Current state and prospects of gout treatment in Korea
Article information
Abstract
Effective management of gout includes the following: appropriate control of gout flares; lifestyle modifications; management of comorbidities; and long-term urate-lowering therapy (ULT) to prevent subsequent gout flares, structural joint damage, and shortening of life expectancy. In addition to traditional treatments for gout, novel therapies have been introduced in recent years. Indeed, new recommendations for the management of gout have been proposed by various international societies. Although effective and safe medications to treat gout have been available, management of the disease has continued to be suboptimal, with poor patient adherence to ULT and failure to reach serum urate target. This review outlines recent progress in gout management, mainly based on the latest published guidelines, and specifically provides an update on efficient strategies for implementing treatment, efficacy and safety of specific medications for gout, and cardiovascular outcomes of ULT. In particular, we reviewed gout management approaches that can be applied to a Korean population.
INTRODUCTION
Gout is the most common inflammatory arthritis, which is induced by hyperuricemia and subsequent monosodium urate (MSU) crystal deposition in joints and other tissues [1]. Gout has a negative impact on the quality of life of patients due to extreme joint pain, and various comorbidities associated with the disease can be life-threatening. The prevalence and incidence of gout are increasing not only in Korea, but also in many other countries worldwide [2–4]. Despite its increasing prevalence and incidence, and the availability of effective and safe medications to treat gout, management of the disease has continued to be suboptimal, with poor patient adherence to urate-lowering therapy (ULT) and failure to attain the therapeutic level of serum urate (SUA) [5,6]. The optimal management of gout requires a multifarious approach, including mitigation of gout flare symptoms, lifestyle modification, patient education, management of comorbidities, and particularly, long-term ULT to dissolve MSU crystals and prevent future gout flares. Moreover, gout management should be individualized for each patient based on their comorbid conditions or concomitant medications. In this review, we provide an update on the treatment of gout mainly based on recently published guidelines, including the 2020 American College of Rheumatology (ACR) guidelines [7], the 2017 British Society for Rheumatology (BSR) guidelines [8], the 2016 European League Against Rheumatism (EULAR) recommendations for the management of gout [9], and the 2021 Asia-Pacific League of Associations for Rheumatology (APLAR) guidelines for the treatment of gout [10]. In particular, we reviewed gout treatment approaches that can be applied to Korean populations based on studies conducted on Korean patients with gout.
GENERAL PRINCIPLES OF TREATMENT FOR GOUT
The treatment of gout mainly consists of non-pharmacological and pharmacological therapies. Non-pharmacological therapy includes lifestyle modification, such as exercise, weight control, diet, and patient education. Pharmacological therapy includes adequate control of acute inflammation, prophylaxis against gout flares, long-term ULT to reverse hyperuricemia, and treatment of comorbidities. Table 1 summarizes the clinical guidelines for the management of gout [7–10].
NONPHARMACOLOGIC TREATMENT FOR GOUT
Lifestyle modification and diet therapy
Results from a diet and genetics meta-analysis showed that the effect of diet or individual food items on SUA levels was small [11]. However, dietary factors may trigger gout flares, and patients with gout frequently seek advice on the dietary management of gout. Above all, a significant dose-response relationship between alcohol consumption, regardless of alcoholic beverage type, and the risk of recurrent gout flares was observed in a case-crossover study [12]. Hence, it is recommended that patients with gout limit their alcohol intake regardless of disease activity [7]. Regarding other dietary factors, a purine diet was reportedly associated with an increased risk of gout flares [13], and a high-fructose diet was associated with a high risk of incident gout [14]. Indeed, purine intake and a high-fructose diet should be limited in patients with gout [7]. Although vitamin C supplementation has been shown to lower SUA levels [15], vitamin C at a modest dose (500 mg/day) was insufficient as monotherapy or adjunct to standard ULT [16]; therefore, it is no longer recommended in patients with gout [7]. Recently, APLAR suggested that the evidence of limiting purine-rich foods to lower SUA levels or prevent gout flares in patients with gout is insufficient [10]. Since excessive food restrictions may reduce patients’ compliance with medical treatment, it is better to focus on treatment using ULT and restrict mainly alcohol and high-fructose intake [17,18]. Weight loss approaches are also conditionally recommended for obese or overweight patients [7]. A large cohort study demonstrated that obesity was associated with a higher risk of incident gout and that changes in body mass index were associated with the risk of recurrent gout flares in a dose-responsive manner [19]. Similarly, weight loss through bariatric surgery or diet also demonstrated clinically relevant reductions in SUA levels and gout flare frequency [20,21].
