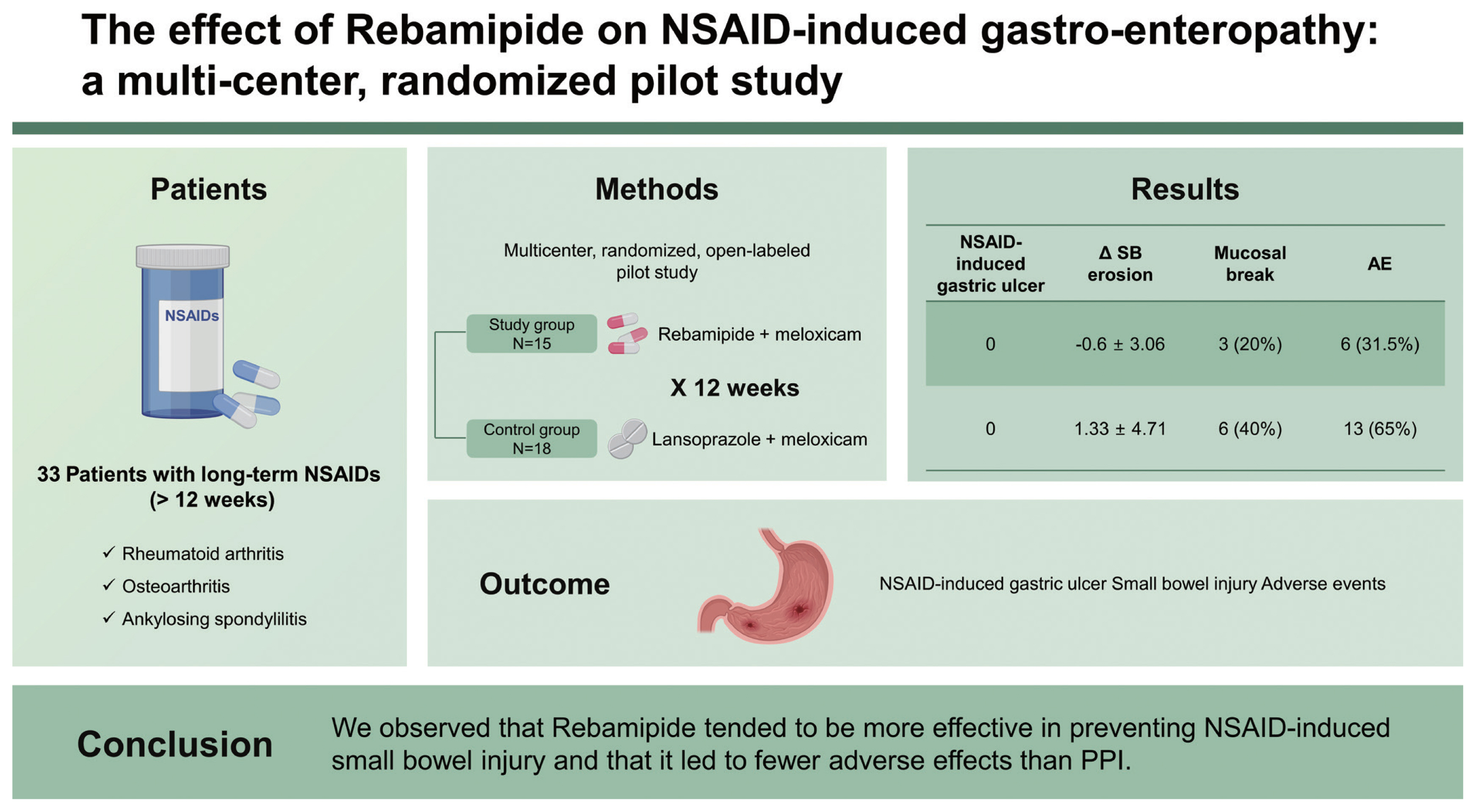
The effect of rebamipide on non-steroidal antiinflammatory drug-induced gastro-enteropathy: a multi-center, randomized pilot study
Article information
Abstract
Background/Aims
Non-steroidal anti-inflammatory drugs (NSAIDs) are commonly-used medications, and ailments such as arthritis or heart disease, require long-term use of these drugs, which can induce gastroenteropathy with bleeding and ulcers. This study investigated the associations between efficacy, safety, and gastrointestinal symptoms linked to rebamipide and proton pump inhibitor administration in patients requiring long-term NSAID use.
Methods
This study was a multi-center, randomized, open-labeled, pilot design.
Results
Thirty-three patients were included. Of these, 15 were included in the study group and 18 were in the control group. NSAID-induced gastric ulcers, which were the primary outcome of this study, did not occur in either the study or control group. Changes in the number of small bowel erosions and ulcers were −0.6 ± 3.06 in the study group and 1.33 ± 4.71 in the control group. The number of subjects with mucosal breaks (defined as multiple erosions and/or ulcers) was three (20%) in the study group and six (40%) in the control group (p = 0.427). No serious adverse events occurred in either group. However, dyspepsia and skin rashes occurred in six patients (31.58%) in the study group and 13 (65%) in the control group (p = 0.036).
