GFR over time can present to track kidney disease progression, and one method is to express it as a GFR slope (average rate of change in GFR over time) (Fig. 1). Through this, we can detect GFR decline. In addition, substantial GFR decline over time can use as an endpoint for DKD outcomes. The details will be described in the following paragraphs.

Glomerular filtration rate (GFR) as an endpoint for diabetic kidney disease outcomes in clinical trials. UACR, urinary albumin-to-creatinine ratio; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; CV, cardiovascular. aNew onset, progression, or regression of albuminuria, b[34], c[32], ddefined as an eGFR of < 15 mL/min/1.73 m2.
Issues in determining kidney endpoints in clinical trials
In real-world practice, most guidelines recommend that albuminuria be regularly tested as a marker for kidney damage and the eGFR be also monitored as an indicator of renal function in patients with diabetes [13,29]. In particular, as there are many patients with diabetes whose renal function declines without the appearance of albuminuria, measurement of the eGFR is emphasized [30]. However, there is still no consensus regarding the selection of endpoints in clinical trials of patients with diabetes. Since the Food and Drug Administration and other regulatory agencies mandate CV safety trials of all new antidiabetic agents, consistent clinical endpoints have been used for CV events. Meanwhile, inconsistent kidney endpoints have been used as one of the prespecified secondary outcomes in most cardiovascular outcome trials (CVOTs). As any new drug, including antidiabetic agents, may potentially be beneficial or harmful to the kidneys, kidney safety must first be assessed in the course of their long-term use. Determining which endpoint to use for clinical trials is a complicated process, and currently, there is no relevant consensus. Therefore, diverse variables and definitions are being used to assess kidney outcomes in clinical research. In addition, reports of kidney outcomes are frequently incomplete [31]. This heterogeneity is still present in clinical trials of new antidiabetic drugs. Recently, efforts have been made to more objectively compare the effects of various antidiabetic drugs on the development and progression of DKD using uniformly defined kidney endpoints [32,33].
Surrogate endpoints as alternatives for hard endpoints
End-stage kidney disease (ESKD) or KF (GFR < 15 mL/min/_1.73 m2 or the initial of renal replacement therapy) is an accepted hard endpoint in clinical trials for the progression of DKD [13]. However, the disadvantage of ESKD as a clinical endpoint is that it requires a long follow-up duration [34]. Thus, biomarkers relevant to clinical trials for DKD progression include parameters such as urine protein/albumin, serum creatinine, or eGFR levels. These surrogate endpoints have been used instead of less frequently used hard clinical endpoints. The use of surrogate endpoints in clinical trials can reduce the number of participants and shorten the follow-up period required to achieve a statistical power to evaluate the effectiveness of new interventions and evaluate interventions for early-phase disease [35]. As a representative surrogate endpoint, changes in albuminuria levels have been used to evaluate DKD progression. The treatment effects on albuminuria were found to have moderately strong correlations with treatment effects on clinical endpoints, based on a joint analysis of 41 treatment comparisons from randomized controlled trials (RCTs) [35]. This association was stronger for participants with high baseline levels of albuminuria (urinary albumin-to-creatinine ratio [UACR] of > 30 mg/g or 3.4 mg/mmol). In addition, recent studies have reported that increases in albuminuria levels reflect the progression of earlier structural glomerular lesions, whereas an early GFR decline may not accurately reflect such lesions in patients with type 2 diabetes with preserved kidney function [36]. However, uncertainty still remains over the reliability of evidence that surrogate therapeutic effects on albuminuria could be used to predict treatment effects on hard endpoints in clinical trials [35,37].
Substantial declines in the GFR as intermediate endpoints
GFR is widely accepted to be the best overall marker of kidney function. Typically, eGFR decline of 30%, 40%, or 50% from baseline and serum creatinine doubling (57% eGFR decline) have been used to assess declining kidney function [32]. The eGFR decline from baseline has both strengths and challenges as a kidney endpoint [38]. As the GFR must decline for subjects to develop KF, a substantial decline (40%, 50%, or 57%) can be used as an “intermediate” endpoint in clinical trials [34]. The advantage of using the rate of changes in the GFR as an endpoint is that it provides a greater statistical power than binary outcomes, such as ESKD. However, several precautions must be taken when using the GFR as an endpoint. A substantial decline in the GFR can be appreciated in advanced kidney disease [39]. In addition, this endpoint is the most useful in trials of rapid progressors [40]. Changes in the GFR can occur as a result of hemodynamic effects on a single-nephron GFR, rather than alterations in a number of nephrons [41]. Therefore, changes in the GFR might not be a specific measure for the progression and regression of kidney disease. Meanwhile, endpoints that are derived from serum creatinine-based GFR estimates might be biased if the treatment or intervention affects creatinine production, secretion, or extrarenal elimination [42]. Moreover, this measure has been shown to have interlaboratory variations and be influenced by the hemodynamics and diet [43].
