Clinical outcomes of and risk factors for secondary infection in patients with severe COVID-19: a multicenter cohort study in South Korea
Article information
Abstract
Background/Aims
Secondary infection with influenza virus occurs in critically ill patients and is associated with substantial morbidity and mortality; however, there is limited information about it in patients with severe coronavirus disease 2019 (COVID-19). Thus, we investigated the clinical outcomes of and risk factors for secondary infections in patients with severe COVID-19.
Methods
This study included patients with severe COVID-19 who were admitted to seven hospitals in South Korea between February 2020 to February 2021. Multivariate logistic regression analyses were performed to assess factors associated with the risk of secondary infections.
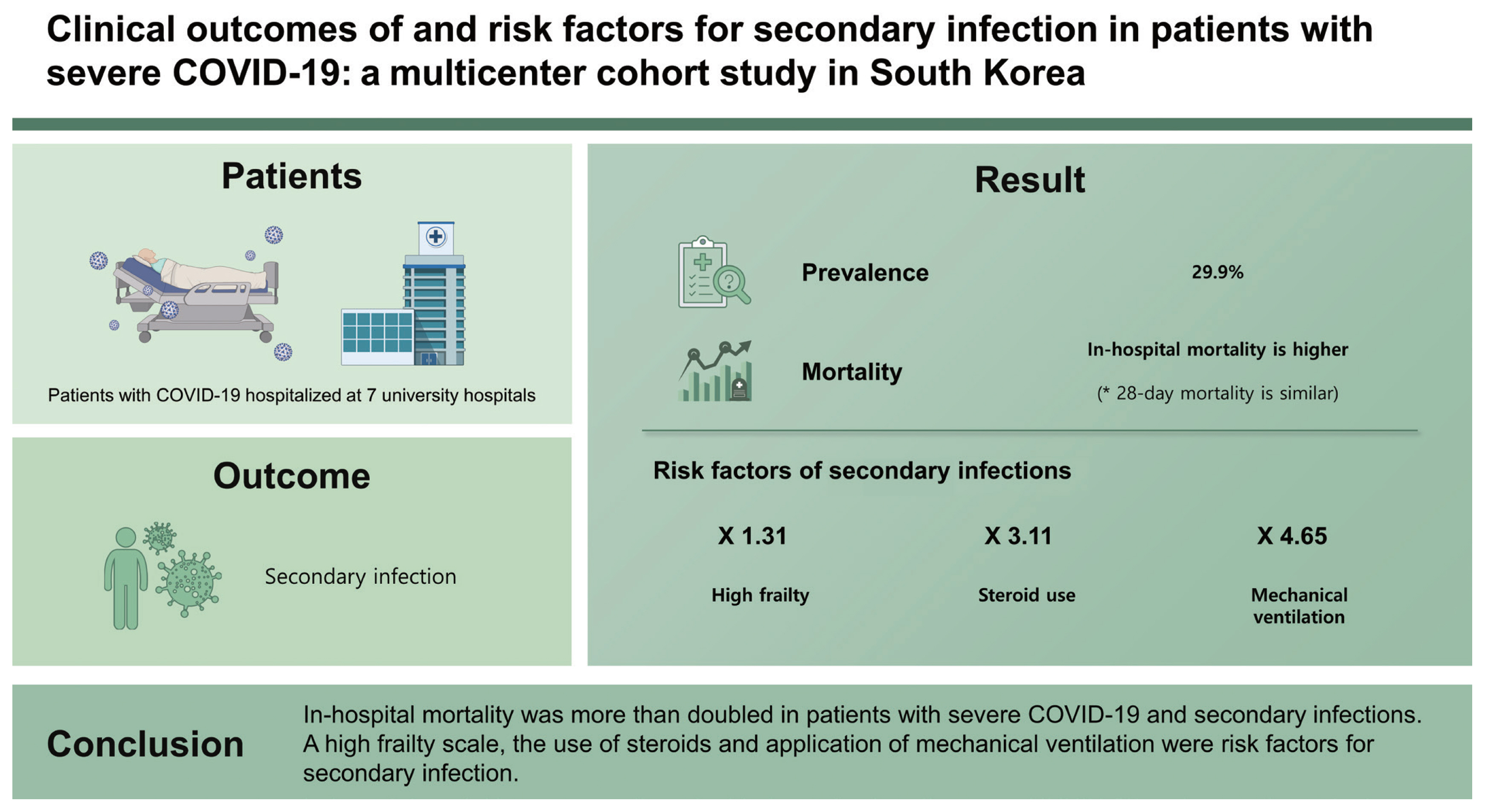
Results
Of the 348 included patients, 104 (29.9%) had at least one infection. There was no statistically significant difference in the 28-day mortality (17.3% vs. 12.3%, p = 0.214), but in-hospital mortality was higher (29.8% vs. 15.2%, p = 0.002) in the infected group than in the non-infected group. The risk factors for secondary infection were a high frailty scale (odds ratio [OR], 1.314; 95% confidence interval [CI], 1.123 to 1.538; p = 0.001), steroid use (OR, 3.110; 95% CI, 1.164 to 8.309; p = 0.024), and the application of mechanical ventilation (OR, 4.653; 95% CI, 2.533 to 8.547; p < 0.001).