Conclusions
Although statistically significant differences were not generated, possibly as a result of the small sample size, mucosal breaks observed via capsule endoscopy revealed that rebamipide was likely to be more effective than lansoprazole in preventing small intestine damage caused by NSAIDs. Furthermore, fewer side-effects emerged with rebamipide.
INTRODUCTION
Non-steroidal anti-inflammatory drugs (NSAIDs) are commonly prescribed to reduce fever, pain, and inflammation. Some of these drugs require a prescription, while others can be purchased over-the-counter [1]. As the ageing population increases, so does the prevalence of chronic diseases like degenerative arthritis and osteoarthritis; this in turn results in an increase in the use of readily available NSAIDs. The main limitation to the use of these medications is the possibility that they may lead to severe upper gastrointestinal (GI) side-effects, such as bleeding and perforation. At the same time, on a global level, the prevalence of NSAID-associated gastric and duodenal ulcers ranges from 9% to 22%, with severe bleeding or perforation occurring in less than 1% of individuals who take NSAIDs per year [2,3].
Many people experience these GI complications, but cannot stop their medications because aspirin use in patients with heart disease or NSAIDs in patients with arthritis are necessary treatments [4,5]. In order to prevent upper GI complications, it is common to use NSAIDs in combination with a proton pump inhibitor (PPI) [6,8]. PPIs reduce gastric acid secretion by inhibiting the proton pumps in stimulated gastric parietal cells. Therefore, PPIs are useful in preventing and healing gastric and duodenal ulcers caused by exposure to NSAIDs [9]. Recently, it has been reported that capsule endoscopy has permitted the detection of small intestinal lesions, proving that NSAID-induced GI problems occur in both the upper and lower GI tracts. However, although the concomitant use of PPIs has been shown to be effective in the upper GI tract, their protective effect in the lower GI tract is controversial. Furthermore, some studies have reported that PPIs adversely affect the small intestine [10–13].
Another preventive treatment for GI complications is the use of mucosal protective agents, such as misoprostol or rebamipide. It is generally considered that prostaglandins are important for the mediation of inflammation and maintenance of mucosa homeostasis, and that prostaglandin depletion is an important mechanism in NSAID-induced GI injury [14,15]. A supplementation with misoprostol (a synthetic prostaglandin) is effective in preventing NSAID-induced GI complications. However, a limitation is that it results in a high incidence of diarrhea in patients who have used it continuously [15]. This consequence has limited the wide use of misoprostol, while facilitating the development of other synthetic prostaglandins and stimulators. Rebamipide, 2-(4-chlorobenzoylamino)-3-[2(1H)-quinolinon-4-yl] propionic acid, is one such agent, possessing the required properties. It stimulates prostaglandin generation in the gastric mucosa and improves ulcer healing. In addition, it protects the gastric mucosa against acute injury caused by ulcerogenic factors like NSAIDs [16–18]. Previous clinical studies have evaluated the effect of medications on NSAID-induced GI complications, and several studies have shown that lansoprazole and misoprostol are effective in the prevention of these problems [19]. Another study proved that rebamipide had similar desired effects and fewer side-effects than those of misoprostol [20].
Some studies have used a combination of rebamipide and PPI, which have been found to be more effective when together, than when PPI is used alone, in preventing NSAID-induced GI lesions [4]. However, there are no studies comparing lansoprazole with rebamipide. Therefore, our research goal was to compare the effectiveness of rebamipide and lansoprazole through a multi-center, randomized, open labeled, pilot study. In this work, cyclooxygenase-1 (COX-1) inhibition was compared between lansoprazole and rebamipide with meloxicam; and the effect on NSAID-induced gastro-enteropathy in patients requiring continuous administration of NSAIDs was explored.
METHODS
Study subjects
A total of 40 subjects with rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, or any other musculoskeletal disease that requires continuous NSAID administration for a minimum of 12 weeks, were enrolled. the study was conducted from September 2014 to December 2015 through these three institutions. This study was a collaborative research conducted in Korean and Chinese hospitals, recruiting a total of 30 patients in Korea (20 patients in Seoul National University Bundang Hospital and 10 patients in Seoul Metropolitan Government Seoul National University Boramae Medical Center), and 10 patients in China (all in Dongfang Hospital, Tongji University).
Inclusion/exclusion criteria
Eligible subjects were adults (≥ 19 years in Korea; ≥ 18 years in China) diagnosed with rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, or any other musculoskeletal disease requiring continuous NSAID therapy for more than 12 weeks. Moreover, patients were required to have been free of GI symptoms for the previous 6 months; and endoscopy results, at the time of screening, were to reflect modified Lanza scores (MLS) of between 0 and 2.