Education for the patients and primary care physicians
Drug adherence in patients with gout worldwide is very poor [22,23]. Drug adherence rates of patients with gout were the lowest when comparing drug adherence rates among patients with gout, hypertension, hypercholesterolemia, type 2 diabetes mellitus, hypothyroidism, osteoporosis, and seizure disorders [24]. To overcome this problem, education for primary care physicians is essential, in addition to education for patients with gout. Therefore, patient education is emphasized in almost all treatment guidelines [7,9,10].
Acute gout flares
The use of topical ice on inflamed joints has been shown to reduce pain [25] and has been conditionally recommended as an adjuvant treatment in patients experiencing a gout flare [7]. Additional non-pharmacological care for gout flares includes rest of acutely affected joints, mobility assistance, and hydration [8].
PHARMACOLOGIC TREATMENT FOR GOUT
Asymptomatic hyperuricemia
There is no universally accepted definition of hyperuricemia; however, it is typically defined as an SUA level > 7.0 mg/dL [26]. Asymptomatic hyperuricemia is a condition characterized by hyperuricemia without any symptoms or signs of MSU crystal deposition disorders, such as gout, urolithiasis, and urate nephropathy [27]. Although several epidemiological studies have indicated that hyperuricemia is associated with an increased risk of hypertension, chronic kidney disease (CKD), and cardiovascular (CV) disease [28–32], hyperuricemia itself has not been established as a causal factor in any of these diseases. Among patients with asymptomatic hyperuricemia, ULT with febuxostat has been shown to significantly reduce incident gout flares over a 3-year period; however, the incidence of gout was low for both the febuxostat and placebo groups (0.9% vs. 5.9%) [33], which would correspond to a 3-year number needed to treat with febuxostat of 24 patients to prevent a single gout flare. In addition, among those with asymptomatic hyperuricemia with SUA levels > 9 mg/dL, only 22% developed gout within 5 years [34]. Given that the benefits of ULT do not outweigh the costs or risks associated with treatment for most patients with asymptomatic hyperuricemia, including those with comorbid CKD or CV disease, initiation of ULT is recommended against in those with asymptomatic hyperuricemia [7]. However, when a patient’s SUA level is > 9 mg/dL, individualized ULT can be considered based on each patient’s lifestyle or comorbidities.
Acute gout flares
Gout flares are induced by the activation of the NLR family pyrin domain containing 3 (NLRP3) inflammasome by MSU crystals with interleukin 1β (IL-1β) production and a subsequent cascade of other pro-inflammatory cytokines and chemokines [35,36]. The major goals of treatment for gout flares are pain control and rapid suppression of inflammation.
Early treatment with colchicine, non-steroidal anti-inflammatory drugs (NSAIDs), or glucocorticoids (oral or injectable) is recommended as a first-line therapy for gout flares [7–9]. Head-to-head clinical trials comparing first-line anti-inflammatory agents with different mechanisms of action have demonstrated similar efficacy between low-dose colchicine, NSAIDs, and oral glucocorticoids for treating gout flares [37–40]. Regarding safety issues, naproxen (750 mg/day for 7 days) caused fewer side effects than low-dose colchicine (1.5 mg for 4 days) [37], while indomethacin (150 mg/day for 2 days followed by 75 mg/day for 3 days) resulted in more minor adverse events than prednisolone (30 mg/day for 5 days) [38]. When colchicine is the chosen agent, low-dose colchicine (1.0 to 1.2 mg immediately followed by 0.5 to 0.6 mg after an hour) is recommended instead of high-dose colchicine (4.8 mg) due to their comparable efficacy and a lower risk of adverse effects associated with low-dose colchicine [7,41].