Conclusions
In-hospital mortality was more than doubled in patients with severe COVID-19 and secondary infections. A high frailty scale, the use of steroids and application of mechanical ventilation were risk factors for secondary infection.
INTRODUCTION
The coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in December 2019 [1,2] and the cumulative number of reported cases worldwide has reached nearly 197 million; the cumulative deaths have reached 4.2 million [3].
Critically ill patients with COVID-19 have variable in-hospital mortality ranging from 29.9% to 53.4% [4–8]. A recent study reported that the risk factors for 90-day mortality in critically ill patients with COVID-19 were older age, obesity, diabetes mellitus, and a lower arterial oxygen partial pressure (PaO2)/fraction of inspired oxygen (FiO2) ratio [8]. Several studies report that bacterial co-infection in patients hospitalized with COVID-19 was low, although more than half of the patients received empirical antibiotics at the time of admission [9,10]. Early empirical antibiotics may lead to secondary bacterial or fungal infections [11,12].
Secondary bacterial infection in patients during the 2009 influenza pandemic contributed to significant mortality and morbidity [13]. A tertiary center in Israel reported that secondary infection in COVID-19 patients had an increased mortality risk compared to influenza patients [14]. However, there are a few studies on concomitant infections in patients with severe COVID-19, and fewer pathogen evaluations for secondary infections in the Republic of Korea. Thus, we aimed to evaluate the incidence, pathogens, risk factors, and outcomes of secondary infection in patients with severe COVID-19.
METHODS
Study design and patients
This was a multicenter, retrospective cohort study that enrolled adult (≥ 17 years old) patients with severe COVID-19 who were admitted to one of the seven tertiary or referral hospitals in South Korea from February 2, 2020 to February 28, 2021. In all participating hospitals, the same ventilator-associated pneumonia (VAP) bundles [15] and central line insertion bundles [16] were performed.
The Institutional Review Boards (IRBs) of the participating hospitals approved the study protocol, and the need for informed consent was waived due to the observational nature of the study (IRB of Chungnam National University Hospital, No. 2021-04-053, IRB of Chosun University Hospital, No. 2021-04-002).
Definition
SARS-CoV-2 infection was confirmed by reverse-tran-scriptase polymerase chain reaction assay; an oxygen saturation level of 94% or less on room air or a need for oxygen support was defined as severe COVID-19 infection [17]. Secondary infections occurring during illness or hospitalization were classified as hospital-acquired pneumonia (HAP) or VAP, bloodstream infection (BSI), central line-associated bloodstream infection (CLABSI), and catheter-associated urinary tract infection (CAUTI) [18,19]. The definitions of BSI, CLABSI, HAP or VAP, and CAUTI are provided in Supplementary Table 1. Definitions of vital signs and laboratory/radiologic data before and after infection are shown in Supplementary Table 2.
Data collection and outcomes
All study data were retrieved from the electronic medical records. Data on basic demographic characteristics, including sex, age, and data regarding initial laboratory, radiology evaluations, the need for invasive support (mechanical ventilation and continuous renal replacement therapy [CRRT], extracorporeal membrane oxygenation [ECMO]), and use of vasopressors were collected. Initial confusion, urea nitrogen, respiratory rate, blood pressure, 65 years of age and older (CURB-65) score, sequential organ failure assessment (SOFA) score, Acute Physiology and Chronic Health Evaluation II (APACHE II) scores, Charlson comorbidity index (CCI), and frailty scale were analysed.
Statistical analysis
All values are expressed as mean ± standard deviation for continuous variables and as percentages for categorical variables. Student’s t test was used for continuous data, and Pearson’s chi-squared test or Fisher’s exact test was used for categorical data. Multivariate logistic regression analyses with a backward elimination procedure, including all predictors showing a p ≤ 0.10 in the univariate analysis, were performed to obtain the adjusted odds ratio (OR) along with 95% confidence interval (CI) and to define the variables that were independently associated with disease severity. All p values were two-tailed, and p < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software version 22.0 (IBM Co., Armonk, NY, USA).
