Real-life experience of ledipasvir and sofosbuvir for HCV infected Korean patients: a multicenter cohort study
Article information
Abstract
Background/Aims
To evaluate the efficacy and safety of ledipasvir/sofosbuvir (LDV/SOF) therapy in hepatitis C virus (HCV)-infected Korean patients in a real clinical setting.
Methods
A total of 273 patients who received LDV/SOF therapy between May 2016 and February 2021 were consecutively enrolled and analyzed. A per-protocol analysis was performed to evaluate the virologic response.
Results
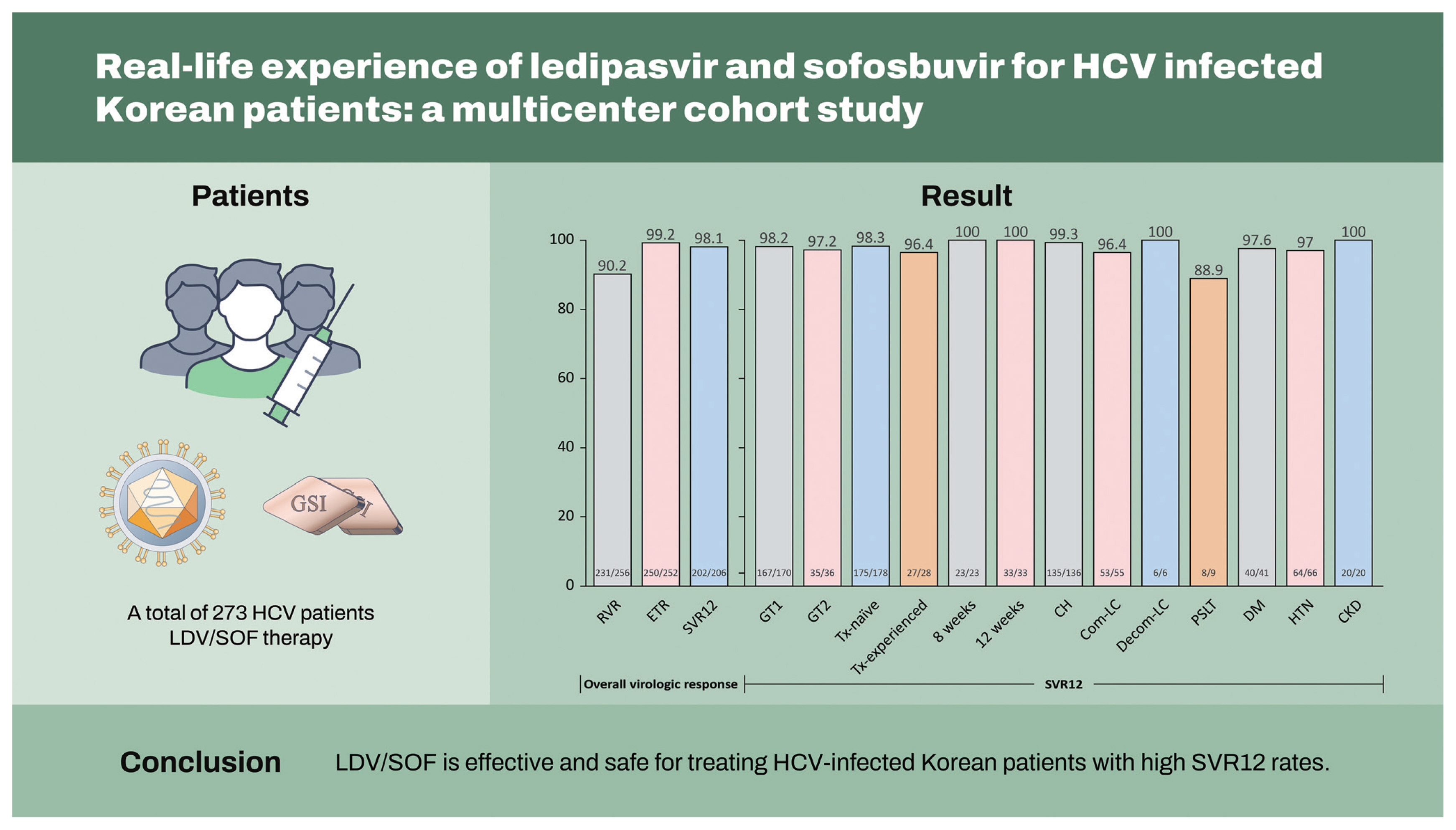
Seventy-five percent were infected with genotype 1, and 25% were infected with genotype 2. A hundred eighty-one (66.3%) patients had chronic hepatitis, 74 (27.1%) had compensated cirrhosis, eight (2.9%) had decompensated cirrhosis, and 10 (3.7%) had undergone liver transplantation. Undetectable HCV RNA at week 4 was achieved in 90.2% (231/256) of patients, 99.2% (250/252) achieved the end of treatment response, and 98.1% (202/206) achieved sustained virologic response at 12 weeks post-treatment (SVR12). According to liver function, the SVR12 rates were 99.3% (135/136) in chronic hepatitis, 96.4% (53/55) in compensated cirrhosis, and 100% (6/6) in decompensated cirrhosis. The SVR12 rates according to the genotype were 98.2% (167/170) for genotype 1 and 97.2% (35/36) for genotype 2. An 8-week LDV/SOF treatment in treatment-naïve chronic hepatitis patients with HCV RNA < 6,000,000 IU/mL at baseline resulted in 100% (23/23) SVR12 rates. Overall, LDV/SOF was tolerated well, with a 0.7% (2/273) discontinuation rate due to adverse events that were unrelated to LDV/SOF.
Conclusions
LDV/SOF is effective and safe for treating HCV-infected Korean patients with high SVR12 rates.
INTRODUCTION
Hepatitis C virus (HCV) infection is a major cause of chronic liver disease worldwide; it can lead to liver cirrhosis (LC), liver failure, and hepatocellular carcinoma (HCC) [1,2]. The global prevalence of HCV infection was approximately 1.0% in 2015, corresponding to 71.1 million patients with chronic viremic infection [3]. Recent advances in the development of direct-acting agents (DAAs) have revolutionized HCV treatment, showing excellent efficacy and safety [4–6]. Based on these remarkable results, the World Health Organization announced a strategy to eliminate HCV infection by 2030 [7,8].
