Safety and effectiveness of direct-acting antivirals in patients with chronic hepatitis C and chronic kidney disease
Article information
Abstract
Background/Aims
To evaluate the effectiveness and safety of direct acting antivirals (DAAs) available in chronic kidney disease (CKD) patients with hepatitis C virus (HCV) infection in Korea.
Methods
In a retrospective, multicenter cohort study, 362 patients were enrolled from 2015 to 2019. The effectiveness and safety of DAAs including glecaprevir/pibrentasvir, sofosubvir/ribavirin, ledipasvir/sofosbuvir, and daclatasvir/asunaprevir were analyzed for patients according to CKD stage. We evaluated sustained virologic response at week 12 after treatment (SVR12) as primary endpoint. The effectiveness and safety were also evaluated according to CKD stage.
Results
Among 362 patients, 307 patients completed DAAs treatment and follow-up period after end of treatment. The subjects comprised 87 patients (62 with CKD stage 3 and 25 with CKD stage (4–5), of whom 22 were undergoing hemodialysis). HCV patients with CKD stage 1 and 2 (estimated glomerular filtration rate [eGFR] ≥ 60 mL/min/1.73 m2) showed SVR12 of 97.2% and 95.4% respectively. SVR12 of CKD stage 3 and 4–5 (eGFR < 60 mL/min/1.73 m2) patients was 91.9% and 91.6% respectively. Patients undergoing hemodialysis achieved SVR12 (90.9%). Treatment failure of DAAs in stage 1, 2, 3, and 4–5 was 2.8%, 2.7%, 1.6%, and 4%. DAAs showed good safety profile and did not affect deterioration of renal function.
Conclusions
DAAs shows comparable SVR12 and safety in CKD patients (stage 3, 4, and 5) with HCV compared with patients with stage 1 and 2. The effectiveness and safety of DAAs may be related to the treatment duration. Therefore, it is important to select adequate regimens of DAAs and to increase treatment adherence.
INTRODUCTION
Chronic hepatitis C virus (HCV) infection is a major cause of liver diseases such as liver cirrhosis and hepatocellular carcinoma. The goal of treatment of HCV is to achieve a sustained virologic response (SVR) and eradicate the virus. In the interferon era, treatment of HCV infection is hampered by poor tolerability and a low SVR rate [1–3]. Interferon-based treatment has an SVR of up to 50% and a high incidence of adverse events (AEs) [4]. HCV treatment has shifted from pegylated interferon-based therapy to direct acting antivirals (DAAs), which have high SVR rates, less toxicity, and good tolerability [5]. DAAs have been used successfully in patients with difficult-to-treat HCV infection, including those with treatment failure, and decompensated cirrhosis, as well as those who have undergone organ transplantation [6,7].
Early detection and treatment of HCV associated with kidney disease is paramount to prevent the progressive loss of kidney function. HCV-infected patients have a 23% greater risk of chronic kidney disease (CKD) compared to uninfected patients [8]. Chronic HCV infection is associated with an increased risk of end-stage renal disease (ESRD) [9]. Therefore, the Kidney Disease Improving Global Outcomes (KDIGO) guidelines suggest screening patients with chronic HCV for creatinine clearance and proteinuria [10]. The duration of chronic HCV infection influences the risk of CKD progression. HCV seroconversion within 12 months is not a major risk factor for CKD, but cirrhosis and other comorbidities contribute to the significantly increased prevalence of CKD among patients with chronic HCV infection [11]. In addition, chronic HCV infection worsens outcomes in patients at every stage of CKD. Moreover, HCV infection accelerates the decline in renal function and increases the mortality rate of patients with CKD stage 4 or 5 [12,13]. Patients on dialysis for ESRD are particularly vulnerable to HCV infection [14]. The estimated prevalence of chronic HCV infection in hemodialysis centers is 4% to 50% [15,16]. Therefore, treatment strategies for difficult-to-treat HCV-infected patients with CKD are necessary to improve renal and liver-related morbidity and mortality.