GFR slope-based endpoint in clinical trials
When the endpoint is based on repeated measurements of the eGFR, the comparison of GFR slopes between treatment groups may provide a better statistical power than any alternative endpoints using the eGFR [34,44]. The eGFR slope can be used as a continuous variable or a cutoff value, allowing dichotomous comparisons. The slope can be assessed before any substantial decline in the GFR occurs, and its accuracy relies on recording multiple values over time. However, the cutoff values for steep or shallow slopes are arbitrary. The GFR slope is worth using as an endpoint only when the following two assumptions are met: first, the mean rate of the GFR decline is constant during the intervention in a trial, and second, the treatment effect on the GFR slope is the same, regardless of the patient’s underlying rate of kidney disease progression [45]. However, there are some issues with these assumptions. Most interventions have short-term hemodynamic effects on the GFR early after randomization, which differ from their long-term effects [46]. Thus, sodium-glucose cotransporter 2 (SGLT2) inhibitors are well known to cause an initial dip in the GFR in the early phase, which contrasts with their long-term kidney protective effects [47].
In addition, the nonlinearity in eGFR slopes complicates interpretations. Several trials have used the GFR slope as an endpoint [48–50]. These early clinical trials revealed a linear decline of kidney function over time. However, these studies were conducted over relatively short periods, enrolled small numbers of subjects, and recorded fewer than 10 longitudinal GFR measurements or estimates per subject. By contrast, a more recent and larger study of many African American patients with hypertensive nephrosclerosis has shown a nonlinear eGFR trajectory or an extended period of nonprogression [51]. Another study reported that approximately half of their patients showed a nonlinear kidney function decline before reaching dialysis [52]. These data challenge the prior paradigm of a linear trajectory of the kidney function decline, which may have important implications for the analysis and interpretation of the data of clinical trials that use the eGFR slope as the endpoint. By contrast, a data analysis of six clinical studies showed that the proportion of patients with the probability of a nonlinear eGFR decline of > 50% was generally low, ranging from 19.3% to 31.7% in diabetes trials and from 15.1% to 21.2% in nondiabetes trials [53]. These data indicate that the eGFR slope can be used as the endpoint if the GFR declines linearly over time during a clinical trial period. However, one should take the proportion of nonlinearity into account when designing clinical trials with subjects who have diabetes [53].
A recent meta-analysis of RCTs showed strong associations between treatment effects on the GFR slope and those on the clinical endpoint [54]. These findings suggest that the GFR slope might be a useful surrogate endpoint in clinical trials of kidney disease progression. The use of the GFR slope can substantially increase the statistical power compared with that of the clinical endpoint, particularly when the baseline GFR is high and there are no acute effects [54]. However, another meta-analysis demonstrated a consistent association between the eGFR slope and subsequent development of ESKD, even when the slope difference was small and observed over a period of only 1 to 3 years [44]. These findings suggested that the change in the GFR slope might be a good surrogate endpoint for ESKD in clinical trials, particularly in longer trials of patients with a rapidly progressing disease.
Application of the GFR as an endpoint in clinical trials
Currently, GFR is used as various endpoints in clinical trials. Using GFR-based parameters as endpoints for kidney disease progression has both advantages and disadvantages in clinical trials of antidiabetic agents. Kidney endpoints should be determined according to the characteristics of the study, such as the stage of kidney injury in the subjects. The application of GFR as an endpoint for DKD outcomes in clinical trials is summarized in Fig. 1. Drawing a consensus on the appropriate use of GFR-based endpoints in clinical trials will require a sustained effort.
EFFECTS OF NEW ANTIDIABETIC DRUGS ON THE GFR
Preventing the GFR decline is important, as KF in DKD leads to poor outcomes in patients, such as death or CV disease. Currently, metformin and several other antidiabetic drugs are used to treat patients with diabetes [55]. In this section, the effects of antidiabetic drugs on the eGFR will be reviewed, mainly with regard to new classes of antidiabetic drugs.
SGLT2 inhibitors
Multiple pathophysiological disturbances, including hemodynamic, structural, and inflammatory processes, contribute to kidney damage and the GFR decline in DKD [56]. An increased activity of SGLT2, which is responsible for approximately 90% of glucose and sodium reabsorption in the proximal tubule, is pivotal to initiating many of the pathophysiological abnormalities in DKD. Therefore, SGLT2 inhibitors play a role in renal protection. According to the current KDIGO guidelines, they are recommended as a first-line treatment, along with metformin, in patients with DKD with an eGFR of ≥ 30 mL/min/1.73 m2 [56].
