Risk factors and clinical impact of COVID-19-associated pulmonary aspergillosis: Multicenter retrospective cohort study
Article information
Abstract
Background/Aims
The risk factors and clinical impacts of coronavirus disease 2019 (COVID-19)-associated pulmonary aspergillosis (CAPA) remain controversial, and no data have been reported in Korea. This study aimed to investigate the epidemiology and importance of CAPA diagnostic efforts and to identify the predictors of CAPA and the impacts on clinical outcomes.
Methods
Between January 2020 and May 2021, data of severely to critically ill COVID-19 patients were extracted from seven hospitals of the Catholic Medical Center through a clinical data warehouse. Corticosteroid use was subcategorized into total cumulative dose, early 7-day dose, mean daily dose, and duration of use.
Results
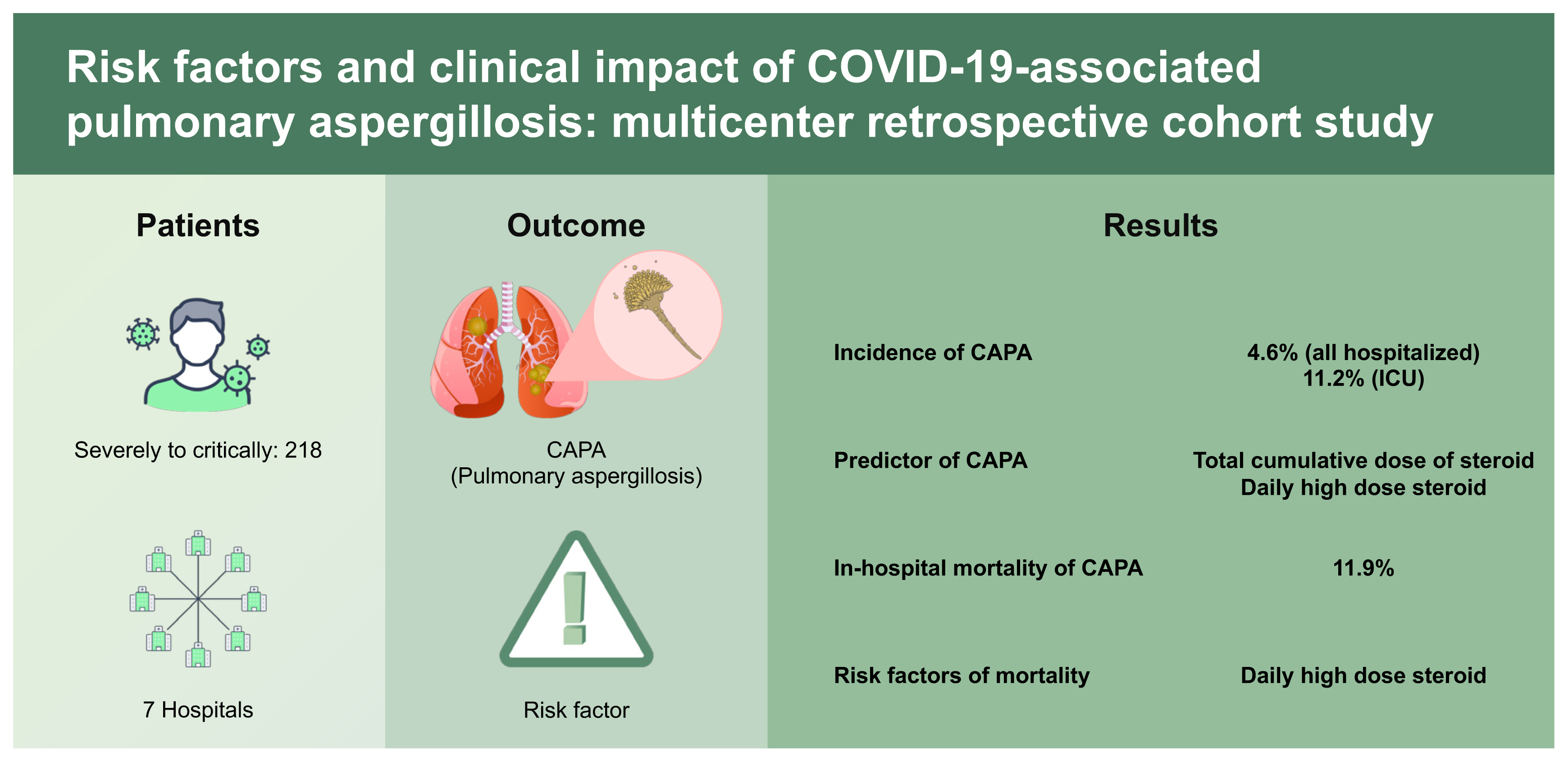
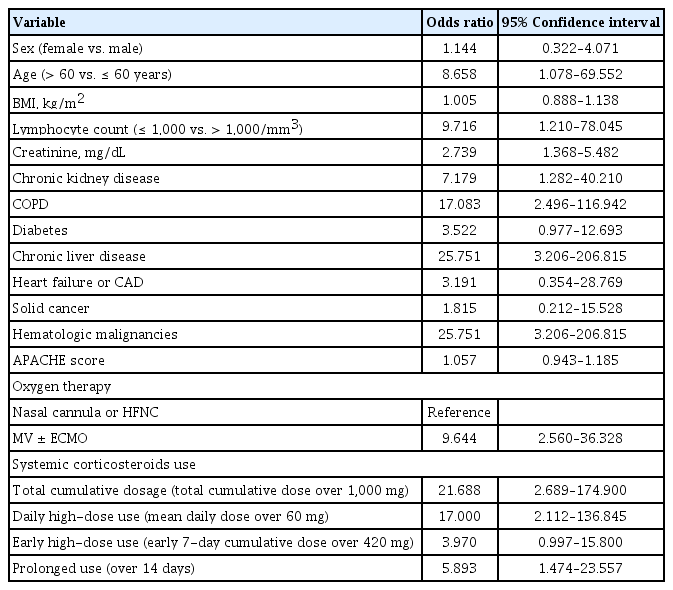
A total of 2,427 patients were screened, and 218 patients were included. CAPA was diagnosed in 4.6% (10/218) of all hospitalized and 11.2% (10/89) of intensive care unit patients. Total cumulative dose (over 1,000 mg as methylprednisolone) and daily high-dose corticosteroid use (over 60 mg/day) were independent predictors but not early 7-day high-dose corticosteroid use (over 420 mg/week) (odds ratio [OR], 1.731; 95% confidence interval [CI], 0.350 to 8.571) nor prolonged use (OR, 2.794; 95% CI, 0.635 to 13.928). In-hospital overall mortality was 11.9% (26 of 218). CAPA itself did not affect the outcome; rather, daily high-dose steroid use significantly increased the 30-day mortality (hazard ratio, 5.645; 95% CI, 1.225 to 26.091).
Conclusions
CAPA was not uncommon, especially in critically ill patients. Daily high-dose corticosteroid use was the predictor of CAPA and associated with high mortality rates. High-dose corticosteroids use after early inflammatory phase should be avoided, and active surveillance methods for CAPA are essential for those high-risk patients.
INTRODUCTION
After the first case of coronavirus disease 2019 (COVID-19) was confirmed in Korea in January 2020, the country had the second-highest number of COVID-19 patients in the world until early February 2020 [1,2]. As of March 11, 2022, a cumulative total of 5,539,650 cases of COVID-19 had been confirmed in Korea, with a case fatality rate of 0.17% [3]. Bacterial and fungal superinfections are life-threatening infectious complications in severe respiratory viral infections [4]. Approximately 5% of patients with COVID-19 are critically ill and require intensive care management; therefore, concerns about superinfections with COVID-19 have persisted. Secondary or co-infection with other respiratory pathogens in severe COVID-19 patients has been reported, and research on COVID-19-associated pulmonary aspergillosis (CAPA) has been documented from the early period of the pandemic [4–6].