While ACR, EULAR, and APLAR do not prioritize among the three first-line therapies, the choice of anti-inflammatory agent generally depends on comorbid conditions and concurrent medications for each patient [7,9,10]. For instance, NSAIDs should be avoided in patients with renal impairment, peptic ulcer disease, cardiac disease, and concomitant anticoagulant use. Colchicine should not be administered to patients with severe renal impairment or severe liver disease, or administered in combination with strong inhibitors of cytochrome P450 3A4 and/or P-glycoprotein, such as cyclosporin, ketoconazole, clarithromycin, and verapamil [9,42]. Moreover, high-dose glucocorticoids are avoided in patients with active infection or uncontrolled diabetes. In contrast, intravenous, intramuscular, or intra-articular injections are preferred in patients who are unable to take oral medications [7]. Intra-articular glucocorticoid injection can also be considered for treatment of acute monoarticular gout [8,43]. For patients who have had recurrent flares, treatment selection is typically driven by patient preference based on past experiences of efficacy or adverse events associated with ULT [7]. In addition, for patients with severe gout flares, for example, when multiple joints are involved, combination therapy, such as colchicine and NSAID, or colchicine and glucocorticoids, may be considered [8,9,44]. Given that IL-1 has emerged as a crucial cytokine in gout flares, IL-1 inhibitors, including canakinumab, anakinra, and rilonacept, have been used to treat gout flares in Western countries [45–47]. However, IL-1 inhibitors are not available in Korea.
Dapansutrile (OLT1177) is a novel anti-inflammatory agent, an orally active β-sulfonyl nitrile molecule that selectively inhibits the NLRP3 inflammasome in neutrophils and human monocyte-derived macrophages and the subsequent activation of IL-1β [48]. Further studies are needed to confirm the clinical potential of dapansurtrile in gout flares.
Prophylaxis against mobilization flares
The experience of gout flares that occur in the first few months of ULT initiation is one reason for stopping ULT [49]. There are two main strategies for decreasing the risk of gout flares during this period. First, concurrent anti-inflammatory prophylaxis therapy is strongly recommended during the first 3 to 6 months of ULT [7–9]. While the most indicated is low-dose colchicine, and a low-dose NSAID as an alternative in cases of intolerance or contraindication to colchicine [8–10], the ACR guidelines also indicate prednisone/prednisolone for prophylaxis therapy [7]. Previous studies have shown that concomitant administration of naproxen 500 mg/day or colchicine 0.6 mg/day for 3 to 6 months effectively reduced gout flares [50–52]. Among gout patients in Korea, colchicine (62.3%) was reported as the most commonly prescribed initial prophylactic agent, followed by NSAIDs (39.9%) in a multicenter retrospective cohort study [53]. Regarding the duration of prophylaxis therapy, the 2017 BSR and 2016 EULAR guidelines recommended to continue prophylaxis during the first 6 months of ULT [8,9], and the 2020 ACR for 3 to 6 months [7]. While there are no currently available Korean guidelines on this issue, prophylaxis therapy more than 6 months from initiation of ULT, and achieving target SUA at the time of stopping prophylaxis was associated with fewer gout flares in Korean patients with gout [53]. In terms of the colchicine dose for prophylaxis therapy, low-dose colchicine (0.6 mg/day) was shown to prevent gout flare with fewer adverse events when compared with regular dose (1.2 mg/day) of colchicine among Korean gout patients [54,55]. Second, ULT should be initiated at a low-dose and gradually increased to reduce the risk of flares. The current guidelines recommend starting doses of allopurinol and febuxostat at ≤ 100 and ≤ 40 mg/day, respectively, and lower allopurinol doses in patients with CKD [7,9]. A randomized open-label trial (FORTUNE-1 study) demonstrated that starting febuxostat at a low-dose with stepwise dose increase, as well as concomitant low-dose colchicine prophylaxis therapy, effectively prevented gout flares compared to fixed-dose febuxostat alone; however, there was no significant difference in the incidence of gout flares between stepwise increases in febuxostat dose and low-dose colchicine prophylaxis [51].