DAAs are reportedly safe and effective in patients with CKD. The first DAA regimen approved in Korea was a 24-week course of daclatasvir (DCV) and asunaprevir (ASV) in 2015 [17,18]. However, there are few data on the effectiveness and safety of DAAs for patients with CKD (particularly those with severe renal impairment [estimated glomerular filtration rate, eGFR < 30 mL/min/1.73 m2]) in clinical practice. DAAs have a low rate of AEs, although there is concern over drug-drug interactions (DDIs) with DAAs in patients with underlying comorbidities [19]. Therefore, we evaluated the effectiveness and safety of DAAs in HCV patients with CKD in Korea.
METHODS
In this retrospective, multicenter study based on prospectively collected demographic, clinical and biochemical data, 362 patients were enrolled from February 2015 to December 2019, i.e., beginning immediately after the introduction of DAAs in Korea. The patients were treated using the regimens covered by National Health Insurance. Subjects who met the following inclusion criteria were enrolled: adult males and non-pregnant females, aged ≥ 18 years; documented evidence of chronic HCV infection (e.g., HCV RNA for > 6 months); and chronic hepatitis or liver cirrhosis. Liver cirrhosis was assessed by radiological imaging (ultrasonography or liver dynamic computed tomography at screening showing coarse liver echotexture with nodularity and a small liver or the features of portal hypertension (HTN) such as splenomegaly or varices). The patients’ Child-Pugh class was A or B. Regarding HCV treatment history, HCV treatment-naïve participants were defined as those who had never received HCV treatment with an approved or investigational drug. HCV treatment-experienced participants were those who had previously received pegylated interferon-based regimen (with or without ribavirin [RBV] and not including DAAs) for HCV treatment.
The exclusion criteria were as follows: decompensated episodes including refractory ascites or hepatic encephalopathy; evidence of a medical condition contributing to chronic liver disease other than HCV or seropositivity for human immunodeficiency virus or hepatitis B virus; diagnosed or suspected hepatocellular carcinoma or other malignancies; significant cardiovascular, pulmonary, or neurological disease and uncontrolled diabetes or HTN; and current alcohol or substance abuse.
Patient records were de-identified and their data were anonymized. This study was conducted in accordance with the Declaration of Helsinki, and approval was obtained from the Ethics Review Board of the Catholic University of Korea (XC20RIDI0186). Written informed consent by the patients was waived due to a retrospective nature of this study.
Treatment protocol
Glecaprevir/Pibrentasvir
For the glecaprevir/pibrentasvir (G/P) regimen, patients received glecaprevir 300 mg and pibrentasvir 120 mg daily for 8 weeks for chronic hepatitis, or for 12 weeks for liver cirrhosis or in case of prior treatment failure. No dose modification was applied according to renal function.
Sofosbuvir/Ledipasvir
In genotype 1 HCV patients, sofosbuvir/ledipasvir (SOF/ LDV; 400 mg/90 mg) daily for 12 weeks was prescribed for chronic hepatitis. In treatment-experienced cirrhotic patients, SOF/LDV for 24 weeks or SOF/LDV with RBV for 12 weeks was prescribed. SOF was not used in patients with an eGFR < 30 mL/min/1.73 m2.
SOF and RBV
For genotype 2 HCV patients, the combination of SOF (400 mg) and RBV for 12 weeks was prescribed. For cirrhotic patients, SOR and RBV for 16 weeks was prescribed. The RBV dose was determined according to body weight (< 65 kg: 1,000 mg; ≥ 65 kg: 1,200 mg). SOF was not used in patients with an eGFR < 30 mL/min/1.73 m2.
DCV and ASV
For genotype 1b HCV patients, the combination of DCV and ASV for 24 weeks was prescribed if NS5A resistance associated variants (RAV), including L31F/I/M/V and Y93H, were absent. In cases of severe renal impairment, the ASV dose was reduced to 100 mg daily. This was the first interferon-free DAA regimen approved in South Korea (in 2015), and it was the only regimen available for patients with CKD (particularly those with severe renal impairment) until approval of the G/P regimen in South Korea in 2018.