Previous studies have reported that CAPA occurs in approximately 10% of critically ill COVID-19 patients admitted to the intensive care unit (ICU), with a mortality rate of 54% [7,8]. The incidence of CAPA was higher than that of invasive pulmonary aspergillosis (IPA) in critically ill patients without a diagnosis of COVID-19 (1% to 7%), and the mortality rate of CAPA was even higher than that of IPA in patients with hematologic malignancies [9,10]. Advanced age, chronic obstructive pulmonary disease (COPD), high Sequential Organ Failure Assessment score, and high Acute Physiology and Chronic Health Evaluation II score have been suggested as risk factors for CAPA [6,10–12].
However, most studies were case series or retrospective studies conducted in Europe with a focus on critical COVID-19 patients. Therefore, regional differences and the varying epidemiology of patients with severe COVID-19 have not yet been fully elucidated. Systemic corticosteroids have been widely used as standard care for severe COVID-19 patients after the clinical study by Randomized Evaluation of COVID-19 Therapy [13], but their impact on the development of CAPA has not been thoroughly determined [8,14]. Furthermore, it remains controversial whether the high mortality rate of CAPA is due to CAPA itself or other factors such as advanced age and comorbidities [10,12].
This study aimed to evaluate the epidemiologic trend of CAPA in Korea and clarify the importance of diagnostic efforts. In addition, we conducted this study to identify the predictors of CAPA and risk factors for mortality in patients who were severely to critically ill COVID-19.
METHODS
Study population and clinical data warehouse
This multicenter retrospective study was conducted at seven Catholic Medical Center (CMC) hospitals in Korea. Of the seven hospitals, three were designated for severely and critically ill patients, two were designated for moderately and severely ill patients during study period. Between January 2020 and May 2021, data of patients admitted for the first time after a diagnosis of COVID-19 were retrospectively analyzed. A clinical data warehouse (CDW) platform was used to access and extract information. The CDW, which incorporates seven affiliated hospitals under CMC in Korea, is a data platform that collects and distributes clinical data to researchers [15]. It includes more than 15 million electronic medical records that were completely anonymized. The target patients were defined as adult patients (18 years or older) with the International Classification of Diseases, 10th revision (ICD-10) codes indicating COVID-19 (U071, 072, 109) and (1) were first hospitalized due to COVID-19; (2) excluded patients who received treatment only at the residential treatment center; (3) excluded mild to moderately ill patients; and (4) excluded patients who were transferred to another hospital immediately after COVID-19 diagnosis (Fig. 1). The study protocol was approved by the Institutional Review Board of the Catholic University of Korea (No. KC21WIDI0484). We used only anonymous clinical data; therefore, informed consent was waived.
Study definitions
To define and manage CAPA, the European Confederation of Medical Mycology and the International Society for Human and Animal Mycology (ECMM/ISHAM) proposed proven, probable, and possible CAPA criteria [16]. The ECMM/ISHAM consensus criteria were applied in this study, and proven and probable categories were only included as CAPA cases in this study. The European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group criteria were also applied in patients with hematologic malignancies. The severity of illness was categorized according to the National Institutes of Health guidelines, and severely to critically ill patients were included in this study [17]. Critically ill patients were defined as patients requiring high-flow oxygen therapy (FiO2 ≥ 0.4 with flow rate ≥ 30 L/min) via nasal cannula (high-flow nasal cannula [HFNC]) or mechanical ventilation (MV).
Chronic liver disease (CLD) was defined as patients with chronic hepatitis B or C or liver cirrhosis taking therapeutic drugs, and chronic kidney disease was defined as stage 3 or higher. Systemic corticosteroid use was analyzed by subcategorized criteria: (1) The total cumulative steroid dose was defined as the total usage from admission to discharge; (2) Early high-dose corticosteroid use was defined as a total of 420 mg or more of methylprednisolone or the equivalent dose of another corticosteroid use within 7 days; (3) Daily high-dose corticosteroid use was defined as 60 mg or more of methylprednisolone or the equivalent use on average per day during the hospitalization as many institutes are widely using corticosteroids up to 60 mg per day [18,19]; (4) Prolonged use was defined as the use of systemic corticosteroids for more than 14 days.