Long-term ULT
All patients with gout should be informed that gout is a chronic disease with MSU crystal deposition and that long-term ULT is required to suppress tophi and prevent subsequent gout flares and joint damage.
Indications for initiation of ULT
The 2020 ACR guidelines for gout management strongly recommend ULT for all patients with frequent gout flares (≥ 2 annually), subcutaneous tophi, and/or evidence of radiographic damage due to gout [7]. Initiation of ULT was conditionally recommended for patients experiencing their first flare with comorbid moderate-to-severe CKD (glomerular filtration rate [GFR] < 60 mL/min/1.73 m2), urolithiasis, or a very high SUA level of > 9 mg/dL [7]. Similar recommendations for ULT indications have been made by the EULAR [9]. All patients with recurrent flares, tophi, urate arthropathy, and/or renal stones were indicated for ULT, and initiation of ULT was also recommended close to the time of the first diagnosis in young patients (< 40 years), a very high SUA level of > 8 mg/dL, and/or comorbidities [9].
There are some discrepancies among clinical guidelines regarding whether ULT should be initiated during an acute gout flare. While the EULAR does not provide guidance with regard to this issue [9], the BSR guidelines discourage the initiation of ULT during a gout flare and recommend postponing the ULT until acute inflammation has resolved [8]. Instead, ACR conditionally recommends ULT initiation during a flare [7] based on two randomized controlled trials (RCTs) that showed that ULT initiation during this period did not significantly extend the duration or severity of the flare [56,57], and also considering the conceptual benefits of time efficiency and flare symptoms that serve as a powerful motivator for ULT initiation. If the patient’s inflammation and pain are severe, we recommend starting anti-inflammatory treatment first and ULT a week after the inflammation subsides; if the patient’s inflammation is not severe and pain is tolerable, simultaneous anti-inflammatory treatment and ULT can be considered.
Treat-to-SUA target
Long-term ULT based on the treat-to-SUA target protocol has been proven to suppress gout flares, reduce urate crystal deposition, and prevent joint damage in gout [58,59]. The treat-to-SUA target approach, with a target SUA level of 6.0 mg/dL, was recommended by the ACR and EULAR [7,9]. A lower target SUA level of ≤ 5.0 mg/dL is recommended by the BSR for all patients with gout [8], and by the EULAR for those with high urate burden, such as tophaceous gout [9]. Following ULT initiation at a low-dose, the dose should be progressively titrated using serial SUA measurements to achieve and maintain the target SUA [7–9].
SUA-lowering agents
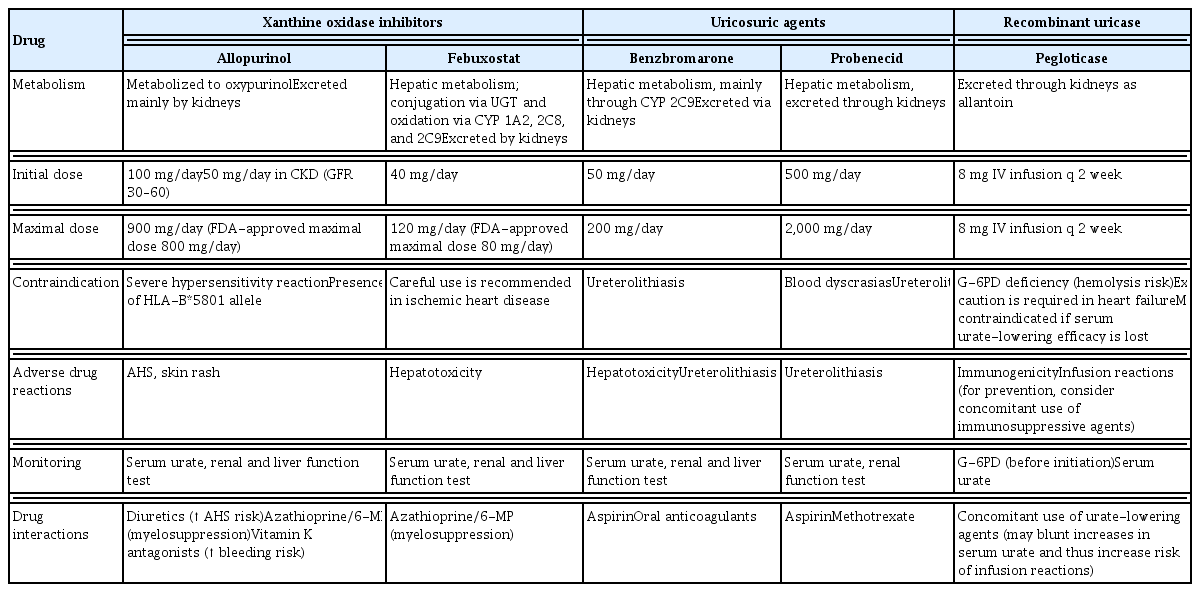