HCV infection is a major chronic liver disease, with an estimated prevalence rate of 0.5% between 2012 and 2016 in Korea. Based on the genotype (GT) distribution, GT1 (52.7% of all infections) and GT2 (45.3%) were dominant in Korea [3,9–11]. More than 90% of the patients infected with HCV were over 40 years of age, and approximately 70% of the patients had chronic hepatitis, followed by 20% with LC [12]. Therefore, for the successful elimination of HCV infection in Korea, a potent DAA showing high efficacy across GT1 and 2 is required regardless of the presence of LC.
Ledipasvir/sofosbuvir (LDV/SOF), a potent DAA, is a combination of an NS5A inhibitor (LDV) and nucleotide analog NS5B polymerase inhibitor (SOF), showing promising efficacy and safety for treating patients with GT1 HCV infection. In phase III clinical trials, the overall sustained virologic response (SVR) after 12 weeks of LDV/SOF were 98%–99% and 96%–97% in treatment-naïve and treatment-experienced GT1 HCV patients, respectively [13–16]. The high efficacy of the LDV/SOF persisted irrespective of LC [17,18]. Moreover, the administration of 12 weeks of LDV/SOF for patients with GT2 HCV infection also provided a high SVR rate (98%), regardless of the fibrosis stage and treatment history, in an integrated analysis of three clinical trials [19].
According to these encouraging results, the Korean Association for the Study of the Liver (KASL) recommends LDV/SOF as a first-line regimen with a single once-daily dosage for treating of HCV infection, except for patients with GT3 [20,21]. However, there have been limited real-world data presenting the treatment efficacy and safety of LDV/SOF in clinical practice. Herein, we assessed the effectiveness and safety of LDV/SOF for patients with HCV infection in a multicenter cohort study in Korea.
