Recognition and attitudes of Korean physicians toward fecal microbiota transplantation: a survey study
Article information
Abstract
Background/Aims
Fecal microbiota transplantation (FMT) represents a treatment option for recurrent Clostridioides difficile infection (CDI). Recently, FMT has been investigated in various clinical settings other than CDI. This study examined Korean physicians’ recognition of FMT and their attitudes toward this procedure
Methods
An online questionnaire included questions on indications for FMT, the FMT process, physicians’ attitudes toward FMT for the treatment of CDI and non-CDI diseases, and possible concerns.
Results
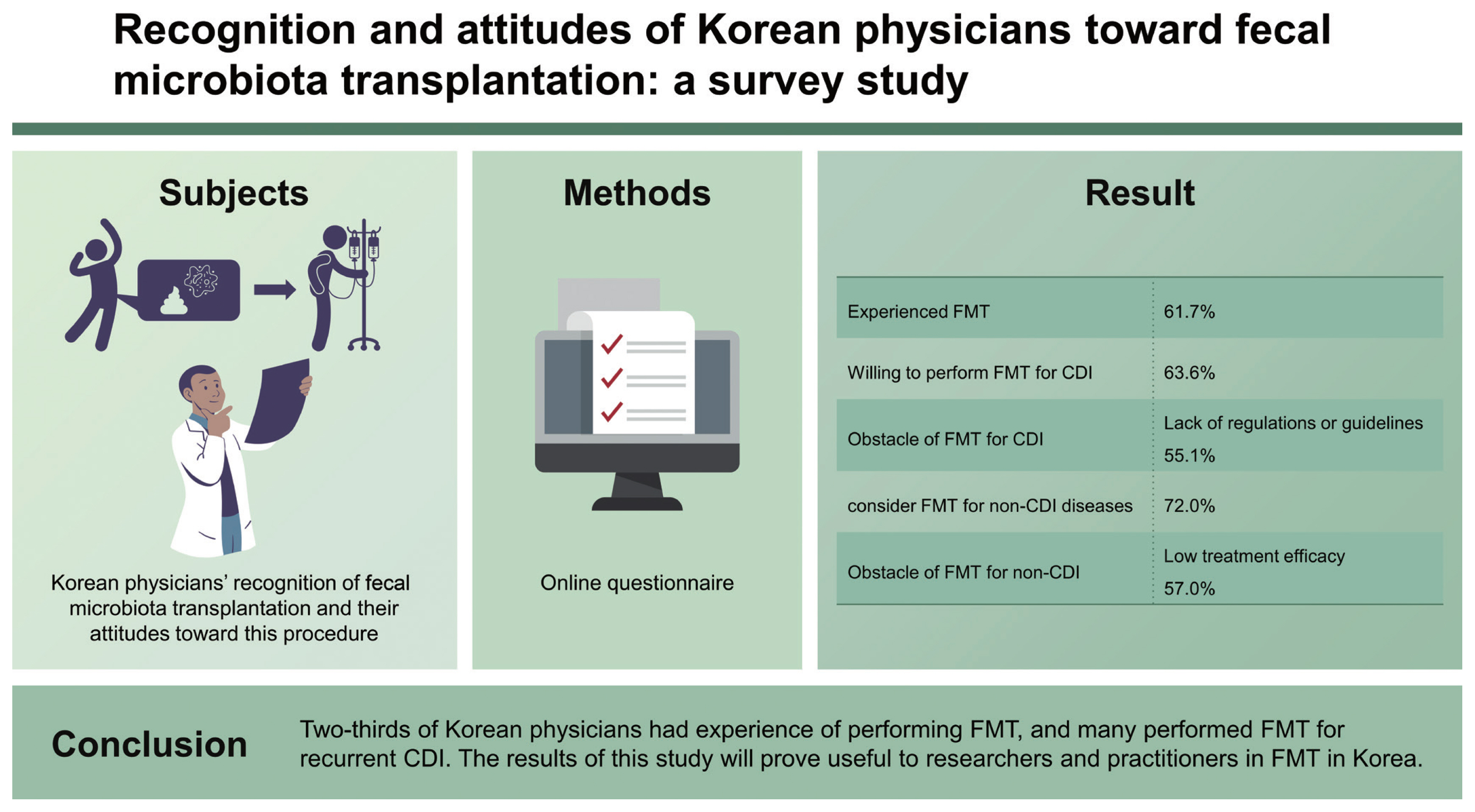
Finally, 107 physicians responded to this survey: 66 (61.7%) had experience of performing FMT, and 86 (80.4%) replied that they were willing to perform FMT for CDI. Two-thirds of physicians (63.6%, n = 68) would perform FMT for recurrent CDI on patients who had at least three recurrences. The most common obstacle to performing FMT for the treatment of CDI was the lack of regulations or guidelines (55.1%, n = 59). Seventy-seven (72.0%) physicians would consider FMT for non-CDI diseases when conventional treatment had failed. The most common obstacle for FMT for the treatment of non-CDI diseases was low treatment efficacy (57.0%, n = 61).