The effects of SGLT2 inhibitors on the kidney outcomes reported in previous studies are summarized in Table 1. Recent CVOTs using SGLT2 inhibitors also identified renal endpoints as secondary outcomes. The Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients-Removing Excess Glucose (EMPA-REG OUTCOME) study was conducted in patients with type 2 DM with an eGFR of > 30 mL/min/1.73 m2 [57]. Analysis of long-term renal outcomes showed that the incidence of nephropathy worsening, including the progression to macroalbuminuria, doubling of the serum creatinine level, initiation of renal replacement therapy, or death from renal disease, was lower in the empagliflozin group than in placebo group (HR, 0.61; 95% CI, 0.53 to 0.70; p < 0.001). The decrease in the eGFR was smaller in the empagliflozin group than in the placebo group over a period of 192 weeks. However, the patients in the empagliflozin group showed a short-term decrease in the eGFR after empagliflozin initiation, followed by eGFR stabilization over time. The eGFR significantly improved after discontinuation of empagliflozin, indicating that the tubuloglomerular feedback (TGF) mechanism was terminated and glomerular hemodynamic changes were reversed. Even when the eGFR decline was analyzed by albuminuria subgroups (normoalbuminuria, microalbuminuria, and macroalbuminuria), the decrease in the eGFR was smaller in the empagliflozin group than in the placebo group in all three albuminuria subgroups, especially in the macroalbuminuria subgroup [58].
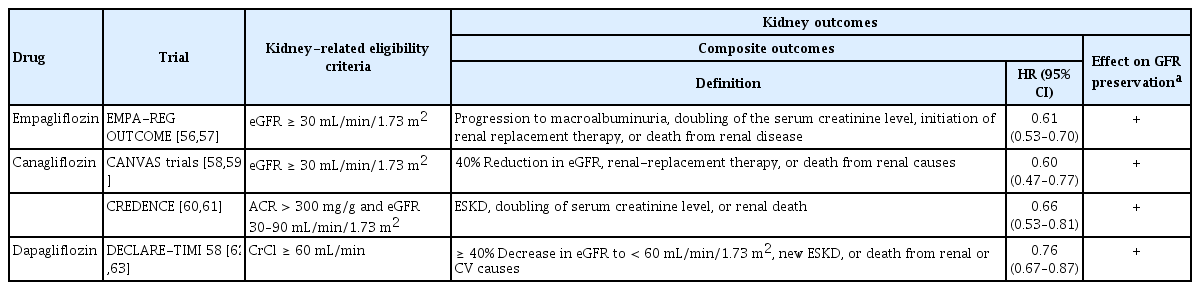
Kidney outcomes from large, placebo-controlled clinical trials in type 2 diabetes mellitus patients using SGLT2 inhibitors
In the Canagliflozin Cardiovascular Assessment Study (CANVAS) program, in which canagliflozin was administered to patients with type 2 DM with an eGFR of > 30 mL/min/1.73 m2, the composite renal outcome (40% reduction in the eGFR, renal replacement therapy, or death from renal causes) was significantly lower in the canagliflozin group than in the placebo group (HR, 0.60; 95% CI, 0.47 to 0.77) [59]. In the CANVAS program, the effects of canagliflozin on the eGFR were similar to those of empagliflozin in the EMPA-REG OUTCOME trial. The eGFR decline was attenuated in the canagliflozin group compared with that in the placebo group, and the eGFR increase after canagliflozin was stopped [60]. Based on the exploratory renal outcome results of the CANVAS program, the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) study was conducted to investigate the renal outcomes of canagliflozin. In patients with type 2 DM with CKD, defined as an eGFR of 30 to < 90 mL/min/1.73 m2 and albuminuria (UACR of > 300 to 5,000 mg/g), the relative risk of the renal-specific composite outcome (ESKD, doubling of the serum creatinine level, or renal death) in the canagliflozin group was 34% lower (HR, 0.66; 95% CI, 0.53 to 0.81; p < 0.001) than that in the placebo group [61]. The decline in the eGFR was faster in the canagliflozin group than in the placebo group in the first 3 weeks but was slower thereafter. According to the post-hoc analysis of the data from the CANVAS program, after week 13, canagliflozin decreased the annual loss of kidney function across the albuminuria subgroups, with a greater absolute reduction in participants with severely increased albuminuria (UACR > 300 mg/g) [61].
The Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) study showed that renal composite events (≥ 40% decrease in the eGFR, to < 60 mL/min/1.73 m2, new ESKD, or death from renal or CV causes) occurred at a lower rate in the dapagliflozin group than that in the placebo group (HR, 0.76; 95% CI, 0.67 to 0.87) [62]. The mean decrease in the eGFR was larger in the dapagliflozin group than in the placebo group 6 months after the randomization [62]. However, the mean changes became comparable after 2 years, and the mean decrease in the eGFR was lower with dapagliflozin than with the placebo after 3 or 4 years. Similar results were observed when the groups were divided according to their baseline eGFR levels. Meta-analyses showed that SGLT2 inhibitors reduced the renal composite outcomes compared to the placebo in individuals with type 2 DM, and the SGLT2 inhibitors were more effective in the group with preserved renal function [63]. Recently, the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial has reported dapagliflozin effects in patients with CKD, with or without type 2 DM [64]. In patients with an eGFR from 25 to 75 mL/min/1.73 m2 and UACR of 200 to 5,000 mg/g, the risk of a renal-specific composite outcome (decline in the eGFR of at least 50%, ESKD, or death from renal causes) was significantly lower with dapagliflozin than with placebo (HR, 0.56; 95% CI, 0.45 to 0.68; p < 0.001). Consistent with the results of previous studies, there was a greater reduction in the eGFR in the dapagliflozin group than in the placebo group during the first 2 weeks. Thereafter, annual changes in the mean eGFR were smaller with dapagliflozin than with the placebo. In the DAPA-CKD study, 32.5% of the patients had CKD without diabetes, and 14.5% had an eGFR of less than 30 mL/min/1.73 m2. Thus, dapagliflozin was shown to be effective at preventing eGFR reductions in patients with CKD and diabetes and in those with advanced CKD.
When SGLT2 inhibitors were initiated, a transient and reversible eGFR decrease, referred to as an eGFR dip, was reversible. However, clinicians are currently worried about an acute dip in the eGFR and how to monitor and manage it. A recent post-hoc analysis of the EMPA-REG OUTCOME results showed a higher proportion of patients with an eGFR dip of ≥ 10% in the empagliflozin group than in the placebo group (28.3% vs. 13.4%, respectively; odds ratio [OR], 2.7; 95% CI, 2.3 to 3.0). However, the long-term eGFR trajectories and safety outcomes, including acute kidney injury (AKI), were similar, regardless of the eGFR dip with empagliflozin treatment [47]. In a post hoc analysis of the CREDENCE study, the eGFR dip of ≥ 10% was more prevalent in the canagliflozin group than in the placebo group (45% vs. 21%, respectively; OR, 3.03; 95% CI, 2.65 to 3.47) [65]. However, the long-term eGFR trajectories and safety outcomes were similar, regardless of the magnitude of the initial eGFR dip. Therefore, when using SGLT2 inhibitors, the initial eGFR dip reflects a protective mechanism of action; it does not increase the risk of AKI and is not considered to be related to kidney injury [66]. There is no need to discontinue the drug or routinely adjust its dosage, unless there is a clinical concern about volume depletion.
Although the underlying mechanisms of the renal protective effects of SGLT2 inhibitors are not completely understood, their glucose-lowering effects and other factors contribute to renal protection [67]. SGLT2 inhibitors have natriuretic and glucose-induced osmotic diuretic effects and consequently, alter renal hemodynamics and reduce blood pressure [68]. In a normal kidney, sodium delivery to macula densa cells of the juxtaglomerular apparatus (JGA) regulates glomerular capillary pressure and the GFR through the phenomenon of the TGF [69]. A decrease in NaCl delivery to the macula densa dilates the afferent arteriole and contracts the efferent arteriole. An increase in NaCl delivery to the macula densa has the opposite effect. Hyperglycemia and diabetes increase glucose levels and the NaCl filtration load, which increases glucose and sodium reabsorption by SGLT2 in the proximal tubule. This decreases the delivery of NaCl to the JGA of the macula densa, thereby causing dilatation of the afferent arteriole and contraction of the efferent arteriole. Eventually, the intraglomerular pressure increases, which can lead to glomerular hypertension, as well as to glomerulosclerosis and decreased renal function, in the long term [70–73]. SGLT2 inhibitors block glucose and sodium reabsorption in the proximal tubule, thus increasing NaCl delivery to the JGA, reversing pathophysiological changes, decreasing intraglomerular pressure, and preserving kidney function [68]. However, there are structural and functional differences in CKD with type 1 and type 2 DM, and patients with type 2 DM are usually older and more obese, with renal hyperfiltration being less likely than in type 1 DM [69]. In a recent randomized study, SGLT2 inhibition in patients with type 2 DM decreased the GFR and filtration fractions without increasing renal vascular resistance, which suggested postglomerular vasodilation rather than preglomerular vasoconstriction [74]. Empagliflozin was shown to attenuate hyperfiltration in patients with type 1 DM with hyperfiltration [75]. However, in patients with type 1 DM with a normal GFR, renal function