METHODS
Study population
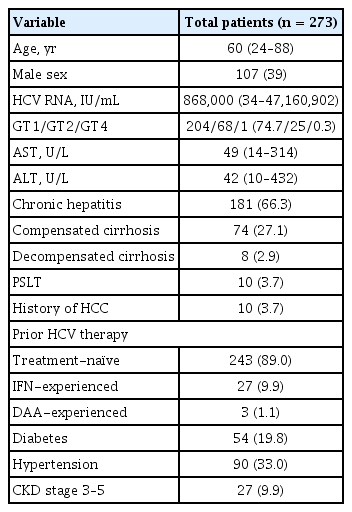
In this retrospective multicenter study, 273 patients with HCV infection who were treated with LDV/SOF at the liver units of eight hospitals of the Catholic University of Korea between May 2016 and February 2021 were consecutively enrolled. Eligible patients were 18 to 70 years old with chronic HCV infection documented based on positive HCV RNA results, irrespective of LC and treatment history of HCV. Patients who were positive for hepatitis A, B, or human immunodeficiency virus and viable HCC at enrollment were excluded from the study. This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Institutional Review Board of the Catholic University of Korea (XC22RIDI0001). Written informed consent by the patients was waived due to a retrospective nature of our study.
Treatment
Patients with GT1, 2, 4, 5, and 6 received a fixed-dose combination of LDV/SOF (LDV, 90 mg; SOF, 150 mg) once daily for 12 weeks regardless of cirrhosis status, according to the guidelines [5,20]. In GT1 patients with decompensated LC, liver transplantation (LT), or treatment-experienced compensated LC, additional weight-based ribavirin was administered at a total daily dose of 1,000 or 1,200 mg for patients weighing < 75 or ≥ 75 kg, respectively. Since June 2018, 8 weeks of LDV/SOF treatment was approved in Korea and administrated to treatment-naïve patients with GT1, whose HCV RNA level was < 6 million IU/mL.
Clinical and laboratory data
The following baseline clinical and laboratory data of all included patients were collected at the start of the treatment: sex, age, complete blood count, aspartate transaminase (AST), alanine transaminase (ALT), total bilirubin, albumin, prothrombin time, history of HCC, treatment history of HCV infection, presence of LC with or without decompensation, diabetes, hypertension, and chronic kidney disease (CKD), which was defined as stage 3 or worse (estimated glomerular filtration rate < 60 mL/min/1.73 m2). Serum HCV RNA levels were quantified using a COBAS TaqMan HCV quantitative test version 2.0 (lower limit of quantification [LLOQ], 15 IU/mL; Roche Molecular Systems, Branchburg, NJ, USA). The HCV GT was determined using the gene sequencing assay. The presence of LC was based on liver biopsy, transient elastography (FibroScan, Echosens, Paris, France), and/or imaging studies using sonography, computed tomography, and magnetic resonance imaging [22]. Laboratory tests, including AST, ALT, and HCV RNA levels, were performed at 4 weeks, end of treatment (EOT), and 12 weeks after EOT.
Outcomes
The primary outcome was the rate of SVR, defined as the proportion of patients with HCV RNA concentration lower than the LLOQ 12 weeks after EOT (SVR12) by per-protocol (patients receiving ≥ 1 dose of DAA with available HCV RNA data at post-treatment week 12). The secondary outcomes were the rate of rapid virologic response (RVR), EOT response (ETR), and SVR12 according to the GT, prior treatment status, treatment period in treatment-naïve GT1 patients with HCV RNA < 6 million IU/mL, presence of LC, and comorbidities. RVR and ETR were defined as undetectable HCV RNA at EOT and 4 weeks, respectively. We also monitored any adverse events (AEs) during LDV/SOF treatment, including severe AEs leading to permanent discontinuation of LDV/SOF or ribavirin.