The agreement rate and appropriateness of the operational definition for CAPA were further analyzed through CDW for further nation-wide study. The operational definition of CAPA was patients with diagnosis codes for COVID-19 (under ICD-10; U071, 072, 109) who were prescribed anti-mold active agents for more than 2 weeks during admission. Anti-mold active agents included voriconazole, isavuconazole, posaconazole, itraconazole as triazole, and amphotericin B deoxycholate and liposomal amphotericin B as polyene. Echinocandins were excluded due to the high possibility of overestimation as they were used as first-line drugs for invasive candidiasis. Patients who had been prescribed anti-mold active agents for more than 2 weeks within the 6 months prior to their diagnosis of COVID-19 and patients with diagnosis code for mucormycosis (B46 in ICD-10) during hospitalization for COVID-19 were excluded. The agreement rate was analyzed by comparing this operation definition with the consensus definition of CAPA (Supplementary Fig. 1).
Active surveillance for invasive fungal disease in patients with COVID-19 in the ICU
Mycological surveillance of ICU patients with severely or critically ill COVID-19 patients who admitted in ICU has been performed only at Seoul St. Mary’s Hospital, since June 2020. To enhance the sensitivity and specificity, serum galactomannan (GM) antigen, (1–3)-β-D-glucan antigen (BDG), and fungal culture from respiratory samples were performed twice a week. Serum GM assay (Platelia Aspergillus EIA, Bio-Rad, Hercules, CA, USA) and BDG test (Gold Mountain River Tech Development, Beijing, China) were performed following the manufacturer’s instructions, using a positivity threshold of ≥ 0.5 optical density index in serum for GM and ≥ 80 pg/mL for BDG. Surveillance for invasive fungal diseases ended after ICU discharge. Diagnosis and treatment of CAPA were determined by an infectious disease physician and an intensive care physician based on consensus criteria.
Statistical analysis
The baseline characteristics are presented as medians with interquartile ranges (IQRs) for continuous variables and as frequencies with percentages for categorical variables. Normal distribution was checked using the Shapiro–Wilk test, and the Wilcoxon rank-sum test was used to compare nonparametric continuous variables. Chi-square or Fisher ‘s exact tests were used for categorical variables. Survival analysis curves were fitted using the Kaplan–Meier method, and the log-rank test was performed to analyze differences. The risk of mortality was assessed using a multivariable-adjusted proportional hazards model with hazard ratio (HR) and 95% confidence interval (CI). The proportional hazards assumption was evaluated by a log-log plot, and there was no significant proportionality in hazards over time. A binary logistic regression model was used to identify the predictors of CAPA. The degree of agreement between CAPA by ECMM/ISHAM consensus criteria and operational definition was measured using Cohen’s kappa. All statistical analysis were performed based on the consensus definition of CAPA. Statistical significance was set at p < 0.05. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Baseline characteristics
Over the study period, 2,427 patients were admitted to the CMC with COVID-19. After excluding mild to moderately ill patients and patients treated only at the resident treatment center, 218 patients were included: 137 patients with severe COVID-19 and 81 patients with critical COVID-19. Patient demographics, clinical findings, and steroid use are shown in Table 1 and Supplementary Table 1. All patients received oxygen therapy upon admission. Only 26.6% (58/218) of patients had serum GM tests regardless of results, and bronchoscopy was performed in 2.3% (5/218). In total, 126 (57.8%) of 218 patients were administered systemic corticosteroids with a median cumulative dose of 300 mg methylprednisolone and 9 days as the median duration. The incidence of CAPA by consensus definition was 4.6% (10/218) of all admitted patients and all the CAPA cases occurred in ICU patients (11.2%, 10/89): nine cases occurred in critically ill COVID-19 patients and only one case occurred in severe patients. The median time to diagnosis after admission was 9.5 days with an IQR of 7.0 to 12.0. In the patients who developed CAPA, median age, lymphopenia (< 1,000/mm3), comorbidities such as COPD, CLD, hematologic malignancy, systemic corticosteroid use (total cumulative dose, early high-dose corticosteroid use, daily high-dose corticosteroid use, and duration), and mortality were higher than in patients without CAPA (all p < 0.05). CAPA occurred only in the systemic corticosteroid use group with median mean daily dose 119.3 mg methylprednisolone (range, 34.3 to 405.7 mg). Of the 218 patients, 32.1% (70/218) received over 1,000 mg of methylprednisolone during their hospitalization, 37.2% (81/218) received daily high-dose corticosteroids, and 38.5% (84/218) received early high-dose corticosteroids. All the mycological evidence for the diagnosis of CAPA was supported by serum GM tests. Nine isolates of Aspergillus species were cultured: five isolates of Aspergillus niger from deeply expectorated sputum (three from adequate specimen and two from inadequate specimen), three isolates of Aspergillus fumigatus from tracheal aspirate (two from adequate specimen and one from inadequate specimen), and one Aspergillus flavus from sputum (from inadequate specimen).