parameters were not altered by empagliflozin. These hemodynamic effects were consistent with afferent vasoconstriction, conveyed by the TGF, rather than with efferent vasodilatation [75]. A post-hoc analysis of a study that used dapagliflozin adjunct to insulin in patients with type 1 DM showed that the extent of albuminuria decreased in the dapagliflozin group compared with that in the placebo group during a 52-week follow-up period, but there was no significant difference in the eGFR changes between the groups [76]. An initial eGFR dip that was observed with dapagliflozin in patients with type 1 DM subsequently stabilized over time, which resulted in no significant differences in the placebo group. Further studies on the renal protective effects of SGLT2 inhibitors in type 1 DM are required. In addition, SGLT2 inhibitors are thought to be effective in renal protection via other mechanisms. A study showed that SGLT2 inhibitors suppressed hepcidin concentrations and increased erythropoiesis [77], which indicates that more research on the reduction of hypoxic stress by SLGT2 inhibitors is required. SGLT2 inhibitors can also alleviate oxidative stress and proinflammatory and fibrotic changes by reducing lipids in the podocytes by controlling lipotoxicity [78,79]. Furthermore, matrix metalloproteinase 7 and fibronectin 1 levels were reduced in patients with diabetes who were treated with canagliflozin for 2 years compared with those treated with glimepiride [80]. These results suggest that SGLT2 inhibitors may attenuate processes related to inflammation and fibrosis. Several mechanisms are involved in the pathophysiology of DKD, and therefore, it is difficult to control this disease via only one mechanism. The renal protective mechanisms of SGLT2 inhibitors have not been fully elucidated for aspects such as endothelial dysfunction, mitochondrial injury, autophagy, and tubular hypertrophy, and therefore, further studies are required.
Glucagon-like peptide 1 receptor agonists
Glucagon-like peptide 1 (GLP-1) is a gut-derived peptide that is secreted from intestinal enteroendocrine cells in response to nutrients, especially glucose and fat [81]. GLP-1 is an incretin that increases glucose-stimulated insulin secretion by pancreatic β-cells and stimulates β-cell neogenesis and inhibits β-cell apoptosis. GLP-1 receptor agonists (RAs) are an important class of drugs with well-established efficacy and safety profiles in patients with type 2 DM. GLP-1 RAs include short-acting agents, exenatide (twice a day) and lixisenatide (once a day), and long-acting agents, exenatide (once a week), liraglutide (once a day), dulaglutide (once a week), albiglutide (once a week), and semaglutide (once a week) [82]. GLP-1 RAs are either derived from the Gila monster salivary peptide exendin-4 (exenatide and lixisenatide) or via modifications of the human GLP-1 active fragment (liraglutide, dulaglutide, albiglutide, and semaglutide). Lower homology with human GLP-1 presents a greater potential for antibody production and injection-site reaction (pruritus or erythema) [82]. The short-acting agents, the first type of receptor agonist, result in a greater delay in gastric emptying and a reduction in insulin secretion and are consequently more potent at reducing postprandial (vs. fasting) plasma glucose concentrations. The long-acting agents are the 2nd type of receptor agonist, which result in a shorter delay in gastric emptying, predominantly by increasing insulin and decreasing glucagon secretion and are more effective at reducing fasting plasma glucose concentrations. All GLP-1 RAs are administered as subcutaneous injections, but semaglutide is also available in an oral form. Currently, albiglutide and semaglutide have not been approved for use in Korea.
The renal benefits of GLP-1 RAs that have been identified in previous studies are summarized in Table 2. In most cases, renal endpoints were used as prespecified secondary outcomes in CVOTs, similar to that in studies on SGLT2 inhibitors. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial showed that composite renal outcomes (new-onset persistent macroalbuminuria, persistent doubling of the serum creatinine level and an eGFR of ≤ 45 mL/min/1.73 m2, ESKD, or death due to renal disease) occurred in fewer patients with type 2 DM in the liraglutide group than in the placebo group (HR, 0.78; 95% CI, 0.67 to 0.92; p = 0.003) [83]. These results were primarily driven by the new onset of persistent macroalbuminuria (HR, 0.74; 95% CI, 0.60 to 0.91; p = 0.004), while other renal events, including a persistent doubling of the serum creatinine level and an eGFR of ≤ 45 mL/min/1.73 m2, ESKD, or death due to renal disease, were similar between the groups. The eGFR decline was slightly slower in the liraglutide group than in the placebo group. The decrease in the eGFR at 36 months was 7.44 mL/min/1.73 m2 in the liraglutide group, as compare with 7.82 mL/min/1.73 m2 in the placebo group. In the subgroup analysis, the effects of liraglutide on the eGFR, compared with those of the placebo, appeared to be higher in patients with a baseline eGFR of 30 to 59 mL/min/1.73 m2 or in those with macroalbuminuria. However, the eGFR slope was not significantly different between the liraglutide and control groups in patients with an eGFR of ≥ 60 or < 30 mL/min/1.73 m2 or in those with microalbuminuria or normoalbuminuria.