Statistical analysis
Continuous variables are documented as mean ± standard deviation or median and range. Categorical variables are expressed as counts with percentages. Differences between categorical variables were analyzed using the chi-square test or Fisher’s exact test. For continuous variables, Student’s t test or the Mann-Whitney U test was performed to analyze the group differences, as appropriate. Statistical significance was set at a two-tailed p value of < 0.05. All statistical analyses were performed using R version 4.0.4 (http://cran.r-project.org) and Prism standard version 8.4.2 (GraphPad Software, San Diego, CA, USA).
RESULTS
Baseline characteristics
Among the 273 patients enrolled between May 2016 and February 2021, 206 patients (75.5%) completed the follow-up for 12 weeks after EOT, with HCV RNA results available for assessing SVR12. The baseline patient characteristics are summarized in Table 1. The median age was 60 years (range, 24 to 88) and 107 (39.0%) patients were male. Most patients were infected with GT1 (n = 204, 74.7%) followed by GT2 (n = 68, 24.9%), and were treatment-naïve (n = 243, 89.0%) at baseline. A total of 181 patients (66.3%) had chronic hepatitis, and 74 patients (27.1%) had compensated LC at baseline. Among clinical comorbidities before LDV/SOF treatment, hypertension (n = 90, 33.0%) was the most prevalent comorbidity followed by diabetes (n = 54, 19.8%) and CKD (n = 27, 9.9%).
Efficacy
Among the 206 patients who were followed up for 12 weeks after EOT, 202 patients (98.1%) successfully achieved SVR12 (Fig. 1A). The rate of RVR in 256 patients was 90.2% (n = 231). ETR was evaluated in 252 patients who completed LDV/SOF treatment, and 250 patients (99.2%) showed ETR in our study. The reasons for patients being unable to evaluate SVR12 (n = 67) were loss to follow-up (n = 65, 97%) and discontinuation of treatment due to AEs (n = 2, 3.0%).
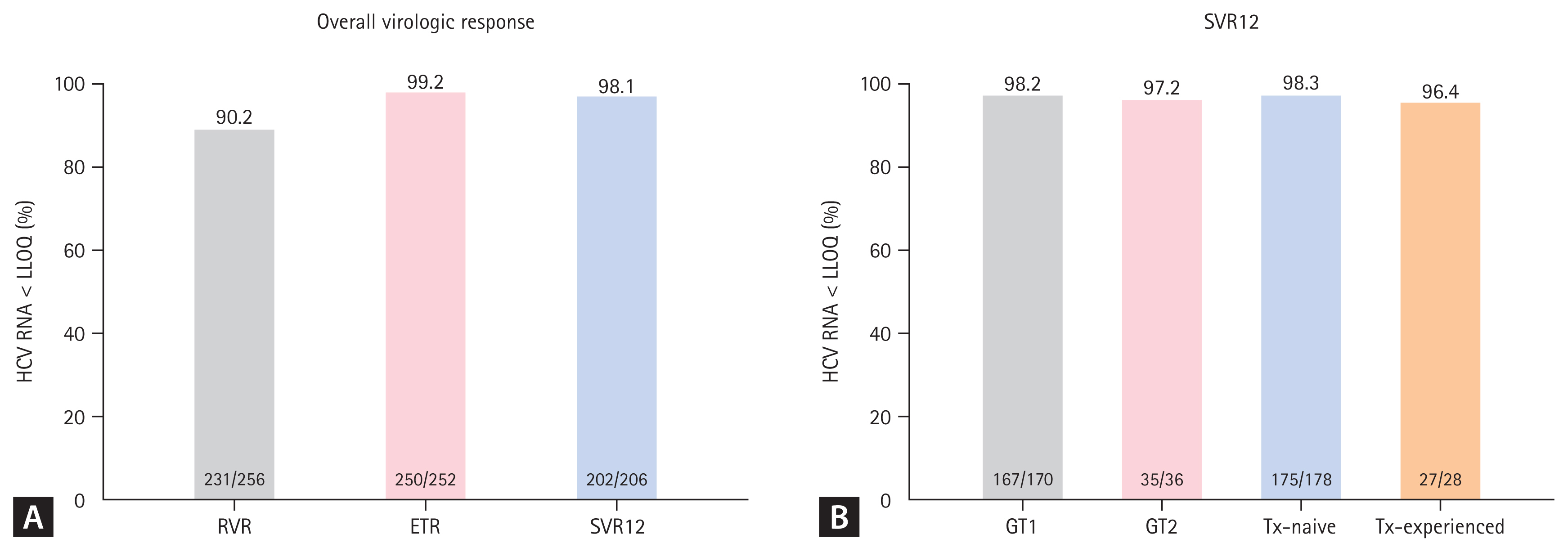
The rates of (A) rapid virologic response (RVR), end of treatment response (ETR), and sustained virological response 12 weeks after end of treatment (SVR12) for the per protocol population after ledipasvir/sofosbuvir treatment and (B) SVR12 according to the genotype (GT) and prior treatment (Tx) status. HCV, hepatitis C virus; LLOQ, lower limit of quantification.