Predictors of CAPA
In the univariate analysis, the development of CAPA was significantly associated with age over 60 years, lymphopenia, the presence of underlying comorbidities, undergoing MV, and systemic corticosteroid use (Table 2). Systemic corticosteroid use was the only modifiable predictor of CAPA, and its use was further analyzed by categorization: total cumulative dose, early high-dose use, daily high-dose use, and duration of use. Total cumulative dose, daily high-dose use and duration of use were predictors of CAPA in univariate logistic regression analysis but not early high-dose use. After adjusting for covariates, total cumulative dose and daily high-dose corticosteroid use were still independent predictors of CAPA, but early high-dose use (odds ratio [OR], 1.731; 95% CI, 0.350 to 8.571 after adjusting oxygen therapy) and prolonged use was not (OR, 2.974; 95% CI, 0.635 to 13.928 after adjusting for oxygen therapy and OR, 3.661; 95% CI, 0.865 to 15.498 after adjusting for age) (Supplementary Table 2).
Outcomes and risk factors for mortality
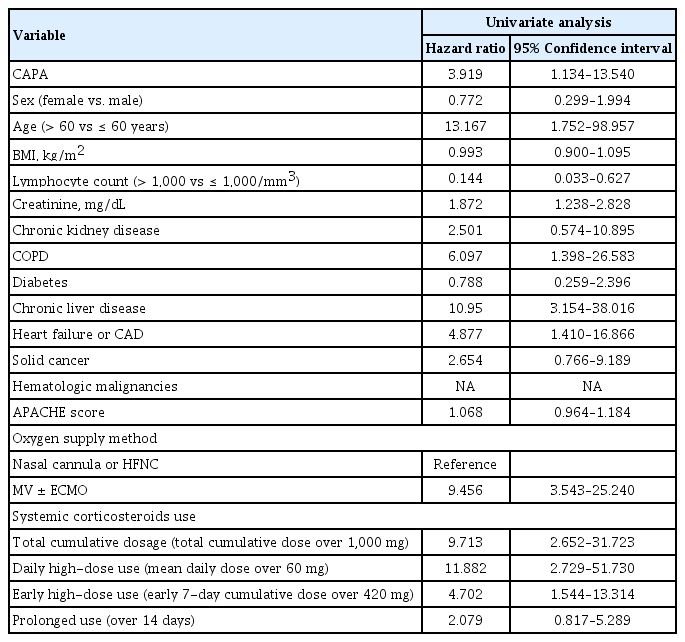
The overall in-hospital mortality for COVID-19 patients was 11.9% (26/218) and the mortality rate was higher in patients with diagnosed with CAPA: 50% (5/10) of patients with CAPA vs. 10.1% (21/208) of patients without CAPA (OR, 8.905; 95% CI, 2.308 to 33.305). The 30-day all-cause mortality in patients with CAPA was also significantly lower than that in patients without CAPA (30% [3 of 10] of patients with CAPA vs. 7.2% [15 of 208] of patients without CAPA), as determined by survival analysis using the Kaplan–Meier method (log-rank test p = 0.019) (Fig. 2). In the univariate proportional hazard model, CAPA was also a significant risk factor for mortality (HR, 3.919; 95% CI, 1.134 to 13.540). Except for CAPA, age over 60 years, lymphopenia, presence of underlying comorbidities, undergoing MV, and systemic corticosteroid use were significantly associated with overall mortality in the univariate analysis (Table 3). In multivariable models, oxygen therapy independently increased mortality, while CAPA only showed an increasing trend in mortality rather than statistical significance. In the categories of steroid use, only daily high-dose corticosteroid use was significantly associated with mortality. Detailed multivariate proportional hazard models are presented in Table 4.