Exclusion criteria included a previous history of GI tract surgery (with the exception of appendectomy); GI malignancies in the previous 5 years, recurrence of active GI ulcers or perforations, gastroesophageal varices, Barrett’s esophagus, and esophageal stricture. If subjects had a severe systemic disease, symptoms or signs of GI obstruction, malnutrition within the previous 3 months, or were pregnant or lactating women, they were excluded. Moreover, patients taking biologics or medications that might affect the study, and those with abnormal blood tests were also excluded.
Study design
This was a multi-center, randomized, open label pilot study as shown in Fig. 1. After completing all the screening procedures, subjects who met the selection criteria were randomly assigned to one of the two treatment groups. The study group received Mucosta (rebamipide, Otsuka Pharmaceutical Co. Ltd., Tokyo, Japan) in association with Mobic (meloxicam, Boehringer Ingelheim Pharma GmbH & Co KG., Origin, Germany), and the control group were administered Lanston (lansoprazole, Takeda Pharmaceuticals, Fujisawa, Japan) and Mobic (meloxicam). Rebamipide 100 mg was administered orally three times a day: after breakfast, after dinner and 30 minutes before sleep; while lansoprazole was administered in a single oral dose of 15 mg before breakfast. Concerning the NSAID treatment, subjects in both groups discontinued previous NSAID medications for 1 week (± 3 days) during the screening, and started Mobic® (meloxicam) 15 mg three times per day after a meal at baseline for 12 weeks. Esophagogastroduodenoscopy (EGD) and video capsule endoscopy (VCE) were performed at visit 1 (V1) and visit 5 (V5), and GI symptoms were evaluated at V1, V3, V4, and V5. Laboratory tests were performed at V2, V3, V4, and V5.
Evaluation
The efficacy of this study was appraised by comparing the baseline data with the results after the 12-week administration of the study regimen. The primary endpoint was the incidence rate of gastric ulcers detected by EGD after 12 weeks of treatment. Secondary endpoints included comparisons of the incidences of small bowel injuries identified by VCE, the intensity of GI symptoms measured by common term criteria for adverse events, and changes in serum concentrations of fatty acid-binding protein 2 (FABP2). The safety of this study was assessed using the adverse events profile, abnormal laboratory values, and vital signs.
Clinical measurement
Esophagogastroduodenoscopy
The results of the EGD were based on the endoscopic judgment of an endoscopist in each center. The degree of ulceration and mucosal injury was assessed by the endoscopist as a static image according to standardized guidelines, and the degree of mucosal injury was graded using MLS of 0 to 5.
Video capsule endoscopy
VCE (PillCam, Given Imaging, Yokneam, Israel; MiroCam, IntroMedic, Seoul, Korea) was used in this study. Two GI specialists (H.Y. and Y.J.C.) who remained blinded to the subjects’ treatment protocol, evaluated all video images for small intestinal injuries. If there were disagreements between specialists, they would discuss it and together determine the outcome. Moreover, the GI specialists verified the total number of small intestinal mucosal lesions on each VCE and compared these changes between the two groups.
GI symptoms
GI symptoms were assessed by categories that were classified based on the U.S. National Cancer Institute (NCI) “Common Terminology Criteria for Adverse Events (CTCAE) v4.03.”
Statistical analysis
The primary goal was to evaluate the incidence rate of gastric ulcers. The frequency and percentage of the subjects who developed gastric ulcers 12 weeks after administration of the study drugs were presented and the difference in the incidence rate of gastric ulcer between each group was calculated using a 95% confidence interval (CI). Pearson’s chi-square test or Fisher’s exact test were conducted to compare the two treatment groups in this regard.
A secondary goal was to compare the incidence of small bowel injuries using VCE. Statistics (the number of analyzed data, the mean ± standard deviation, median [range]) for change in the incidence of small bowel injuries from baseline to week 12 were presented for each treatment group. Comparisons between the means of the treatment groups were analyzed by the two sample t test or Wilcoxon’s rank sum test, according to the distribution of the variables.
Another secondary goal was a comparison of the intensity of total GI symptoms. The number of analyzed data, the mean ± standard deviation, median (range) for the total score and sub-assessments of GI symptoms’ intensity at each visit were presented according to each treatment group. The comparisons of total scores and sub-assessments of GI symptoms between treatment groups were performed via repeated analysis of variance (ANOVA).