The rate of SVR12 was also evaluated according to the GT and prior treatment status (Fig. 1B). First, the SVR12 rate was not significantly different between GT1 (n = 170) and GT2 (n = 36) (98.2% vs. 97.2%, p = 0.539) patients. The effectiveness of LDV/SOF treatment was both high in treatment-naïve (n =178) and treatment-experienced (n = 28) patients without significant group differences (98.3% vs. 96.4%, p = 0.445).
We analyzed the efficacy of LDV/SOF in relation to the treatment duration. Among the 204 patients with GT1, 60 patients (29.4%) were treatment-naïve patients with HCV RNA levels < 6 million IU/mL, who could be treated with LDV/SOF for 8 or 12 weeks. Of the 60 patients, 56 patients (8 weeks of treatment, n = 23; 12 weeks of treatment, n = 33) were evaluated for achieving SVR12, and the SVR12 rate was 100% in both groups (p = 1.000) (Fig. 2A).
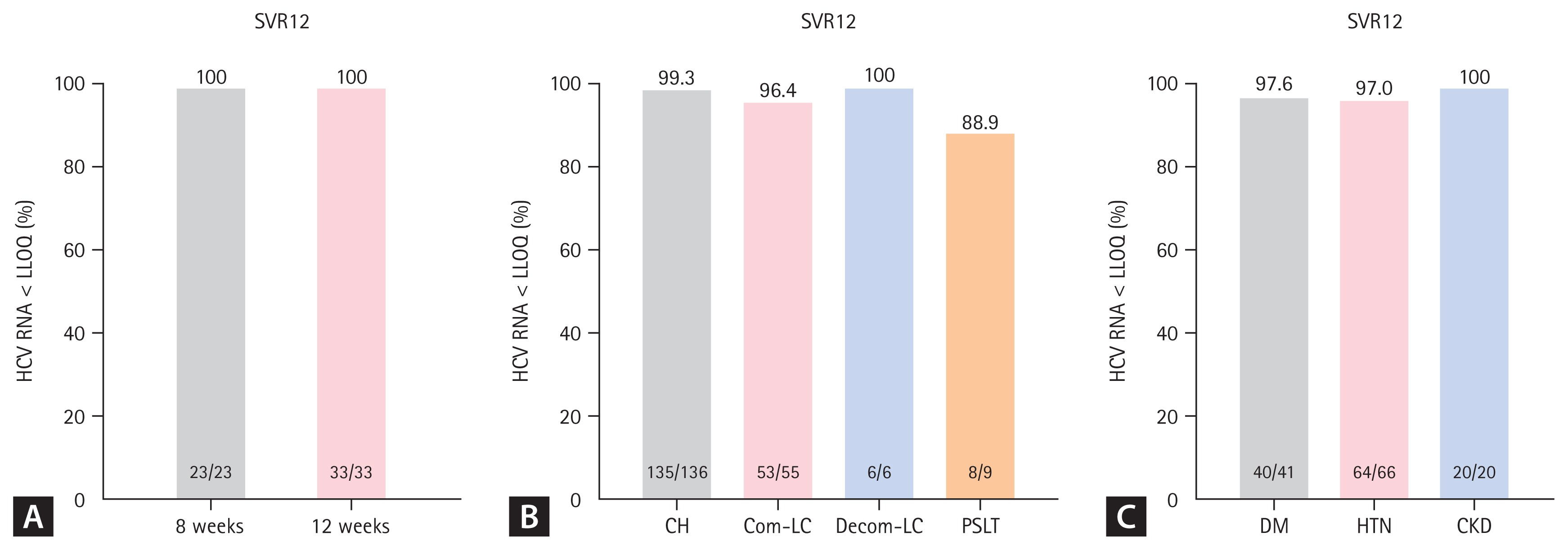
The rate of sustained virological response 12 weeks after end of treatment (SVR12) according to (A) the treatment duration in treatment-naïve genotype (GT) 1 patients with hepatitis C virus (HCV) RNA < 6 million IU/mL, (B) the liver cirrhosis (LC) and liver transplantation status, and (C) comorbidities in patients with HCV infection. LLOQ, lower limit of quantification; CH, chronic hepatitis; PSLT, post-status liver transplantation; DM, diabetes mellitus; HTN, hypertension; CKD, chronic kidney disease.
The treatment effectiveness of LDV/SOF according to the presence or absence of cirrhosis was also evaluated (Fig. 2B). Regardless of cirrhosis status, all patient groups had SVR12 rates above 95%, with results of 99.3% (135/136) and 96.4% (53/55) in patients with chronic hepatitis and compensated cirrhosis (p = 0.200), respectively. All six patients with decompensated cirrhosis reached SVR12; among the nine patients who underwent LT, eight (88.9%) could achieve SVR12 after treatment with LDV/SOF.