Kidney outcomes from large, placebo-controlled clinical trials in type 2 diabetes mellitus patients using GLP-1 RA
The dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND) trial, showed similar results to those in the LEADER trial [84]. Composite renal outcomes (first occurrence of new macroalbuminuria, a sustained decline in the eGFR of 30% or more from baseline, or chronic renal replacement therapy) occurred less frequently in the dulaglutide group than in the placebo group (HR, 0.85; 95% CI, 0.77 to 0.93; p = 0.0004). These effects were primarily driven by new-onset macroalbuminuria (HR, 0.77; 95% CI, 0.68 to 0.87; p < 0.0001), whereas other renal events were not significantly different between the groups. The eGFR slope was similar between the dulaglutide and placebo groups (p = 0.12). The Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN 6) showed new or worsening nephropathy (persistent macroalbuminuria, persistent doubling of the serum creatinine level and an eGFR of ≤ 45 mL/min/1.73 m2, or a need for renal replacement therapy) in fewer patients with type 2 DM in the semaglutide group than in the placebo group (HR, 0.64; 95% CI, 0.46 to 0.88; p = 0.005) [85]. Across the total study population involved in the SUSTAIN 1–7 trials, semaglutide was associated with initial reductions in the eGFR, followed by a plateau, with a lower long-term decline (week 12 or 16 to week 30 to week 104) [86].
The Exenatide Study of Cardiovascular Event Lowering (EXSCEL) assessed the effects of once-weekly exenatide (EQW) on CV outcomes in patients with type 2 DM [87]. A post-hoc analysis of the EXSCEL trial [88] was performed to examine the EQW effects on the eGFR slope, and changes in albuminuria levels were stratified using the baseline UACR. The mean eGFR decline was similar between the placebo and EQW groups. However, a subgroup analysis showed that EQW treatment slowed the progression of eGFR decline in participants with elevated baseline albuminuria levels (> 100 mg/g) but not in those with a lower degree of albuminuria [88]. The EQW treatment effects on the eGFR slope were higher in participants with baseline albuminuria levels of > 200 mg/g than in those with baseline albuminuria levels of > 100 mg/g.
The dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7) trial was performed in patients with type 2 DM and moderate-to-severe CKD (stage 3–4) to assess the efficacy of once-weekly dulaglutide in glycemic control, its overall safety, and effects on the eGFR decline compared with those of once-daily insulin glargine [89]. Overall, over 52 weeks, the decline in the eGFR was significantly lower in the dulaglutide group than in the insulin glargine group. The results were consistent in the macroalbuminuria group (UACR of > 300 mg/g), but in patients without macroalbuminuria, the eGFR decline did not differ between the dulaglutide and insulin glargine groups at 52 weeks. A reduced eGFR decline was also observed in GLP-1 RA-treated patients with type 2 DM with moderate-to-severe CKD. In addition, the effects of dulaglutide were greater in the group with macroalbuminuria, even in patients with the underlying decreased eGFR. Furthermore, there were no overall between-group differences in glycemic control or blood pressure in the AWARD-7 trial. These findings suggested that GLP-1 RA exerted eGFR preservation effects, regardless of its effects on glycemic control or blood pressure. Taken together, the available data suggest that GLP-1 RAs slow the eGFR decline in participants with moderate-to-severe CKD. This is different from the effects of SGLT 2 inhibitors, which reduce the eGFR slope, regardless of the albuminuria level or eGFR [61,62]. There are several ongoing studies of primary kidney outcomes with GLP-1 RA treatments, which may help address unanswered questions.