Currently available ULTs have three different mechanisms of action: inhibition of urate production by xanthine oxidase (XO) inhibition (allopurinol and febuxostat), promotion of renal urate excretion (probenecid and benzbromarone), and catalysis of uric acid to water-soluble allantoin (pegloticase). Table 2 summarizes each of these agents for the treatment of gout.
1) Allopurinol
The first-line ULT recommended for patients with gout is allopurinol, which is a purine-based inhibitor of XO that was first used in 1966 [7–9]. Although rare, potentially life-threatening allopurinol hypersensitivity syndrome (AHS) typically develops within the first few months of treatment with allopurinol [60]. In particular, AHS, which appears in Koreans is unique and life-threatening [61]. Risk factors for AHS include the presence of the HLA-B*5801 allele, CKD, old age, concomitant diuretic use, and a high initial dose of allopurinol [62]. Therefore, in subgroups of Southeast Asian and African ethnicities, with a relatively high prevalence of HLA-B*5801, pre-testing for HLA-B*5801 is conditionally recommended before starting allopurinol [7,62]. The positive rate of HLA-B5801 in Koreans is reportedly 12.2% [63] while the positive rate in Caucasians is only 0.7% [63,64]. In addition, HLA-B5801 genotyping prior to treatment with allopurinol was less costly and more effective than treatment without genotyping among gout patients with CKD in Korea over a time period of 12 months [65]. Hence, it is recommended that Korean patients with gout, especially those with renal insufficiency, undergo the HLA-B58*01 test before starting allopurinol. Recently, Korean national health insurance has begun to cover this test at a reasonable cost. Although genetic factors (presence of HLA-B*5801) or reduced renal function are not modifiable, the initial allopurinol dose can be adjusted. Hence, allopurinol should be started at a low-dose, such as 100 mg/day in general, or ≤ 50 mg/day for those with CKD (GFR < 60 mL/min/1.73 m2) [7]. Once patients with gout are established on an initial low-dose of allopurinol, the dose can be safely increased by 100 mg increments every month, or by 50 mg increments for those with renal impairment, until the target SUA is reached [66]. The average dose of allopurinol needed to achieve a target SUA of < 6.0 mg/dL was reported to be approximately 400 mg/day [66]. Although the United States Food and Drug Administration (FDA)-approved maximal dose of allopurinol is 800 mg/day [67], many patients with gout are not treated with the maximum permitted doses of allopurinol in real-world clinical settings [68,69]. A retrospective healthcare claims database study in Korea showed that the mean maximal dose of allopurinol used was 248 mg/day, with only 6.9% of allopurinol users receiving a maximal dose of > 300 mg/day [69].