Regarding patients with comorbidities, the SVR12 rate was 97.6% among patients with diabetes (n = 41) and 97.0% among those with hypertension (n = 66) (Fig. 2C). Patients with CKD (n = 20) also showed a high SVR rate (100%), and there were no statistically significant differences in the SVR rate according to the type of comorbidity (p = 1.000).
Virologic failure
Among 273 included patients, four (1.5%) showed virologic failure after LDV/SOF treatment (Supplementary Table 1). Of four patients showing virologic failure, three were treatment-naïve and the other was interferon (IFN)-experienced. One treatment-naïve patient with GT2 did not have LC and showed non-response at 4 weeks treatment with an increase in HCV RNA. The other two treatment-naïve patients had compensated LC with GT1, which resulted in virologic failure, including one patient who achieved ETR. The other patient, who underwent LT and was IFN-experienced, had a high level of initial HCV RNA (9,116,410 IU/mL) and showed virologic relapse at 12-week post-treatment after an achievement of ETR. Among the four patients showing virologic failure, one was successfully treated with glecaprevir/pibrentasvir and the other three were waiting for approval of potent regimens such as SOF/velpatasvir/voxilaprevir in Korea.
Safety
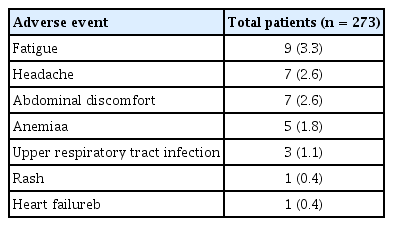
During the treatment with LDV/SOF, 33 of all the patients (12.1%) experienced AEs. The most common AEs was fatigue, followed by headache, abdominal discomfort, anemia due to ribavirin, upper respiratory tract infection, rash, and heart failure due to underlying severe aortic stenosis, which occurred in nine (3.3%), seven (2.6%), seven (2.6%), five (1.8%), three (1.1%), one (0.4%), and one patient (0.4%), respectively (Table 2).