Definition and classification of CKD and severe renal impairment
The eGFR was calculated using the Modification of Diet in Renal Disease study equation: GFR (mL/min/1.73 m2) = 175 × (Scr)−1.154 × (age)−0.203 × (0.742 if female) × (1.212 if African American); where: Scr is the serum creatinine level in mg/dL. All the patients in this study were Korean.
CKD was classified according to KDIGO Clinical Practice Guidelines for CKD based on the eGFR for ≥ 3–6 months. The eGFR was assessed at 3–6 months before and at the time of treatment. Only patients with an eGFR < 60 mL/min/ 1.73 m2 for all measurements were considered to have CKD. This definition follows the recommendations of the CKD guidelines. The CKD stage was classified according to the eGFR as follows: stage 1, eGFR ≥ 90 mL/min/1.73 m2; stage 2–4, 15 to 90 mL/min/1.73 m2; and stage 5, eGFR < 15 mL/min/1.73 m2 or dialysis. The definition of severe renal impairment was an eGFR < 30 mL/min/1.73 m2 (i.e., CKD stage 4–5).
Endpoints and laboratory evaluations
The primary endpoints were sustained virologic response at week 12 after treatment (SVR12) after treatment, and safety. SVR12 was defined as an HCV RNA level less than the lower limit of quantification (15 IU/mL). Safety, i.e., AEs (anemia, skin rash, and discontinuation of study medication), was also evaluated.
The secondary endpoints were virological response to treatment at week 4 and end of treatment (EOT); normalized alanine transferase (ALT); and treatment failure including viral breakthrough and relapse. Any patient who met one of the following criteria was considered to have had on-treatment virologic failure and was required to discontinue treatment: an increase in the HCV RNA level of > 102 IU/mL after achieving undetectable HCV RNA, or a confirmed increase in the HCV RNA of > 1 log10 from the nadir during the treatment period.
Treatment regimens were determined by baseline characteristics including CKD stage, liver cirrhosis, and genotype, and physicians’ selection of available regimen approved by medical insurance during the study period. All patients were followed-up every 4 weeks, EOT and after the treatment period. Physical examinations, including tolerability assessment and laboratory tests were performed at baseline and 4-week intervals thereafter.
Statistical analysis
Continuous variables are presented as medians and ranges, and categorical variables as numbers and percentages. The Friedman test was used to evaluate changes in the eGFR level over time. To compare groups with respect to treatment responses and AEs, Fisher’s exact test was used. Data are medians and ranges or means ± standard deviations. The primary endpoint was evaluated by conducting per-protocol and modified intention-to-treat (ITT) analyses. The per-protocol analysis was computed as SVR12 for patients who completed treatment per the schedule. The modified ITT analysis was conducted under the assumption that all treatment interruptions were protocol failures in all patients treated with at least one dose of DAAs. A multivariate logistic regression analysis was performed using variables with a p < 0.05 in the univariate analyses to evaluate factors predictive of SVR12. Statistical analyses were performed using SPSS software version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Baseline characteristics
Among 362 patients, 307 completed DAA treatment, of whom 291 patients were followed after EOT, and 16 were excluded due to follow-up loss (Fig. 1). The subjects comprised 87 patients (62 with CKD stage 3 and 25 with CKD stage 4–5), of whom 22 were undergoing hemodialysis. The baseline characteristics of the enrolled patients are listed in Table 1. Most patients were infected with genotype 1b HCV (67.7%). The median age of the patients was 61 years, and 36.1% (n = 111) were male. Eighty-four patients had liver cirrhosis (27.3%); the others had chronic hepatitis. HCV patients with CKD stage (eGFR < 60 mL/min/1.73 m2) had significantly higher rates of diabetes mellitus (DM) and HTN than patients with an eGFR ≥ 60 mL/min/1.73 m2 (34.4% vs. 17.2%, 64.3% vs. 28.6%, p = 0.005 and p = 0.000, respectively). Major causes of CKD were also DM and HTN. Forty-seven patients (15.3%) had previously received pegylated IFN-based or DAA therapy; the others were treatment-naïve. Patients with CKD stage 1–2 received G/P and SOF/LDV (40.9% and 59.1%, respectively). Patients with CKD stage 3 were administrated SOF-based regimens (67.7%), DCV + ASV (22.5%) and G/P (9.6%). Patients with CKD stage 4–5 received G/P (60%) and DCV + ASV (40%) regimens.