Conclusions
Two-thirds of Korean physicians had experience of performing FMT, and many performed FMT for recurrent CDI. The results of this study will prove useful to researchers and practitioners in FMT in Korea.
INTRODUCTION
Fecal microbiota transplantation (FMT) has been established as an effective treatment option for recurrent or refractory Clostridioides difficile infection (CDI) [1]. The clinical manifestations of CDI may differ between countries; for example, the incidence of CDI is lower in Asia-Pacific countries than in Western countries [2,3]. The incidence of community-associated CDI is reported to be up to 50% in Western countries [4]. By contrast, its proportion in Korea is reported to be less than 5% [2,5].
Because FMT provides a rapid resolution of CDI and is associated with a lower recurrence compared with conventional treatment [6], it is recommended for recurrent or refractory CDI [1,7,8]. The process of FMT includes patients selection (= FMT indication), donor screening, stool processing, and fecal microbiota administration. Despite its high efficacy for the treatment of CDI, physicians as well as patients are rather reluctant to pursue FMT as a treatment option for non-CDI diseases [9,10]. Nevertheless, with growing evidence of the relationship between dysbiosis of the gut microbiota and various diseases, including gastrointestinal and metabolic diseases, FMT has been investigated in a number of clinical settings other than CDI [11–14].
The first FMT procedure in Korea was reported in 2013 and it has since been performed in several academic centers [15–18]. In this nationwide survey, we investigated physicians’ recognition of FMT and their attitudes toward this procedure for treatment of both CDI and non-CDI diseases.
METHODS
A nationwide online survey was conducted that contained 20 questions in six categories. The survey focused on demographic characteristics of physicians’ experience of FMT, and their attitude toward FMT for the treatment of CDI and non-CDI diseases. The perception of safety of FMT and stool banks was investigated. The perception of safety of FMT was compared with that of a blood transfusion. Overall safety of FMT was investigated using a 5-point scale. Details of the questionnaire are presented in Appendix 1. The anonymous survey was conducted online by Survey Monkey (https://www.surveymonkey.com). The email invitation to participate was sent three times to members of the Korean Society of Neurogastroenterology and Motility, the Korean Association for the Study of Intestinal Diseases, and the Korean Society of Gastroenterology. Responses were collected from May to October 2019. Ethical approval for the study was obtained from Bucheon St. Mary’s Hospital (IRB approval number: HC22QASI0028). Informed consent was waived by the board.
RESULTS
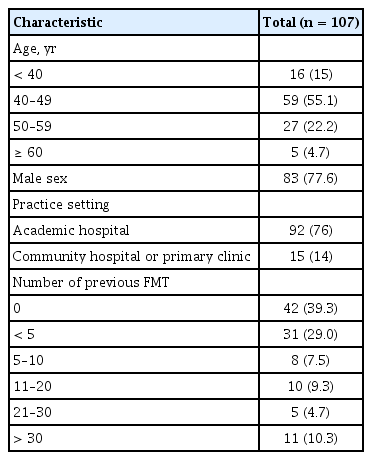
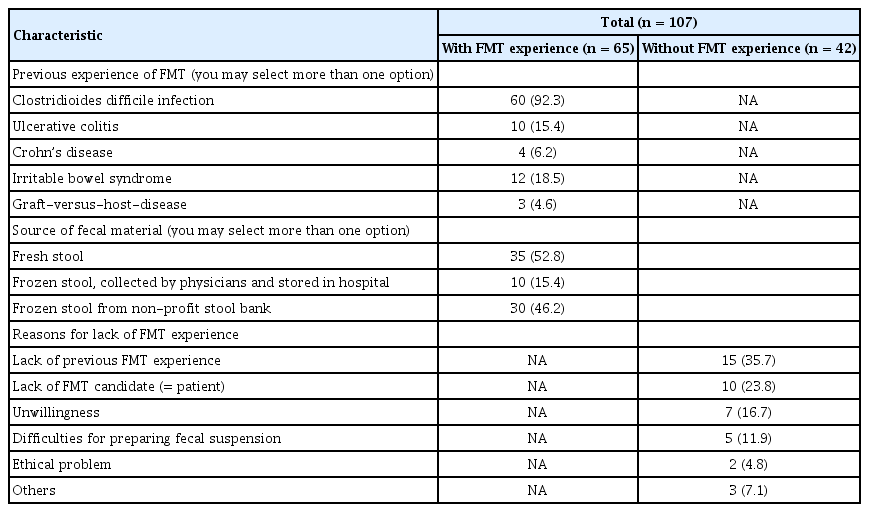