Among the 33 patients experiencing AEs, only two (0.7%) discontinued the treatment due to AE. One patient was an 83-year-old man without LC, who developed severe aortic stenosis. He died due to heart failure related to aortic stenosis after 7 weeks treatment with LDV/SOF. The other patient was a 64-year-old woman with decompensated LC. She discontinued LDV/SOF plus ribavirin treatment after 4 weeks treatment due to anemia and general weakness. The other 14 patients continued LDV/SOF treatment, managing their AEs successfully. Consequently, none of the patients discontinued the treatment because of AEs related to LDV/SOF.
DISCUSSION
To the best of our knowledge, this multicenter cohort study is the first to evaluate the efficacy and safety of LDV/SOF for HCV treatment in a real-world setting in South Korea. Overall, the SVR12 rate of LDV/SOF treatment was very high, with a low occurrence of AEs. LDV/SOF treatment consistently demonstrated high rates of SVR12 irrespective of GT, cirrhosis, or comorbidities. Treatment for 8 weeks with LDV/SOF also showed a high SVR12 rate in treatment-naïve GT1 patients whose HCV RNA level was < 6 million IU/mL. Thus, our results suggest that LDV/SOF treatment is effective and safe for treating HCV patients in a real-world setting in South Korea, a country with a high prevalence of GT1 and GT2.
Similar to the promising results of LDV/SOF, including a phase IIIb study in Korea [18], the LDV/SOF treatment showed a 98.1% SVR rate in the real-world setting in our study. The high rate of SVR over 95% sustained regardless of the GT and previous treatment history supporting the HCV treatment guidelines of the KASL [20]. The high efficacy of LDV/SOF in GT1 was well evaluated in previous studies, and the SVR rate of 98.2% in our study is comparable to those of previous studies [23–25]. However, there has been limited real-world data regarding the efficacy of LDV/SOF in GT2. Our multicenter cohort study revealed that, for the first time in Korea, 12-week LDV/SOF treatment in GT2 had a high SVR rate of 97.2%, which is similar to that obtained in previous clinical trials [19]. Based on our results, LDV/SOF could be used equally effectively in not only GT1 patients but also GT2 patients. Moreover, prior HCV treatment experience, especially IFN-based therapy, did not affect the SVR12 rate of the LDV/SOF treatment, supporting the notion that LDV/SOF could be used regardless of the treatment history [17,26].
One of the key findings emerging from our real-world study is the consistent efficacy of LDF/SOF, especially in the 8-week treatment for selected patients and patients with decompensated LC. Based on a post hoc analysis of the ION-3 study, the efficacy of the 8-week regimen of LDV/SOF was compared with a 12-week treatment in treatment-naïve GT1 patients with HCV RNA levels < 6 million IU/mL. Similar to the 12-week treatment, the 8-week treatment with LDV/SOF in selected patients showed a high SVR12 rate (95% to 96%) [27–31], which is in accordance with our results. These results provide an insight into the reduction in the economic burden with short treatment duration, while simultaneously preserving high efficacy. Moreover, LDV/SOF treatment with or without ribavirin demonstrated a high SVR12 rate in compensated and decompensated LC. Although there were few patients with decompensated LC in our study, high SVR12 rates in both compensated and decompensated LC support the treatment guidelines of KASL and previous studies [20,32]. As glecaprevir/pibrentasvir is contraindicated for decompensated LC and SOF/velpatasvir, an effective DAA for decompensated LC [33], is still not approved in South Korea, our promising results of LDV/SOF plus ribavirin in decompensated LC provide the possibility of successful HCV treatment in these Korean patients.