Thirty-day survival for patients with coronavirus disease 2019 (COVID-19) by COVID-19-associated pulmonary aspergillosis (CAPA) diagnosis.
Agreement analysis between operational definition and consensus definition
We assessed the consistency between the operational definition of CAPA and ECMM/ISAHM consensus definition. The incidence of CAPA by operational definition was 5.5% by operational definition and 4.6% by ECMM/ISHAM consensus definition. The degree of agreement by Cohen’s kappa was 0.713 with 95% CI 0.494 to 0.931 which showed substantial level of agreement (Supplementary Fig. 1).
DISCUSSION
In this retrospective multicenter study, the incidence of CAPA was 4.6% in all hospitalized patients and 11.2% in all ICU patients. We also demonstrated the importance of clinical awareness and surveillance methods for CAPA diagnosis based on the incidence between study hospitals. Systemic use of corticosteroids was the only modifiable predictor for CAPA development (total cumulative dose over 1,000 mg and daily high-dose use over 60 mg) and risk factor (daily high-dose use over 60 mg) for mortality related to COVID-19. However, CAPA itself did not independently affect the outcome. The results of this study highlighted that daily high-dose corticosteroid use for more than 7-day may strongly affect the occurrence of CAPA and the overall mortality. It is mandate to include the superinfections such as CAPA and potential adverse effects of overusing systemic corticosteroid based on accumulated data in the guidelines for COVID-19.
IPA following respiratory viral infection has been reported in patients with influenza, severe acute respiratory syndrome, and adenoviral pneumonia [20,21]. In previous studies, the incidence of CAPA in COVID-19 patients admitted to the ICU varied from 3%–39% and 0.7%–7.7% in all hospitalized patients [7,10]. This was mainly based on studies conducted in Europe, where experienced medical staff and equipment are readily available for IPA diagnosis. Therefore, it was difficult to estimate the differences according to medical resources and regional differences. The results of this study showed that the incidence of CAPA (4.6% among all hospitalized COVID-19 patients and 11.2% in the ICU) and median time to CAPA diagnosis from admission (9.5 days) were consistent with those of previous studies, and it was difficult to find significant regional differences [7,10,22]. However, the incidence in this study could be underestimated due to a couple of reasons: First, some of the time period included in this study was before the widespread use of immunomodulating agents such as corticosteroids, interleukin 6 (IL-6) monoclonal antibodies, IL-1 receptor antagonists, and Janus kinase inhibitors which are known to affect CAPA occurrence [7,23]. Second, there was a lack of awareness of CAPA in the early period of the COVID-19 pandemic, and only one hospital performed a surveillance under high index of suspicion for CAPA in ICU patients.
Although the overall incidence was consistent with previous studies, there was a notable difference in CAPA incidence between hospitals. In the one hospital that performed active surveillance for CAPA, the incidence was similar to that found in previous studies (13.4%, 9/67), but in the other hospitals that did not perform active surveillance, the CAPA incidence was substantially lower (0.6%, 1/151). There are limitations in early diagnosis due to atypical features of imaging tests and difficulties in defining mycological factors such as GM and polymerase chain reaction (PCR) tests [6,11,12]. Therefore, active surveillance has been emphasized in high-risk patients for early diagnosis and treatment of CAPA, but there is no universal surveillance protocol [16,22]. In one of the hospitals in this study, active surveillance was performed twice a week with serum GM, BDG, and culture (sputum or tracheal aspirate) for severely and critically ill COVID-19 patients in the ICU receiving oxygen therapy via HFNC or mechanically ventilated. If the test was positive, CAPA was diagnosed after a consultation with an infectious disease specialist based on ECMM/ISHAM consensus criteria. The incidence of CAPA in the hospital that performed active surveillance was similar to that of previous studies, but it showed a distinct difference in other hospitals that did not undergo surveillance, confirming the importance and appropriateness of the surveillance method using serologic tests and culture in severely or critically ill COVID-19 patients [6,11,22,24].