In total, 107 physicians responded to this survey (response rate: 11.7%, 107/917). The response rate of physicians in the academic hospital and the primary clinic or community hospital was 30.3% (92/304) and 2.4% (15/613), respectively. Demographic characteristics of responders are presented in Table 1 (males: 77.6%, 83/117). All responders were gastroenterologists. Most responders worked as professors in university-affiliated hospitals, and 60.7% had FMT experience. Details of their experience of FMT are presented in Table 2. Indications for FMT experience were CDI, inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), and graft-versus-host disease. FMT was mostly performed for the treatment of CDI (92.3%). Mostly fresh stool was chosen for FMT. Frozen stool from non-profit stool banks was the second most used fecal product. The reasons for lack of FMT experience were investigated: 35.7% physicians (15/42) chose lack of previous FMT experience.
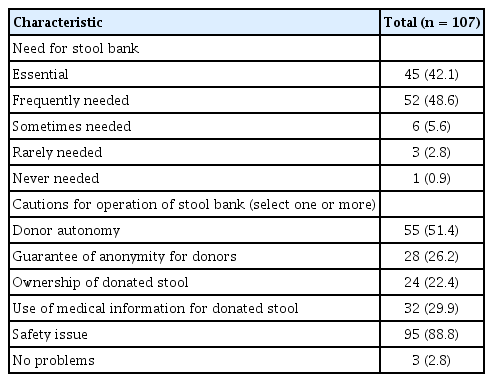
Attitudes toward FMT for the treatment of CDI are shown in Table 3. About 80% of physicians responded that they performed FMT when indicated; 17 (15.9%) responded that they would transfer patients to specialized treatment centers when FMT was indicated for a patient. Except for four physicians (3.7%), most responders replied that FMT could be considered for the treatment of CDI. The most common reason for trying FMT was due to its favorable treatment efficacy (89.7%). Where there was a possible indication of refractory (defined as unresponsiveness after at least two weeks of conventional treatment) or recurrent CDI, FMT was considered by 92.5% and 72.9% of responders, respectively. About one-third of physicians considered that FMT could be considered in severe CDI or fulminant CDI. For recurrent CDI, 63.6% considered FMT in three or more cases of CDI recurrence. Where FMT was not used to treat CDI, the two major obstacles were lack of guidelines (59.8%) and the aesthetically unappealing nature of the FMT procedure itself (57.9%).
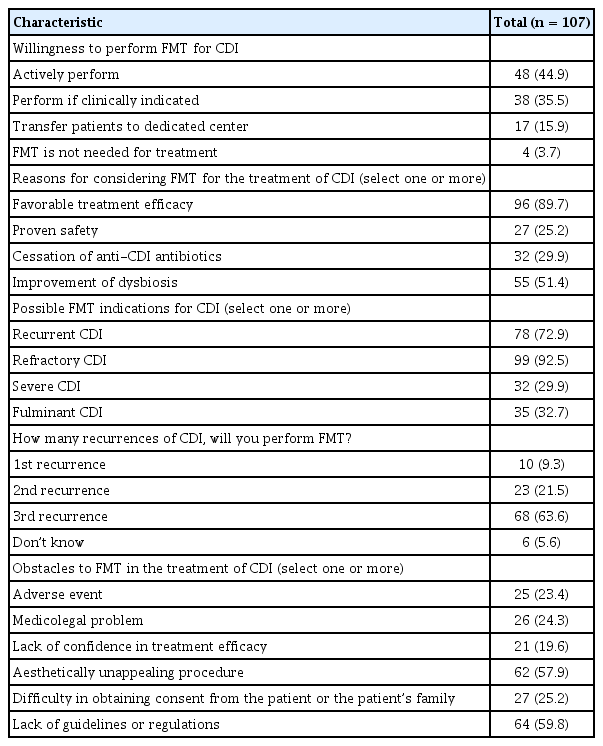
Attitude toward fecal microbiota transplantation for the treatment of Clostridioides difficile infection
Attitudes toward FMT for the treatment of diseases other than CDI are presented in Table 4. About one-third of physicians thought that FMT was not indicated for diseases other than CDI. The most common and second most common indications for FMT were ulcerative colitis (42.1%) and IBS (30.8%), respectively. For Crohn’s disease, 21.5% replied that FMT might be indicated; this response level was half that for ulcerative colitis. The most common obstacle for FMT for the treatment of diseases other than CDI was its low treatment efficacy (57%). Regarding safety issues, transmission of infectious diseases and adverse events related to the FMT procedure were considered as obstacles in 40.2% and 39.2% of cases, respectively.