Flow chart of patients enrolled in this study. F/U, follow-up; SVR12, sustained virologic response at week 12 after treatment.
Treatment effectiveness
During follow-up, the overall SVR12 rate was 94.7% (291/ 307) in the per protocol analysis. HCV patients with CKD stage 1 and 2 (eGFR ≥ 60 mL/min/1.73 m2) showed an SVR12 rate of 97.2% and 95.4%, respectively. The SVR12 rate of CKD stage 3 and 4–5 (eGFR < 60 mL/min/1.73 m2) patients was 91.9% and 91.6%, respectively. Also, 90.9% of patients undergoing hemodialysis achieved SVR12 (Fig. 2A). The modified ITT analysis yielded SVR12 rates in patients with CKD stage 3 and 4–5 (83.8% and 78.5%, respectively) comparable with those in patients with CKD stage 1 and 2, (82.3% and 79.4%, respectively) (Fig. 2B). Although HCV patients with CKD stage 4 showed a low SVR12, the SVR12 rate did not differ significantly according to CKD stage (p = 0.094). Subgroup analyses showed no effect of age, gender, DM, HTN, cirrhosis, genotype, HCV RNA level (> 106 IU/mL), treatment experience, or treatment regimen on the SVR12 rate (p = 0.139, p = 0.076, p = 0.492, p = 0.248, p = 0.578, p = 0.227, p = 0.126, p = 0.545, or p = 0.073, respectively) (Fig. 3). In HCV patients with severe renal impairment, the SVR12 rate did not differ according to any of these factors, including treatment regimen (G/P vs. DCV + ASV).
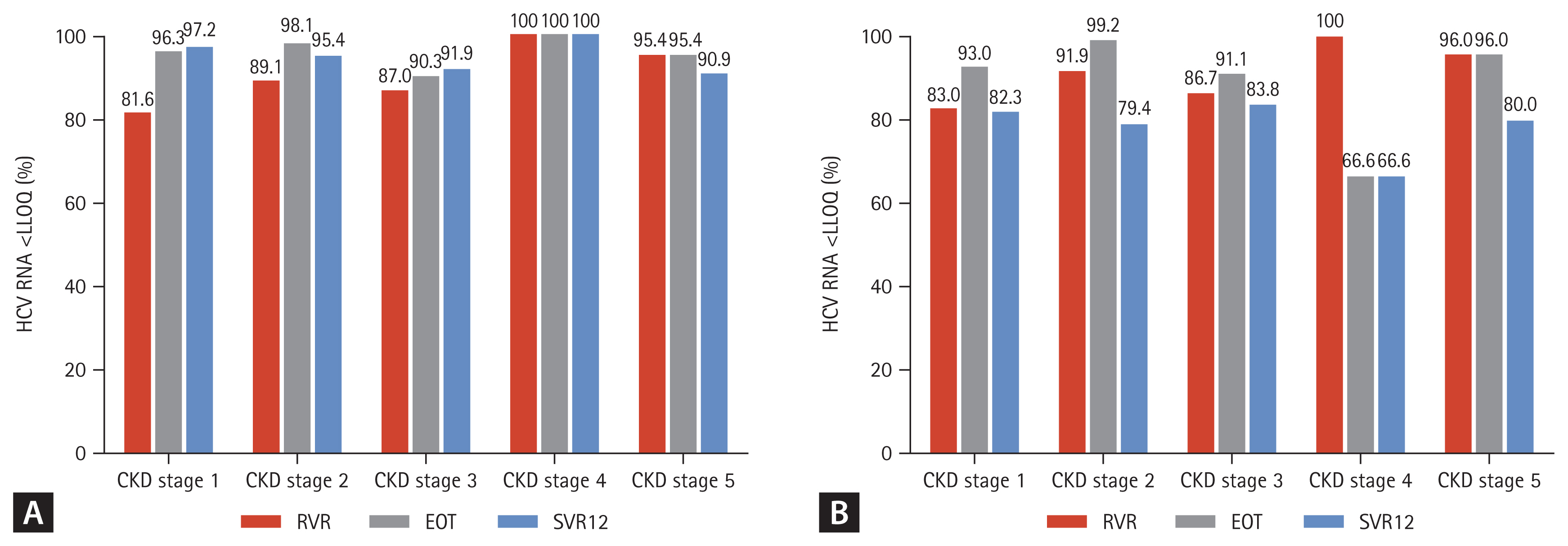
The virological response at week 4 (rapid virological response [RVR]), end of treatment and after 12 weeks of treatment with direct acting antivirals according to chronic kidney stage. (A) Per-protocol, (B) modified intention-to-treat (ITT). HCV, hepatitis C virus; LLQQ, lower limit of quantification; EOT, end of treatment; SVR12, sustained virologic response at week 12 after treatment.