The cornerstone of the mycological diagnosis of CAPA in the ECMM/ISHAM criteria is culture, GM, and PCR using bronchoalveolar lavage. However, many institutions in this study cannot actively perform bronchoscopy for CAPA diagnosis because of safety issues such as aerosol generation and lack of professional medical staff and equipment [16,22]. Bronchoscopy was performed in only 5% of cases in this study, and all mycological evidence for probable CAPA was based on serum GM. Serum GM is readily available in many institutions, and its effectiveness has already been confirmed in influenza-associated pulmonary aspergillosis diagnosis, similar to CAPA [25,26]. Since low sensitivity of serum GM was reported due to the lack of angioinvasion of Aspergillus in non-immunocompromised patients, previous studies suggested the use of serum GM along with serum BDG or PCR results to enhance sensitivity and specificity [5,9,27,28]. Thus, diagnostic efforts through high index of suspicion with serological tests may be effective supplementary method for diagnosing CAPA in a resource-limited setting [22,27,29].
Monoclonal antibody and remdesivir were widely used in mild to moderate COVID-19 patients, whereas systemic corticosteroids became the standard treatment in severe to critical COVID-19 patients [14,30,31]. Previous studies reported that no significant difference in secondary infections, including CAPA, due to systemic corticosteroid use, but there was still limitation in the analysis between systemic corticosteroid and CAPA development as the type, doses, frequency and duration of corticosteroid administration were not well described [8]. Furthermore, it was difficult to estimate the effect of long-term and high-dose corticosteroid use in real-world as most of these results were derived from studies with a low-dose use (100 mg as prednisolone in patients with CAPA vs. 107 mg without CAPA in prospective study) or short period of use (dexamethasone 6 to 10 mg up to 10 days in clinical trial) [13,32,33]. In the univariate analysis in this study, age over 60 years, lymphopenia, MV, and comorbidities such as COPD, chronic kidney disease, CLD, and hematologic malignancy had a statistically significant impact on CAPA development, which is consistent with the findings of previous studies [5,7,8]. As predictors of CAPA, the dose and duration of systemic corticosteroids were the only significant and modifiable factors. In multivariate analysis adjusted for covariates, higher total cumulative doses of corticosteroid use and daily high-dose corticosteroid use were significant independent predictors and CAPA only diagnosed when the mean daily dose exceeded 30 mg as methylprednisolone. However, an early high-dose corticosteroid use did not independently affect the occurrence of CAPA. Previous studies have raised concerns about the association between systemic corticosteroid use and the incidence of CAPA [27]. In the influenza-associated pulmonary aspergillosis study, similar to CAPA, it was confirmed that systemic corticosteroid use is a significant risk factor for the occurrence of IPA in patients with severe influenza [21]. Therefore, limited use of systemic corticosteroids is recommended in patients with severe influenza, and administration after IPA exclusion through GM is recommended for acute respiratory distress syndrome (ARDS) patients who need corticosteroid use [21]. In this study, we presented the risk of systemic corticosteroid not only by the total cumulative dose, also the cumulative dose over 7 days, mean daily dose and duration of use. Early use of high-dose steroids due to control hyperinflammatory response with COVID-19 might have minimal effect on the development of CAPA, but high-dose systemic corticosteroid therapy after early inflammatory phase is strongly associated with the development of CAPA. Although, administration of high-dose corticosteroids to control the initial inflammatory response is inevitable, efforts to reduce and stop systemic corticosteroid is important when treating severe COVID-19 patients [34]. As in previous clinical trial [13], we recommend not to exceed an mean daily dose of 30 mg/day or less or at least 60 mg/day to prevent CAPA. Furthermore, rigorous surveillance for CAPA is necessary for the patients with prolonged administration of high-dose corticosteroids.