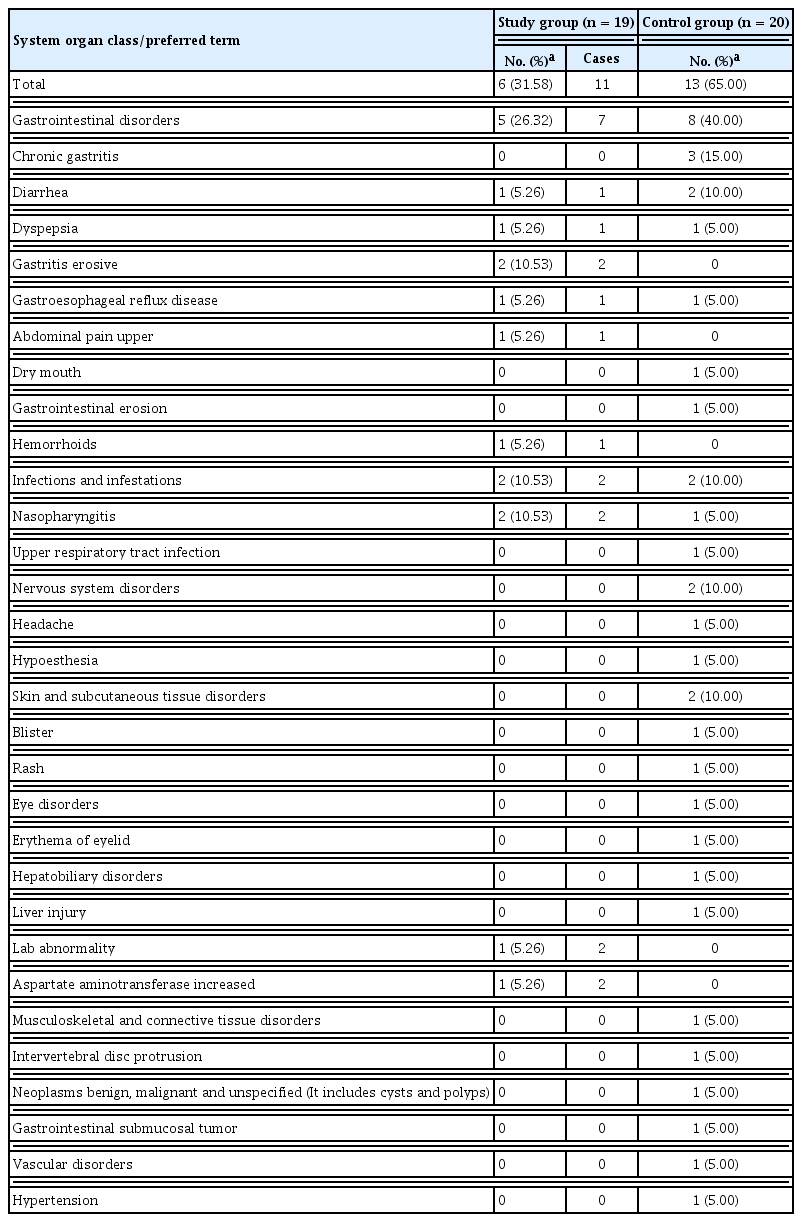
For a safety assessment, we presented each adverse event with a correspondent total number of occurrences and cases, and the occurrence rate in each treatment group was set at a 95% CI. The difference in the proportion of patients who experienced adverse events between groups was analyzed using Fisher’s exact test. All laboratory values and vital signs were compared within and between the treatment groups.
Statistical significance was defined as p < 0.05. All statistical analyses were performed using SAS version 8.2 (SAS Institute, Cary, NC, USA).
Ethical considerations
This study complied with all ethical considerations involving human subjects, as adopted by the 18th World Medical Assembly, Helsinki, Finland. Furthermore, it was approved by the Institutional Review Committees of Seoul National University Bundang Hospital (B-1312-229-005) and Boramae Hospital (16201416), and Shanghai East Hospital Affiliated Tongji University ([2014]临审第 (005) 号修正1) aspiring to protect the lives, health, privacy, and dignity of the study participants. Written informed consent was obtained from all patients.
RESULTS
Forty-four patients were screened. However, one patient was dropped due to a screening failure and three patients abandoned the study. As a result, 40 patients were included. The enrolled 40 patients were divided randomly into two groups composed of 20 participants each. In the study group, one patient then discontinued due to prohibited medication use, and four others withdrew from treatment. In the control group, two patients abandoned the study. Finally, 15 patients in the study group and 18 patients in the control group were analyzed. Patients’ demographics and baseline characteristics are shown in Table 1. There were no differences in the patient’s demographics and baseline characteristics between the two groups.
NSAID induced gastric ulcer
In order to evaluate the primary outcome of this study―NSAID-induced gastric ulcer, EGD was performed before and after drug administration in both groups. No gastric ulcers were found in both groups at baseline and after 12 weeks of NSAID use.