Sustained virologic response at week 12 after treatment (SVR12) according to subgroup. HCV, hepatitis C virus; LLQQ, lower limit of quantification; DM, diabetes mellitus; HTN, hypertension.
Eighty-seven patients with CKD completed DAAs therapy and 79 patients had an SVR at 12 weeks. Six patients discontinued treatment for administrative reasons (nonadherence due to AEs, n = 5; loss to follow-up, n = 1). Two patients (CKD stage 3 and 5, respectively) showed treatment failure. All patients received DCV and ASV. One patient (stage 3) showed viral breakthrough at EOT. The other patient (stage 5) showed viral relapse after 12 weeks of treatment. The rates of treatment failure and cessation of DAAs were 1.6% (1/62) and 6.4% (4/62) in stage 3, and 4% (1/25) and 8% (2/25) in stage 4 and 5 compared with 2.8% (3/109) and 0% in stage 1 and 2.7% (3/111), and 1.8% (2/111) in stage 2.
Safety and tolerability
In total, 307 patients (84.8%) completed DAA treatment. The majority of AEs was of mild or moderate severity; the most common (> 2%) were dizziness, and gastrointestinal side effects (nausea, vomiting, and anemia).
There was one episode of elevated ALT in one patient with CKD stage 2 during the treatment period. Anemia occurred in nine patients (2.9%), possibly due to RBV-induced AEs or underlying renal disease (Table 2). Four patients with CKD stage 3 discontinued DAAs due to AEs (6.4%). The AEs related to treatment discontinuation were dizziness, and gastrointestinal side effects (nausea, vomiting, and anemia). The regimens of these patients were G/P (n = 1), the SOF-based regimen (n = 2), and DCV + ASV (n =1). Two patients with CKD stage 4 and 5 discontinued treatments due to symptom-related nonadherence (8%); they were treated with G/P and DCV + ASV, respectively.
Side effect of the G/P regimen comprised nausea or vomiting, fatigue, and dizziness (1.8%, 0.9%, and 0.9%, respectively). Anemia was the most frequent (6.2%) side effect of SOF-based regimens containing RBV, followed by dizziness (4.0%), fatigue (2.9%), nausea (1.7%). DCV + ASV also caused gastrointestinal symptoms, including nausea or vomiting (4.1%).
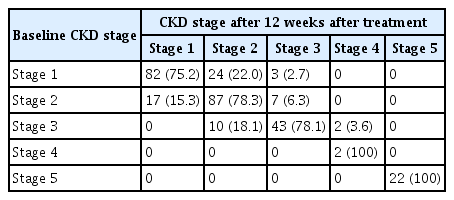
Kidney function deterioration is a concern when using DAAs, although clinical trials showed a comparable safety profile. In our cohort, the eGFR and serum creatinine levels were generally stable during treatment. The progression of CKD stage from baseline to week 12 after treatment is detailed in Table 3. Although most patients remained at the same CKD stage, 10 (9%) with CKD stage 1–2 progressed to stage 3, although there was no significant difference in CKD progression according to CKD stage (p = 0.522).
DISCUSSION
Chronic HCV-infected patients with renal impairment are a special population for HCV treatment. Although current guidelines recommend various first-line DAA therapies such as elbasvir/grazoprevir, G/P, there are several medical issues (e.g., comorbidity of advanced liver disease) and other therapies may not be available under contemporary National Health Insurance coverage in South Korea [20]. Therefore, we evaluated the effectiveness and safety of DAAs available for patients with renal impairment in South Korea.