The overall mortality of patients with CAPA was 54.9%, and some studies have reported up to 100%, which was higher than the mortality of IPA in patients with hematologic malignancies [10]. Previous studies found that CAPA was associated with a 16% to 25% excess in mortality, which is similar to that found in patients with influenza-associated pulmonary aspergillosis [27,32,35]. However, there was a lack of analysis on whether the high mortality rate was the impact of CAPA itself, or whether it was due to age and underlying diseases that affect the development of CAPA [5,7,10]. In this study, the in-hospital overall mortality of patients with CAPA was 50%, and the occurrence of CAPA had a significant impact on 30-day overall survival in univariate analysis. However, the impact of CAPA on mortality only showed an increasing trend in multivariate analysis using the proportional hazard model. In univariate analysis, age over 60 years, COPD, elevated creatinine, CLD, heart failure or coronary artery disease, oxygen therapy and systemic corticosteroid use (total cumulative dose, early high-dose use and daily high-dose use) were associated with high mortality rates, consistent with previous studies [36–38]. However, oxygen therapy and daily high-dose of systemic corticosteroids use were identified as independent risk factors for mortality in the multivariate analysis. The survival benefit of using systemic corticosteroids in severely to critically ill COVID-19 patients in previous studies was about 6 mg of dexamethasone (30 mg of methylprednisolone) for 10 days, but the actual use of steroids in clinical practice was higher than this [8,13]. This trend seems to be due to results of previous studies indicating that the use of high-dose corticosteroids has improved mortality in ARDS due to COVID-19 [18]. However, the effect of high-dose corticosteroid use on the survival rate of COVID-19 remains controversial [19]. In this study, daily high-dose systemic corticosteroid use increased mortality after adjusting for covariates and this was the only modifiable risk factor for mortality but not early high-dose use. In this regard, we suggest that it is necessary to reduce or stop systemic corticosteroid after early inflammatory phase in patients with severe to critical COVID-19 to improve survival.
This study had some limitations. First, although this study was performed based on a multicenter design, confounders could not be fully adjusted due to a limited number of patients with CAPA. Second, we did not evaluate the impact of the delta variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and immunomodulating agents such as IL-6 inhibitors on CAPA development as the delta variant was not a dominant strain and immunomodulators were not widely used during much of the study period. Finally, this study was a retrospective study based on a big-data platform. This makes it difficult to determine whether there is a causal relationship between high-dose corticosteroid use and excess mortality. In addition, it was not possible to analyze the antifungal prescription pattern or the agreement rate of the operational definition by study hospital. Despite these limitations, our study has several strengths. The results reflect the incidence and predictors of CAPA in Korea with a sizable cohort based on clinical data. Second, we highlight the importance of active surveillance and clinical suspicion of CAPA for severely and critically ill COVID-19 patients through comparisons of incidence between study hospitals. Lastly, we identified and specified the impact of systemic corticosteroids on the occurrence and outcome of COVID-19 and CAPA.
In conclusion, CAPA is not uncommon in Korea, and clinical awareness with active surveillance methods for CAPA diagnosis is essential for early diagnosis and treatment especially in ICU patients. The use of systemic corticosteroids is inevitable for the management of severely and critically ill COVID-19 patients in the early inflammatory period, and early high-dose corticosteroid use may be acceptable as it did not significantly influence the occurrence of CAPA and the mortality. However, daily high-dose corticosteroids use after the early inflammatory phase should be avoided to prevent CAPA and reduce mortality.
KEY MESSAGE
1. Clinical awareness of and active surveillance for coronavirus disease 2019 (COVID-19)-associated pulmonary aspergillosis (CAPA) in severely to critically ill COVID-19 patients are essential as the incidence of CAPA was up to 11.2% in intensive care unit patients.
2. Daily high-dose corticosteroid use (over 60 mg as methylprednisolone per day) was significantly associated with the occurrence of CAPA and an independent risk factor for the outcome but not early high-dose use (over 420 mg as methylprednisolone per week).
3. High-dose corticosteroid use after early inflammatory phase needs to be avoided to prevent CAPA and improve survival, and rigorous CAPA surveillance is necessary for high-risk patients with COVID-19.
Acknowledgments
This work was supported by the Korea National Enterprise for Clinical Trials grant funded by the Korea government (Ministry of Health and Welfare) (No.HE21C0006). This study was supported by the Research Fund of Seoul St. Mary’s Hospital, The Catholic University of Korea. We acknowledge the Catholic Information Convergence Institute for its assistance in providing and analyzing the CDW data. We also would like to thank the department of Occupational and Environmental Medicine and Jun-Pyo Myong for their consultation, statistical and technical support for this study.
Notes
No potential conflict of interest relevant to this article was reported.