The incidence of the small bowel injuries
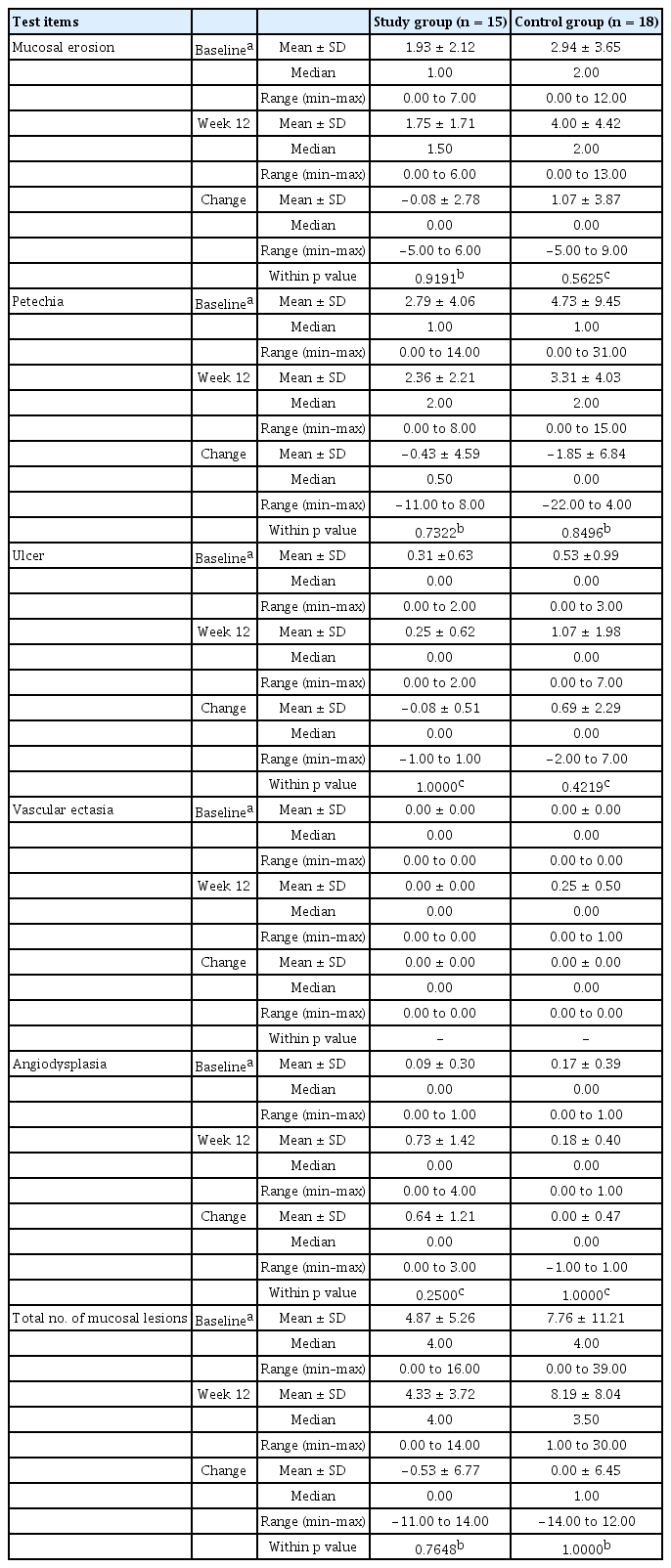
Using VCE, the small bowel injuries before and after taking NSAIDs are shown in Table 2. In the study group, mucosal erosion was 1.93 at baseline and 1.75 after 12 weeks of therapy, and changes in the number of erosions was −0.08. In the control group, erosion was 2.94 at baseline and 4.00 after 12 weeks of treatment, indicating that the difference in the number of erosions was 1.07. In the study group, ulcer was 0.31 at baseline and 0.25 at 12 weeks (difference of −0.08), while in the control group, ulcer was 0.53 at baseline and 1.07 after 12 weeks (difference of 0.69).
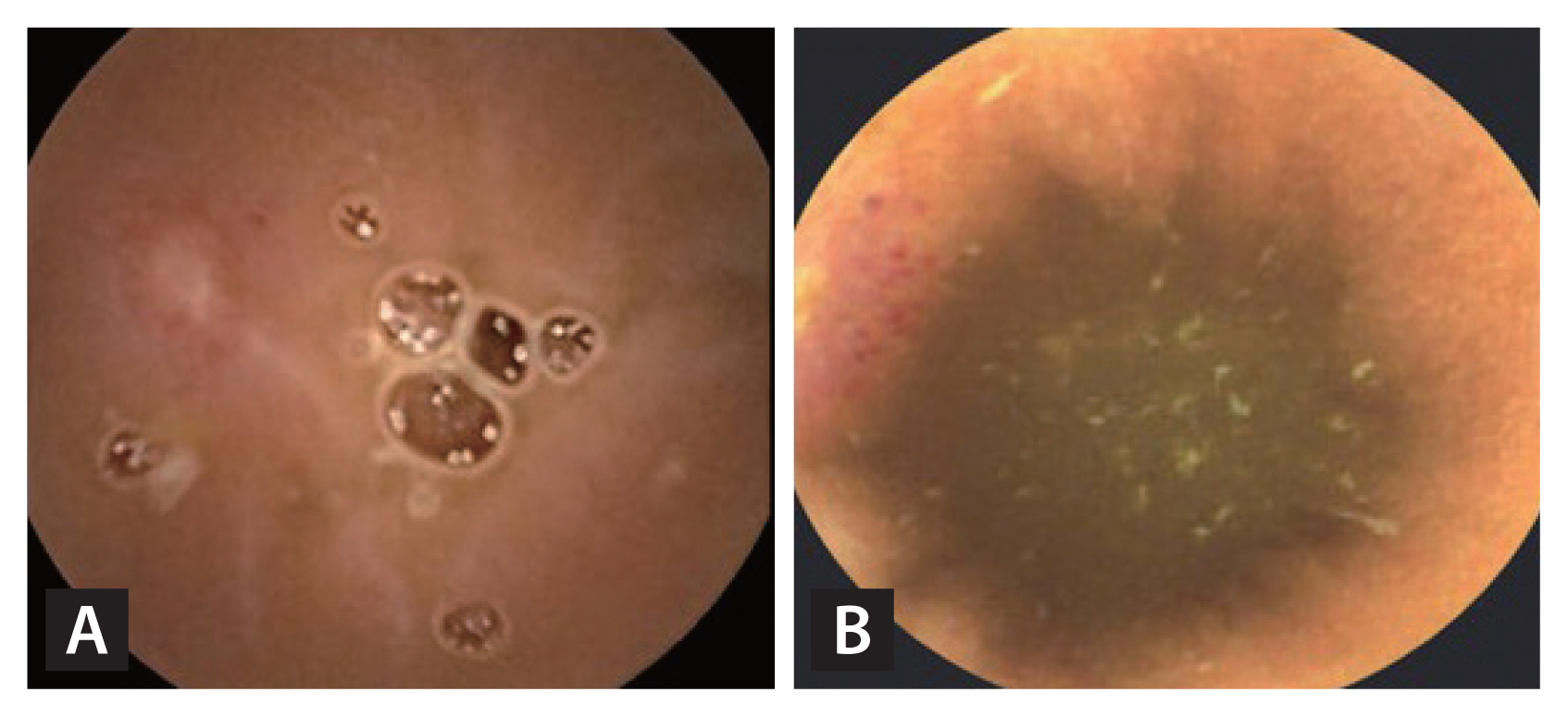