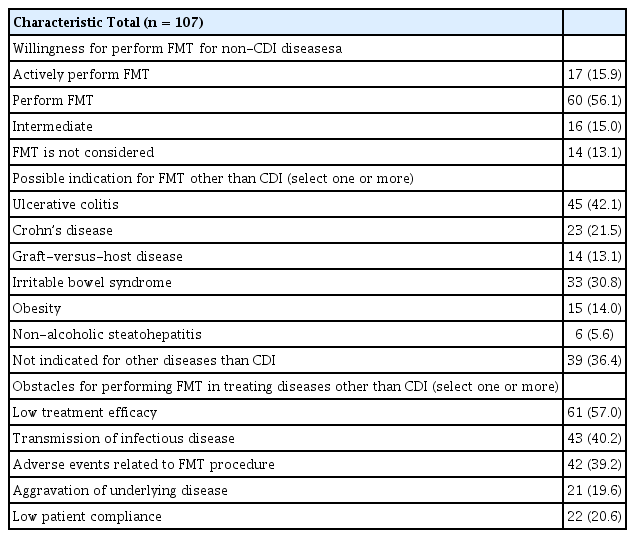
Attitude toward fecal microbiota transplantation for the treatment of diseases other than Clostridioides difficile infection
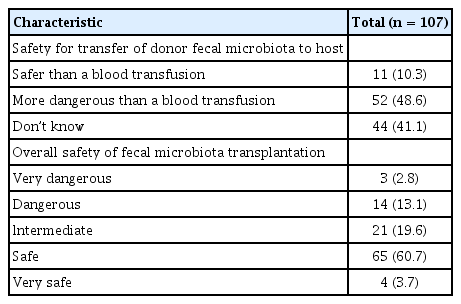
Table 5 indicates physicians’ awareness of safety issues. The safety of FMT was compared with the safety of a blood transfusion. Almost 40% of physicians replied that they could not judge the safety of the FMT procedure compared with that of a blood transfusion; 11 physicians (10.3%) believed that FMT was safer than a blood transfusion, while about half considered that FMT was more dangerous than a blood transfusion. In terms of overall safety, 17 physicians (15.9%) thought that FMT was dangerous or very dangerous.
Table 6 shows physicians’ attitudes toward a stool bank. About 90% responded that a stool bank was necessary to provide fecal material for FMT. Serious concerns regarding the operation of a stool bank involved safety issues (88.8%) and preservation of donor autonomy (51.4%).
DISCUSSION
In this survey, we investigated physicians’ current recognition of FMT and attitudes toward this procedure for treatment of CDI and various other diseases. About 60% of physicians had experience in FMT, and most considered FMT favorably for the treatment of CDI, and more so than for non-CDI diseases.
In recent years, FMT has shown favorable treatment efficacy for the treatment of CDI and is recognized as the standard of care for recurrent CDI. We first investigated physicians’ experience of performing FMT. About 60% of responders had previous FMT experience and CDI was the most common indication for previous FMT experience (92.3%). About half of respondents used frozen stool for FMT.
We also investigated the reasons for lack of FMT experience. Interestingly, the most common reason for not performing FMT was lack of previous FMT experience (35.7%). FMT procedures include donor screening, patient selection, fecal microbiota administration, and post-FMT care. In Western countries, the clinical characteristics of CDI may differ from those in Korea where community-acquired CDI is very low [2,5]. This means that CDI can be complicated because patients are elderly and often extremely ill. Our group reported the first experience of FMT in Korea and included older patients with multiple comorbidities [18]. In such cases, performing FMT is challenging.