As recurrent HCV infection after LT can lead to graft loss and death [34], successful HCV treatment is an important for LT patients with HCV infection. In accordance with the results of previous studies showing a high SVR12 rate (96% to 98%) [35,36], our study also documented a high SVR rate (89%) in post-LT patients. Moreover, a 12-week treatment with LDV/SOF in patients with comorbidities, such as diabetes, hypertension, or CKD, also demonstrated an SVR12 rate of 97% to 100% in our study. Indeed, more than 30% of the patients in our cohort had comorbidities, suggesting the presence of concomitant drugs during HCV treatment. Consequently, the high SVR rate in these patients might be attributable to fewer drug-drug interactions between the concomitant medication and LDV/SOF [25,37]. In addition, an increase in toxicity associated with IFN-based therapy made it challenging to treat HCV infection in patients with CKD. However, LDV/SOF treatment demonstrated a high SVR12 rate even in patients with CKD in our study, similar to the results of previous studies [38–40]. Therefore, the high efficacy of LDV/SOF irrespective of the GT, cirrhosis, LT, and comorbidities make it possible to eradicate HCV infection in Korea. Despite the high SVR12 rate of LDV/SOF treatment, patients showing virologic failure after LDV/SOF therapy can be treated with SOF/velpatasvir/voxilaprevir or glecaprevir/pibrentasvir according to the treatment guidelines of the KASL and the American Association for the Study of Liver Diseases [20,41].
In addition to the high efficacy of LDV/SOF, it is important to evaluate the safety of LDV/SOF in real practice. During the LDV/SOF treatment, only 12.1% of the entire population experienced AEs in our study. Among these AEs, fatigue, headache, and abdominal discomfort were the main ones, which were not severe enough to cause discontinuation of LDV/SOF. Moreover, only 0.7% of the patients discontinued treatment unrelated to LDV/SOF. These AE profiles were similar to those in previous studies showing good safety profiles irrespective of cirrhosis, comorbidities, or treatment history [13,15,29]. Consequently, 8 or 12 weeks of LDV/SOF might be a safe regimen for HCV patients in a real-world setting in Korea.
This study has several limitations. First, this study was retrospective in nature. Second, the number of patients included in the subgroups, such as the decompensated LC and LT groups, was small. Third, the long-term SVR and clinical outcomes of SVR12 were not analyzed in our study. Because of the retrospective design of our study, there have also been inevitable difficulties in collecting data regarding AEs during LDV/SOF treatment, which contributed to the low AE rate seen in our study. However, to the best of our knowledge, this study is the first to evaluate real-world data of LDV/SOF with the largest number of patients in South Korea. Although the patients with decompensated LC or LT included were small, our results could provide a rationale for treating those patients with LDV/SOF. However, further studies are required with a larger sample size. Moreover, the small number of patients with a history of HCC was due to these patients not being covered under the Korean national health insurance. In the future, further studies with long-term follow-up are necessary to evaluate long-term SVR and its effects.
In conclusion, LDV/SOF in clinical practice in Korea showed high efficacy and good safety profile regardless of the GT, prior treatment status, LC status, LT, and comorbidities. Our study also proved the effectiveness of LDV/SOF in GT2 and in decompensated patients. Moreover, the promising treatment outcomes of the 8-week LDV/SOF treatment in selected patients might decrease the economic burden of HCV treatment in Korea. Therefore, LDV/SOF could be an effective treatment option for HCV infection especially in GT1 and 2 prevalent areas where the use of SOF/velpatasvir is not possible. Consequently, these encouraging results provide insights into the increased possibility of successful HCV eradication in South Korea.
KEY MESSAGE
1. In Korean real-world data, ledipasvir/sofosbuvir (LDV/SOF) treatment showed high rates of sustained virologic response at 12 weeks after treatment (SVR12) regardless of genotype 1 or 2, previous treatment status, underlying liver function and co-morbidities.
2. LDV/SOF also demonstrated consistent efficacy after 8-week treatment in treatment-naïve genotype 1 non-cirrhotic patients with hepatitis C virus RNA levels < 6 million IU/mL.
3. LDV/SOF treatment showed high tolerability with low rates of adverse events during treatment.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2021R1I1A1A01050954; Soon Kyu Lee).
Notes
No potential conflict of interest relevant to this article was reported.