The number of subjects with mucosal breaks (multiple erosions and/or ulcer) that were thought to have a meaningful mucosal break after taking the NSAIDs for 12 weeks were three (20%) in the study group and six (40%) in the control group (p = 0.427). Examples of NSAID-induced small bowel injuries in our study are shown in Fig. 2.
The intensity of GI total symptoms
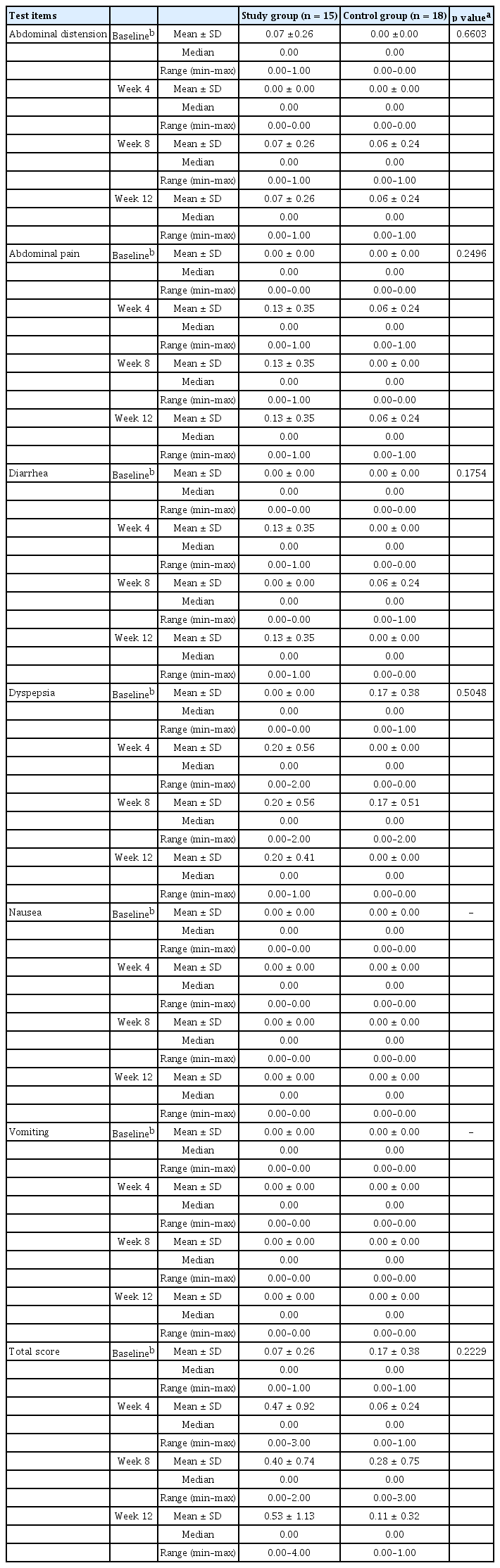
The intensity of GI symptoms such as abdominal distension, abdominal pain, diarrhea, dyspepsia, nausea, and vomiting were recorded according to the CTCAE v4.03 at each visit in both groups. Changes in the GI symptoms after administration of the drug were examined in the control and study groups. The results are shown in Table 3. There were no significant GI symptoms after treatment in both groups.