In our cohort, 307 patients received four DAA regimens (G/P, SOF/RBV, LDV/SOF, and DCV/ASV). The pooled SVR12 rate in patients with renal impairment was satisfactory (91.8%). Patients on hemodialysis had an SVR12 rate of 90.9%. The small number of patients with renal impairment might explain the lower SVR12 compared with those without renal impairment.
G/P is an effective option for HCV patients with severe renal impairment. Glecaprevir, a nonstructural viral protein 3/4A protease inhibitor, combined with pibrentasvir, a nonstructural viral protein 5A inhibitor, is a potent DAA regimen approved for all HCV genotypes [21]. Metabolism and clearance of G/P occur primarily within the biliary system with negligible kidney excretion, so G/P is suitable for patients with kidney disease including those on dialysis for whom dosage adjustment is not required [22]. G/P showed a comparable SVR12 rate (90.4%) in patients with renal impairment (eGFR < 60 mL/min/1.73 m2). G/P is reportedly effective in treating patients with renal impairment and HCV infection (SVR12 > 98%) [23–25]. Especially, in patients with CKD stage 4 or 5, the SVR12 rates in the G/P group were 100% and 92.8%, respectively. Our results are consistent with previous study in Taiwan that reported SVR12 rates of 100% and 98.7% in patients with CKD stages 4 and 5, respectively [26]. With respect to safety, none of the patients in our G/P group showed treatment failure, and one patient discontinued the regimen due to AEs. G/P reportedly results in an SVR12 rate of 100% in genotype 2 HCV patients with severe renal impairment [26]. In this study, G/P also showed comparable SVR12 rate (90.9%) in patients with CKD stage 4–5. The G/P regimen as pangenotypic combination therapy is covered by medical insurance for patients with HCV genotype 2.
DCV + ASV showed an SVR12 of 83.3% in patients with HCV genotype 1b in this study. The treatment failure rate was 8.3% (2/24) and two patients discontinued the regimen due to AEs. DCV + ASV is no longer the recommended regimen because of the relatively longer treatment duration, and due to pre-existing and novel RAV of NS5A, resulting in a low SVR12 rate [27]. Therefore, G/P is a better treatment option than DCV + ASV in patients with severe renal impairment.
SOF is renally excreted and its accumulation results in renal dysfunction. The use of SOF in patients with CKD stage 4–5 (eGFR < 30 mL/min/1.73 m2) was not indicated on the label during the study period [28]. Therefore, we compared patients receiving an SOF-based regimen for CKD stage 3 to patients of CKD stage 1–2. Patients with CKD stage 3 showed a 95% SVR12, similar to the control group (96.1%). Moreover, none of them showed treatment failure, although two discontinued the treatment due to AEs.
Although DAAs are the first-line treatment for HCV, the choice of DAA regimen depends on HCV genotype, treatment history, eGFR, and hepatic fibrosis stage [29]. Additionally, careful review of the patient’s other medications is needed due to possible DDIs. When selecting DAAs based on renal function, the CKD stage is important. Although international guidelines (American Association for the Study of Liver Diseases) recommend the use of any DAA for CKD patients with an eGFR > 30 mL/min/1.73 m2, the use of DAAs for patients with CKD 4–5 was not approved until 2016 [30]. The following regimens are recommended for genotype 1 patients with eGFR < 30 mL/min/1.73 m2: G/P, elbasvir/grazoprevir, ombitasvir/paritaprevir/ritonavir + dasbuvir, and SOF/LDV. For genotype 2, HCV patients, G/P and SOF/LDV are recommended.