2) Febuxostat
Febuxostat is a non-purine XO inhibitor (XOI) that is more selective and potent than allopurinol [70]. It is used as second-line ULT for patients with gout [7–9], mainly due to CV safety of febuxostat versus allopurinol in patients with gout and CV comorbidities (the Cardiovascular Safety of Febuxostat and Allopurinol in Patients with Gout and Cardiovascular Morbidities [CARES] trial) [71]. However, Koreans have a much higher risk of AHS than Westerners; therefore, the Korean FDA continues to maintain febuxostat as a first-line ULT along with allopurinol, and it is the most commonly used ULT in Korea [72]. Moreover, febuxostat showed a significantly higher persistence rate than allopurinol among Korean patients with gout after adjusting for confounding factors [73]. Dose adjustment of febuxostat is not necessary for patients with mild or moderate renal impairment, since its main route of elimination is the liver [70]. Among Korean patients with gout, febuxostat demonstrated good urate-lowering efficacy and renal safety even in cases of stage 4–5 CKD (GFR < 30 mL/min/1.73 m2) not yet on dialysis [74], and was also efficacious and well tolerated in those undergoing dialysis [75]. The initial dose of febuxostat suggested for Korean gout patients on dialysis was 20 to 40 mg/day [75]. The maximal dose of febuxostat approved by the FDA is 80 mg/day, and co-administration of azathioprine or 6-mercaptopurine with febuxostat is contraindicated [76]. Febuxostat at a dose of 80 or 120 mg/day has shown better urate-lowering efficacy than allopurinol at a dose of 300 mg/day in RCTs [77–79].
3) Comparative CV risk between allopurinol and febuxostat
The CARES trial showed comparable rates of adverse CV events between febuxostat (up to 80 mg/day) and allopurinol (up to 600 mg/day); however, all-cause mortality and CV-related death were higher with febuxostat than with allopurinol among gout patients with coexisting CV disease [71]. This result led to the FDA black box warning that patients taking febuxostat should be monitored for signs and symptoms of myocardial infarction (MI) and stroke [76]. However, limitations of the CARES trial should be considered when interpreting the results, including a high dropout rate and the fact that most deaths (approximately 85%) occurred after ULT cessation [71]. Moreover, the absolute CV risk of febuxostat is uncertain due to the absence of a control group. In contrast, a large observational study, including 99,744 older medicare patients with gout, demonstrated no difference in CV risk, including MI, stroke, coronary revascularization, heart failure, or all-cause mortality between febuxostat and allopurinol initiators [80]. In addition, the Febuxostat versus Allopurinol Streamlined Trial (FAST) trial among 6,128 patients with gout aged 60 years or older, with at least one additional CV risk factor, also showed a comparable risk of adverse CV events or all-cause and CV mortality between febuxostat and allopurinol [81].
4) Benzbromarone
Uricosuric agents, including probenecid and benzbromarone, can be used alone or in combination with an XOI in patients who are resistant or intolerant to XOIs [8,9,82,83]. Uricosurics can induce urolithiasis due to their mechanism of action and, therefore, should be avoided in patients with a history of or who currently have urolithiasis. Furthermore, all patients who are on uricosurics should receive adequate hydration; however, neither checking urinary uric acid levels nor receiving alkalinizing agents is recommended [7]. Benzbromarone has been shown to be effective and safe in general, even for gout patients with CKD; however, it was not approved in the USA and was withdrawn from several European countries due to hepatotoxicity associated with its use [84]. Nevertheless, the estimated risk of hepatotoxicity of benzbromarone in Europe is less than 1:17,000 [85]. Hence, patients treated with benzbromarone should undergo liver function tests. Benzbromarone is the only uricosuric agent available in Korea. This drug can be used as a second-line ULT in Korea.
Lesinurad, a urate transporter-1 inhibitor indicated in combination with an XOI, was discontinued in the USA in February 2019 by the marketing-authorization holder [86] and was withdrawn in Europe in July 2020 [87]. Clinical trials have been conducted to determine the efficacy and safety of dotinurad, another novel drug with selective urate reabsorption inhibitor property [88].