Safety assessment
Safety was assessed by recording all clinical adverse events, including frequency and intensity, on an adverse event record table, as well as laboratory values including hematology, chemistry, blood pressure, pulse, and vital signs. Serious adverse effects, which required hospitalization or had significant limitations on general activities, did not occur in either group. Adverse effects such as GI trouble or infection occurred in six of the 19 (31.58%) patients in the study group, and in 13 out of 20 (65%) patients in the control group (p = 0.036). Details are summarized in Table 4.
DISCUSSION
In this study, we compared the effects of rebamipide and PPI in the prevention of NSAID-induced gastroenteropathy in patients with long-term NSAID use. The NSAID-induced GI tract injury is largely caused by three mechanisms. First, NSAIDs inhibit COX-1, resulting in a decrease in prostaglandin levels, which are GI protective agents. Second, direct cytotoxic action leads to changes in the cell membrane permeability of the gastric mucosa and to induction of apoptosis and necrosis of the mucosal epithelium. Finally, there is an increase in production of proinflammatory mediators such as leukotriene [21–23]. Along these three pathways, the major pathophysiology of a peptic ulcer due to repeated NSAID exposure is caused by the inhibition of COX-1 activity in the GI mucosa due to the systemic action of NSAIDs [21–23]. These drugs inhibit the activity of COX-1, resulting in decreased bicarbonate secretion, mucosal blood flow, epithelial cell proliferation and reduced resistance to injury, causing gastric mucosal damage and ultimately, a peptic ulcer [24–26]. As a result, when using long-term NSAID therapy, you need a way to minimize the side-effects of these medications. Current guidelines recommend that in high-risk patients, PPI or misoprostol in combination with COX-2 selective inhibitors, one of them a NSAID, be used [27,28].
PPI irreversibly binds to the proton pump of the secreting canaliculi in parietal cells, the last stage of gastric acid secretion. This prevents gastric acid secretion and ulcers [7]. PPI has been shown to have a protective effect against NSAID-induced peptic ulcers by 80% (gastric) and 61% (duodenal), when compared with a placebo. In addition, PPI is effective in improving indigestion symptoms [29]. Therefore, PPI is the most widely used treatment for patients with long-term NSAID therapy. However, while the combination of PPI and NSAIDs has been shown to reduce NSAID-induced gastric ulcers, the incidence of lower GI side-effects increased steadily [30,31]. COX-2 selective inhibitors can theoretically preserve GI mucosal protection of prostaglandin mediated by COX-1 and GI mucosal injury may still be avoided by the inhibition of COX-1 at the clinically recommended dose [32,33]. Also, COX-2 selective inhibitors may lead to cardiovascular side-effects such as myocardial infarction and thrombosis [22,28].
Mistoprostol is the first drug approved for use in NSAID-induced gastric ulcers [28] but its use is limited due to GI side-effects such as diarrhea and dyspepsia. Some studies showed that rebamipide was as effective as misoprostol for the prevention of NSAID-induced gastric ulcers and that they could be considered for use, given their fewer side-effects [20]. Several studies proved that rebamipide regulates endogenous prostaglandin levels, inhibits reactive oxygen species, suppresses inflammation in the gastric mucosa, and controls neutrophil activation. Because of this, rebamipide was shown to be effective in NSAID-induced gastro-duodenal injury in several clinical and animal studies [34–36]. We have, therefore, compared rebamipide and PPI for their protective effects on NSAID-induced peptic ulcers.
In our study, no ulcer was found at baseline, or after 12 weeks of NSAID use, in either group. This study was the first to compare rebamipide to PPI in NSAID-induced peptic ulcers. However, since it was a pilot study further research is required.
NSAID-induced small bowel injury was also evaluated. It is known that NSAID-induced side-effects in the upper GI tract decrease with the use of PPI, while the incidence of lower GI side-effects continuously increase [30]. Hence, NSAID-induced small bowel injuries have been studied since their observation became possible using VCE [2]. Lower GI complications caused by NSAIDs may range from symptomless inflammation to severe problems such as bleeding, ulceration, obstruction and perforation. As these may result in longer hospitalizations and higher mortality rates, attention should be paid to them [37]. NSAIDs are absorbed in the jejunum and then undergo glucuronidation in the liver during enterohepatic circulation, separating into NSAID-glucuronide and bile. Once in the intestine it is de-conjugated by intestinal bacteria. Given this entero-hepatic circulation, the concentration of NSAID’s exposure to the intestinal mucosa is increased [38].