The SVR12 rates in this study may be relatively lower than those reported in HCV patients with impaired renal function [25,31]. Not only G/P but also paritaprevir/ombitasvir/ ritonavir + dasabuvir showed favorable SVR12 rates (100%) in genotype 1 HCV patients with severe renal impairment [32–34]. This inconsistency with our results can be explained as follows. First, there might be ambiguity regarding the baseline NS5A RAV for DCV + ASV. It is important to identify the baseline NS5A polymorphism before treatment with DCV + ASV. Although we assessed mutations at baseline in patients with CKD stage 4–5, indeterminate NS5A polymorphisms at baseline might have played a role in the virologic breakthrough. Second, managing tolerability and safety and increasing treatment adherence in difficult-to-treat patients are critical. Discontinuation of DAAs due to symptom-related nonadherence is an important cause of treatment failure. In addition, the number of patients with CKD stage 4–5 was small. The different numbers of patients hamper comparisons among studies.
Tolerability is also an important issue in difficult-to-treat patients. DAAs have a low frequency of AEs. The patients who discontinued antiviral medication in our study experienced AEs, including dizziness, dyspnea, and neutropenia. These AEs were not likely to be associated with DAAs. According to previous studies, common AEs of DAAs are fatigue, headache, pruritis, and nausea. These AEs do not affect the deterioration of renal or liver function [26]. In addition, monitoring of anemia is important because anemia is a common complication in patients with CKD. Anemia itself reportedly does not modulate the effectiveness of DAAs in hemodialysis, CKD, or kidney-transplant patients [34]. However, these patients had more comorbidities (e.g., HTN and DM) and a high risk of DDIs between DAAs and medications prescribed for underlying diseases, even with consideration of pharmaceutical reactions. Because interactions between metabolic pathways via the cytochrome P 3A4 enzyme and/ or drug efflux pumps can alter the therapeutic effect of DAAs, thorough investigation of medications is needed before they are started [19,35]. Treatment failure or resistance to DAAs can be caused by DDIs resulting in subtherapeutic concentration [36]. Therefore, close monitoring and management of tolerability and DDIs is critical to the success of HCV therapy in HCV patients with CKD.
Our study had several strengths and limitations. First, it was the first Korean cohort study to analyze HCV-infected patients according to CKD stage. There are few data on the treatment effectiveness and safety of DAAs in patients with severe renal impairment in South Korea. Our results suggest that DAAs are effective and safe in HCV-infected patients with CKD. Although renal safety is an important issue for DAAs, especially in CKD patients, data thereon are lacking. Our results are in agreement with a report that DAA treatment does not affect renal function [37]. However, the number of patients in this study was small, and all patients had HCV genotype 1 or 2, so further studies are required to ascertain the safety profile of DAAs in CKD patients. Second, our findings show that G/P is the better option for Korean patients with chronic HCV and ESRD, although DCV + ASV may be suitable for genotype 1b HCV infection. However, other approved regimens (including paritaprevir, ritonavir, ombitasvir, dasabuvir, and elbasvir/grazoprevir) were not evaluated in this study because of the lack of patients with renal impairment. Third, we performed a comparative analysis with a control group. The substantial heterogeneity among studies might be attributable to variations in sample size and treatment regimens. Despite the small number of patients with severe CKD analyzed in this study, our results suggest a changing therapeutic landscape, with more DAAs becoming available for treating HCV in patients with chronic renal impairment.
In conclusion, DAAs show comparable SVR12 rates and safety profiles in CKD patients (eGFR < 60 mL/min/1.73 m2) with HCV compared with patients with an eGFR ≥ 60 mL/ min/1.73 m2 (91.8% vs. 96.3%, respectively). The effectiveness and safety of DAAs may be related to the treatment duration. Our findings highlight the importance of selecting an effective DAA regimen for CKD patients with HCV.
KEY MESSAGE
1. This is the first analysis of multiple direct acting antivirals (DAAs) in Korean patients with hepatitis C virus infection and renal impairment.
2. DAAs showed comparable sustained virologic response at week 12 after treatment among chronic kidney disease patients (estimated glomerular filtration rate [eGFR] < 60 mL/min/1.73 m2) with HCV compared with patients with eGFR ≥ 60 mL/ min/1.73 m2.
3. Most adverse events of DAAs were mild or moderate, suggesting comparable safety and tolerability profile of DAAs.
4. In this cohort, the majority of patients maintained renal function during and after DAA treatment.
Notes
No potential conflict of interest relevant to this article was reported.