We investigated the willingness of physicians to perform FMT for the treatment of CDI; about 80% were willing to do so when clinically indicated. The two most common obstacles to performing FMT for CDI treatment were a lack of guidelines or regulations (59.8%) and FMT being aesthetically unappealing in nature (57.9%). However, multidisciplinary national FMT guidelines have recently been published [8], and FMT is recommended for recurrent CDI after at least two recurrences. In the present survey, two-thirds of responders considered performing FMT to treat the third recurrence. The survey was completed before the development of Korean FMT guidelines, and FMT can now be performed as a ‘New Health Technology’ only for the treatment of CDI in Korea.
We also investigated physicians’ attitudes toward FMT for diseases other than CDI. FMT is currently being tested in clinical trials for non-CDI diseases because the efficacy of FMT is lower than that of CDI in these diseases. Therefore, we questioned physicians’ willingness to perform FMT for non-CDI diseases when conventional treatments have failed, which was a more conditional position. After CDI, ulcerative colitis and IBS were the most common possible indications for FMT. At the time of this survey, some randomized controlled trials for ulcerative colitis and IBS were published [19–21]. The most common obstacle to performing FMT for non-CDI diseases was low treatment efficacy. Although dysbiosis was proven in various diseases, the pathogenesis of IBS and IBD is multifactorial. In FMT trials for IBS, IBS-related symptoms did not improve after FMT even though dysbiosis improved [21].
Safety is a very important issue for FMT. Although FMT is widely performed in the USA, the Food and Drug Administration (FDA) has announced that fecal material used for FMT should be regulated as an ‘investigational new drug’ [22]. Physicians have raised concerns that the policy of the FDA could restrict FMT and argue that FMT should be managed in the same way as a blood transfusion rather than as a drug or as a solid organ transplantation [23]. We questioned the safety of FMT by comparing it to that of a blood transfusion. Except for those who did not choose either FMT or blood transfusion, 82.5% (52/63) of physicians thought that FMT was more dangerous than a blood transfusion. For overall safety, 17 physicians (15.9%) thought that FMT was dangerous or very dangerous.
We investigated physicians’ thoughts on stool banks, and about 90% of physicians agreed that they were necessary for FMT. FMT is an aesthetically unappealing procedure. Among the multiple steps, the handling and manufacturing of stool are the most unappealing. To counter these issues, nonprofit stool banks can help physicians by providing prescreened and manufactured fecal material [24], and when frozen stool is used, the foul odor is less severe compared with that of fresh stool [25]. In their overall perception of stool banks, the aspect of greatest concern for physicians’ was safety (89%). Although the FMT screening process is very strict and the qualification rate very low [26], transmission of pathogenic microorganisms is a major concern in FMT [27]. Recently, two universal stool banks have been established in Korea to provide stool product, which might help facilitate the FMT procedure.
Our study has several strengths. First, we included both CDI and non-CDI diseases in the survey, whereas similar previous surveys focused on CDI alone [9,28,29]. Given that FMT has recently been investigated for non-CDI diseases, it was logical to investigate physicians’ attitudes toward FMT in both CDI and non-CDI diseases. Second, attitudes to safety were questioned in detail. Third, over 100 gastroenterologists, more than in previous studies, participated in this survey [9,28]. Nevertheless, there are some limitations that should be addressed. First, the survey was carried out before the coronavirus disease 2019 (COVID-19) pandemic. During the pandemic, a guideline was released that recommended that FMT might be best performed to a limited degree [30]. Thus, our survey does not represent the attitudes of the pandemic era toward FMT. Second, because the survey targeted only gastroenterologists, recognition of FMT and attitudes toward the procedure were not investigated among other physicians. Third, the response rate of 11.7% was low. FMT is mostly performed in dedicated academic centers. Physicians in the primary clinic or community hospital may not be interested in FMT. Certainly, the response rate differed among physicians in the academic centers.
In conclusion, this study was the first to investigate Korean gastroenterologists’ recognition of FMT and their attitudes toward this procedure in for the treatment of CDI and non-CDI diseases. It is hoped that the results of this study will prove useful to researchers and practitioners in FMT in Korea.
KEY MESSAGE
1. The most common obstacle to performing fecal microbiota transplantation (FMT) for the treatment of Clostridioides difficile infection (CDI) was the lack of regulations or guidelines.
2. Two-thirds of physicians would perform FMT for recurrent CDI on patients who had at least three recurrences.
3. Three-fourth of physicians would consider FMT for non-CDI diseases when conventional treatment had failed.
Acknowledgments
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, (grant number: HI19C0481, HC20C0099) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (grant number: NRF-2021R1G1A1094049), Republic of Korea.
Notes
No potential conflict of interest relevant to this article was reported.