The mechanism of lower GI injury, which differs from that of upper GI tract injury, is that NSAIDs increase the permeability of the intestinal mucosa by uncoupling the oxidative phosphorylation of the mitochondria, resulting in intestinal mucosal damage due to intestinal bacteria, proteolytic enzymes, and bile [39]. The use of PPIs is known to be of no help for these lower GI side-effects [40]. In addition, some studies have shown that PPIs increased NSAID-induced small bowel injuries [41]. In a laboratory study published in 2011, a combined administration of NSAIDs and PPIs in mice resulted in increased small bowel damage due to intestinal bacterial dysbiosis, increased gram-negative bacteria and decreased actinobacteria. These changes in the intestinal environment due to PPIs were shown to exacerbate small bowel damage by NSAIDs [42]. Some clinical studies have also reported a high incidence of small bowel injuries in 60% to 80% of subjects who had taken nonselective NSAIDs and PPIs simultaneously. In particular, PPIs were shown to exacerbate intestinal mucosal damage caused by NSAIDs [43–46].
We further investigated whether rebamipide, which was effective in preventing NSAID-induced peptic ulcers, was effective in preventing NSAID-induced small bowel injuries. Mizoguchi et al. [47] suggested that rebamipide was effective in preventing NSAID-induced small bowel injuries in rats. Following this, several clinical studies have obtained the same results [4,48]. Kurokawa et al. [49] used VCE in 61 patients in order to analyze changes in small bowel erosion and ulcers in the rebamipide group (NSAID + rebamipide) and a placebo group (NSAID + placebo), after 4 weeks of treatment. Changes in the number of small intestinal erosions in the rebamipide group were −2.5 ± 3.4, and 2.1 ± 3.9 in the placebo group (p < 0.0001). Changes in the number of small intestinal ulcers in the rebamipide group were −0.5 ± 1.6, and 0.1 ± 0.7 in the placebo group (p = 0.024).
In our research, statistical significance could not be confirmed due to the small samples involved. In the study group, the small bowel erosions tended to decrease, and ulcers showed no significant changes 12 weeks after administration of the NSAIDs and rebamipide, as compared to the baseline values. In contrast, in the control group, both small bowel erosions and ulcers tended to increase after 12 weeks of treatment, as compared to the baseline values.
Niwa et al. [50] reported that NSAID-induced small intestinal mucosal injuries (multiple erosions, ulcer, bleeding, redness) occurred in eight out of 10 patients in the placebo group taking Omeprazole, and in only two out of 10 patients (p = 0.023) in the rebamipide group. Similarly, although there was no statistical significance, the results of our study were similar. In our research, subjects with mucosal breaks (defined as multiple erosions and/or ulcers) were three (20%) in the study group and six (40%) in the control group (p = 0.427). These results suggest that rebamipide is effective in the prevention of NSAID-induced small bowel injuries.
Theoretically, there are three mechanisms by which rebamipide is effective in preventing NSAID-induced small bowel injuries. First, rebamipide reduces intestinal permeability in a dose-dependent manner. Secondly, the loss of villi and tight junction damage caused by NSAIDs is restored [51]. Finally, the use of NSAIDs and PPIs cause an intestinal flora imbalance and worsen small intestinal damage. Rebamipide has the effect of inhibiting the intestinal flora imbalance (dysbiosis) by increasing lactobacillus and decreasing bacteroides and clostridium in intestinal bacterial composition [52].
Our study was the first to examine the preventive effects of lansoprazole and rebamipide on the upper and lower GI tracts in order to prevent NSAID-induced gastroenteropathy. Moreover, this study is also the first to compare the safety and efficacy of the two drugs, as well as, the preventive effects of NSAID-induced gastroenteropathy.
The limitation of this research is the small sample sizes involved. As a pilot study, it was difficult to garner more participants. Nevertheless, we found that NSAID-induced gastric ulcers occurred when long-term NSAIDs were taken by participants, even while receiving PPIs or rebamipide, and gastric ulcers did not occur in either group. For the same reason, it is difficult to obtain any statistically significant results with regard to the other observed variables.
In conclusion, although it has not been demonstrated that rebamipide helps prevent NSAID-induced gastric ulcers compared with the control group, it is likely to be more effective than lansoprazole in preventing small intestine damage caused by NSAIDs. To date, no effective drugs have been clearly found to prevent NSAID-induced small bowels injuries. Based on these results, further studies with larger sample sizes need to be conducted in order to prove that rebamipide is a more effective and safe drug than PPI for the prevention of NSAID-induced gastroenteropathy.
KEY MESSAGE
1. It is known that the use of proton pump inhibitor (PPI) is preventive for complications arising from the upper gastrointestinal tract of non-steroidal anti-inflammatory drug (NSAID)-induced gastroenteropathy, but the effectiveness of PPIs in preventing lower gastrointestinal complications is unclear.
2. Among those who take NSAIDs over the long-term, we compared the preventive effects and safety of PPIs and rebamipide on NSAID-induced gastroenteropathy.
3. As a result, we observed that rebamipide tended to be more effective in preventing NSAID-induced small bowel injury and that it led to fewer adverse effects than PPI.
Notes
No potential conflict of interest relevant to this article was reported.