5) Comparative CV risk between uricosuric agents and allopurinol
Unlike XOIs, data on the CV safety of uricosuric agents are limited. In a large medicare study, probenecid was associated with a reduced risk of CV events and all-cause mortality compared with allopurinol [69]. Similarly, a large population-based cohort study of Korean patients with gout reported a decreased risk of composite CV events and all-cause mortality associated with benzbromarone compared with allopurinol [69]. However, these studies could not prove causality, and further studies are required to confirm whether uricosuric agents are favored over XOIs in terms of CV outcomes.
6) Recombinant uricase
Pegloticase is a recombinant uricase conjugated to monomethoxypolyethylene glycol, which is administered as an intravenous infusion every 2 weeks [89]. RCTs of pegloticase over 6 months resulted in a reduced frequency of gout flares, resolution of tophi, and improved patient-reported outcomes, including pain, physical function, and health-related quality of life, among chronic patients with gout who were refractory or intolerant to conventional ULTs [90]. Overall, 41% of the patients treated with pegloticase developed anti-pegloticase antibodies with loss of SUA-lowering efficacy [91]. Moreover, pegloticase infusion reactions were associated with anti-pegloticase antibodies and loss of response [89]. Therefore, for patients treated with pegloticase, SUA levels should be monitored prior to each infusion of pegloticase, and treatment should be stopped if the SUA level increases to > 6 mg/dL, specifically on two consecutive measurements. Due to concerns of toxicity and cost, pegloticase is recommended for patients with severe symptomatic tophaceous gout in whom target SUA cannot be reached with standard ULTs, including XOIs and uricosuric agents, alone or in combination [7–9]. The concomitant use of immunosuppressive agents, such as methotrexate, azathioprine, and mycophenolate, has been attempted to reduce the development of anti-drug antibodies and infusion reactions [92]. However, pegloticase is not yet available in Korea.
MANAGEMENT OF COMORBITIES AND CONCOMITANT MEDICATIONS
Medications for associated metabolic conditions, including losartan, fenofibrate, and SGLT2 inhibitors, have shown modest urate-lowering efficacy and a lower risk of incident gout [93–95]. Regarding the concurrent use of these medications for patients with gout, losartan was recommended for the treatment of hypertension when feasible; however, adding or switching cholesterol-lowering agents to fenofibrate was conditionally recommended against, despite its urate-lowering effects, considering the side effects of the medication [7]. Moreover, given that thiazide diuretics are associated with increased SUA levels [96], it is recommended that hydrochlorothiazide be switched to an alternative antihypertensive agent if such a change is feasible in patients with gout [7]. However, since there are few practical alternatives to low-dose aspirin, discontinuing low-dose aspirin is not recommended among those receiving this medication [7].
CONCLUSIONS
Gout is a chronic disease characterized by MSU crystal deposition, which requires long-term ULT. Patients with recurrent gout flares, tophaceous gout, or structural joint damage due to gout should undergo ULT to achieve and maintain a target SUA level of < 6 mg/dL. Currently available SUA-lowering agents include XOIs, uricosuric agents, and recombinant uricase. To date, allopurinol has had a higher incidence of life-threatening side effects than febuxostat in Koreans. There is no direct evidence that febuxostat increases the risk of CV disease in Korean patients with gout. Therefore, febuxostat is used as first-line ULT along with allopurinol in Korea. HLA-B58*01 tests should be performed to prevent AHS in Korean patients with gout prior to ULT with allopurinol. Administration of concomitant anti-inflammatory prophylaxis therapy is recommended during ULT to prevent gout flares. The first-line anti-inflammatory agents used to treat gout flares include low-dose colchicine, NSAIDs, and glucocorticoids. Additionally, adequate management of lifestyle factors and concomitant medications is recommended. In particular, educating patients and primary care physicians about gout should be emphasized to increase compliance with long-term ULT. This review is not an official guideline for the Korean College of Rheumatology (KCR), and the KCR will publish the Korean guidelines for the early management of gout in 2022. Further studies are needed to address the efficacy and safety of novel ULTs and anti-inflammatory agents in Korean patients with gout.
Notes
No potential conflict of interest relevant to this article was reported.