Several mechanisms are thought to be involved in the renal protective effects of GLP-1 RAs, including glucose control, as well as the reductions in blood pressure and the body weight [90]. However, GLP-1 RAs may also have glucose-independent renoprotective actions. GLP-1 RAs cause natriuresis via the inhibition of sodium–hydrogen exchanger 3 (NHE3), which is localized to the renal proximal tubule, or cause pressure diuresis [90,91]. This natriuretic effect was eliminated after 12 weeks of treatment with liraglutide [92]. However, lixisenatide, a short-acting GLP-1 RA, maintained natriuresis after 8 weeks of treatment, which may reflect its preserved efficacy via intermittent GLP-1 receptor stimulation [93]. GLP-1 RAs seem to have renal hemodynamic effects, independent of natriuretic effects, in individuals without diabetes. In healthy conditions, GLP-1 RAs cause renal afferent arteriole vasodilation and only mildly influence the TGF [94,95]. However, in diabetes, GLP-1 RAs decreased the GFR and ameliorated glomerular hyperfiltration in both experimental [96] and clinical studies [97,98]. This amelioration may have been due to indirect inhibition of putative vascular and tubular factors that are involved in glomerular hyperfiltration in diabetes. The integrated effects of GLP-1 RAs on renal hemodynamics seem to be the result of direct vasodilative actions and the inhibition of pathways for glomerular hyperfiltration [90]. In addition, the mechanisms of GLP-1 RA-associated kidney protection may include anti-inflammatory effects and the amelioration of oxidative stress [99–101]. Glucose-independent anti-inflammatory and antioxidant effects of GLP-1 RA treatments have been described in patients with type 2 DM. Thus, the levels of C-reactive protein [102], markers of oxidative stress [103], transforming growth factor β1, and type IV collagen [104] are reduced by GLP-1 RA treatment.
The threshold of eGFR for human GLP-1 RA safety is an eGFR of less than 15 mL/min/1.73 m2, but there are no sufficient clinical trial data in patients with DKD with stage 5 CKD or kidney transplantation. Therefore, safety in these specific patients with CKD has not been clearly established. In addition, it is unknown whether SGLT2 inhibitors and GLP-1 RAs will have synergistic renal protective effects when used together in patients with moderate-to-advanced DKD. Thus, more studies are required in patients with advanced CKD and type 2 DM.
Dipeptidyl peptidase-4 inhibitors
Dipeptidyl peptidase-4 (DPP-4) inhibitors are classified as an incretin-based therapy, along with GLP-1 analogs. Following glucose ingestion, the distal small bowel releases incretins, such as GLP-1 and gastric inhibitory polypeptide, which stimulate insulin secretion by β-cells of the pancreas. While GLP-1 analogs exert their action, DPP-4 inhibitors prevent the degradation of incretins [105]. The currently used DPP-4 inhibitors include sitagliptin, vildagliptin, saxagliptin, alogliptin, linagliptin, and gemigliptin.
The effects of DPP-4 inhibitors on renal outcomes, including changes in the eGFR, are summarized in Table 3. The effects of DPP-4 inhibitors in relation to renal outcomes, similar to those of SGLT2 inhibitors or GLP-1 RAs, are usually studied as secondary outcomes in CVOTs. The Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE) trial evaluated the CV safety profile of alogliptin in patients with type 2 DM who had had a recent acute coronary syndrome [106]. Of 5,380 patients, 29.1% had a baseline eGFR of < 60 mL/min/1.73 m2. During the study, changes in the eGFR according to the baseline kidney function were similar between the alogliptin and placebo groups.
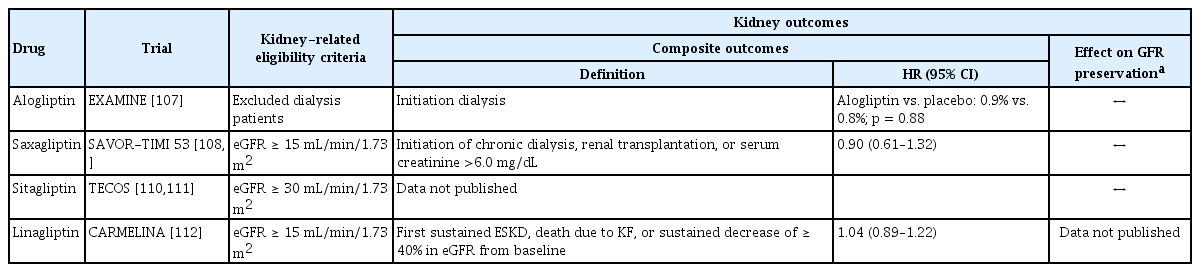
Kidney outcomes from large, placebo-controlled clinical trials in type 2 diabetes mellitus patients using DPP-4 inhibitors
The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction 53 (SAVOR-TIMI 53) trial primarily evaluated the CV safety of saxagliptin versus a placebo in patients with type 2 DM with a history or a significant risk of CV disease [107]. Of 16,492 patients, 15.6% had a baseline eGFR of < 50 mL/min/1.73 m2. According to a post hoc renal outcome analysis for the SAVOR-TIMI 53 trial, compared with the placebo, treatment with saxagliptin was associated with improvement or less deterioration in the albuminuria categories [108]. Furthermore, effects were also observed within the normoalbuminuric range, regardless of glycemic control. However, the overall changes in the eGFR during follow-up were similar between the saxagliptin and placebo groups (p = 0.5794). Renal outcomes, including the doubling in the serum creatinine level, initiation of chronic dialysis, renal transplantation, and a serum creatinine level of > 6.0 mg/dL, were also all found to be similar between the groups.
The Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) was performed to assess the CV safety of sitagliptin versus placebo in patients with type 2 DM and established CV disease [109]. Analysis of renal outcomes in the TECOS study [110] showed that the mean eGFR reduction from baseline over a 4-year period was higher in the sitagliptin group than in the placebo group (−4.0 ± 18.4 mL/min/1.73 m2 vs. −2.8 ± 18.3 mL/min/1.73 m2, respectively). However, the mean eGFR values were slightly lower in the sitagliptin group at the first postrandomization visit and remained consistently lower thereafter. Kidney function decreased at the same rate in both treatment groups, with marginally lower but constant eGFR differences in the participants assigned to the sitagliptin group.
The Cardiovascular and Renal Microvascular Outcome Study with Linagliptin (CARMELINA) trial primarily evaluated the CV safety of linagliptin in patients with type 2 DM with high CV and renal risks [111]. Of 6,979 patients, 47.1% and 15.2% had baseline eGFR values of 30–59 and < 30 mL/min/1.73 m2, respectively, and patients with more advanced CKD were included in the CARMELINA trial. Renal composite outcomes (first sustained ESKD, death due to KF, or a sustained eGFR decrease of ≥ 40% from baseline) were similar between the linagliptin and placebo groups (HR, 1.04; 95% CI, 0.89 to 1.22; p = 0.06). The progression in the albuminuria category occurred less frequently in the linagliptin group than in the placebo group (35.3% vs. 38.5%, respectively; p = 0.003). However, the CARMELINA study did not report differences in the eGFR changes between the two groups. According to a meta-analysis that included RCTs and non-RCTs to evaluate the effects of DPP-4 inhibitors on renal outcomes in patients with type 2 DM, DPP-4 inhibitor treatments were associated with small but significant decreases in the eGFR (p = 0.001) [112].
Evidence from preclinical studies showed that DPP-4 inhibitors reduced fibrosis, oxidative stress, endothelial dysfunction, and inflammation in the kidneys [113]. These effects may be contributing factors to the reduction in albuminuria levels by DPP-4 inhibitors in DKD. In addition, DPP-4 inhibitors could stimulate natriuresis in patients with type 2 DM, exerting their effects on the distal tubule [114], which differs from the effects of SGLT2 inhibitors that act on the proximal tubule. However, there are several conflicting studies. DPP-4 inhibitors were found to promote natriuresis in animals [115,116] and in patients with type 2 DM [114]. By contrast, the natriuretic response to a DPP-4 inhibitor was found to be blunted in obese type 2 DM model mice in a separate study [96]. The natriuretic effects of sitagliptin lasted for up to 2 weeks and then disappeared before the 12-week assessment in patients with type 2 DM [92]. Therefore, DPP-4 inhibitors could alleviate albuminuria via anti-inflammatory, antioxidant, and antifibrotic effects, without significant changes in renal hemodynamics.
CONCLUSIONS
Tracking eGFR changes in DKD may improve the ability to predict the development of hard renal endpoints, such as KF or renal death. In this respect, understanding the trends in GFR changes could be crucial for developing clinical management strategies for the prevention of diabetic complications. Several mechanisms cause a decline in renal function in DKD; therefore, it is difficult to control the decline using only one drug, and drug combinations will likely be required to slow down or prevent renal events in patients with type 2 DM. Previous studies on the use of new drugs in patients with DKD have been heterogeneous, and most renal outcomes were prespecified secondary outcomes from recent CVOTs. Consequently, more research is required to understand the beneficial effects of these drugs on kidneys. Based on the GFR-related clinical endpoints that have been used so far, the striking development of new innovative medical technologies is expected to lead to the discovery of suitable markers for effective kidney protection in the near future, followed by the development of novel drugs.
Acknowledgments
This study was supported by National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIT: 2021R1F1A1061372 to Hyo Jin Kim; 2018R1C1B6002854 and 2021R1A2C1095259 to Sang Soo Kim; and MSIP: 2016R1A2B4008243 to Sang Heon Song).
Notes
No potential conflict of interest relevant to this article was reported.