RESULTS
Characteristics of patients
From February 2, 2020 to February 28, 2021, 1,565 patients were admitted to the hospital for COVID-19 pneumonia. After excluding 1,077 non-severe COVID-19 patients and 140 patients without any type of culture, 348 patients were included in the analysis. Among the 348 patients, 145 patients were positive for any type of culture or bacterial polymerase chain reaction. Among them, 41 patients were diagnosed as colonization because they did not meet the definition of infection mentioned in Supplementary Table 1, and we classified them as the noninfected group. Of the 348 patients, 104 (29.9%) patients had at least one infection and were classified as an infected group; 244 (70.1%) patients without secondary infection were classified as a noninfected group (Fig. 1).
The baseline characteristics of patients at hospital admission are summarized in Table 1. Patients in the infected group were slightly older than those in the noninfected group (70.0 ± 13.1 years vs. 68.1 ± 12.7 years, p = 0.198). There was no statistical difference between the symptoms at admission and the period from symptom onset to hospitalization between the two groups. The CURB-65 (1.6 ± 1.1 vs. 1.2 ± 1.1, p < 0.001), SOFA (4.3 ± 4.0 vs. 2.7 ± 3.1, p < 0.001), and APACHE II scores (12.4 ± 7.3 vs. 9.8 ± 5.1, p = 0.001), CCI (3.5 ± 1.6 vs. 3.1 ± 1.8, p = 0.045), and frailty scale (4.1 ± 2.4 vs. 3.2 ± 1.8, p < 0.001) were higher in the infected group than in the noninfected group. Diabetes mellitus (DM, 39.4% vs. 27.0%, p = 0.022) was more common in the infected group than in the noninfected group; other comorbidities showed no statistically significant difference. Additionally, the body temperature (36.8°C ± 0.8°C vs. 37.1°C ± 0.9°C, p = 0.001) and Glasgow coma scale (13 ± 4 vs. 14 ± 2, p = 0.002) score were lower in the infected group, but the duration of fever was slightly longer in this infected group (3.0 days [interquartile range, IQR, 0.0 to 8.0] vs. 2.0 days [IQR, 1.0 to 6.0], p = 0.076).
Laboratory data and radiologic findings at hospital admission are presented in Supplementary Table 3. While comparing laboratory data from the infected group with that from the noninfected group it was observed that the PaO2/FiO2 ratio, pH, albumin, and d-dimer levels were lower, and the neutrophil lymphocyte ratio, potassium, C-reactive protein (CRP), and prothrombin time were higher in the infected group. Radiologic evaluation revealed fewer unilateral cases and more bilateral cases in the infected group.
Treatment and clinical outcomes of patients
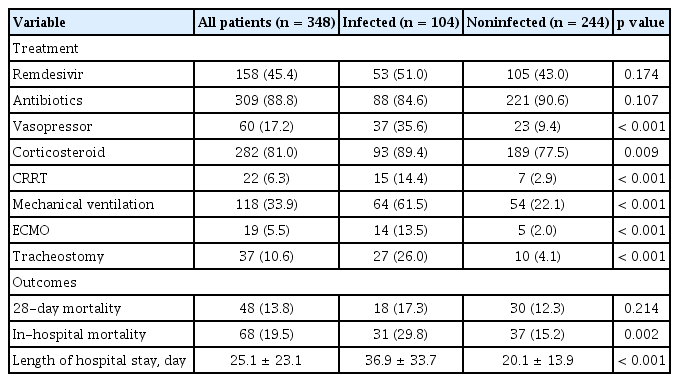
The treatment and clinical outcomes of the patients are presented in Table 2. Vasopressors (35.6% vs. 9.4%, p < 0.001) and corticosteroids (89.4% vs. 77.5%, p = 0.009) were more frequently used in the infected group. The most used steroid type was dexamethasone, followed by methylprednisolone and corticosteroid. There was no statistical difference in the use of steroid type, initial dose, and duration between the two groups (Supplementary Table 4). The use of antibiotics and remdesivir did not significantly different between the two groups. The most used antibiotics were 3rd generation cephalosporine (39.1%) and quinolone (32.5%). Among the types of antibiotics, piperacillin/tazobactam, carbapenem, aminoglycoside, vancomycin, teicoplanin, metronidazole, and colistin were used more in the infected group than in the noninfected group (Supplementary Table 4). Adequacy of antibiotic use was 58.7% in the infected group and 17.2% in the noninfected group (p < 0.001). CRRT (14.4% vs. 2.9%, p < 0.001), mechanical ventilation (61.5% vs. 22.1%, p < 0.001), ECMO (13.5% vs. 2.0%, p < 0.001), and tracheostomy (26.0% vs. 4.1%, p < 0.001) were also more commonly performed in the infected group.
There was no statistically significant difference in 28-day mortality (17.3% vs. 12.3%, p = 0.214) between the two groups. However, in-hospital mortality was higher (29.8% vs. 15.2%, p = 0.002) and the length of hospital stay was longer (36.9 ± 33.7 days vs. 20.1 ± 13.9 days, p < 0.001) in the infected group than in the noninfected group.
Factors associated with secondary infection
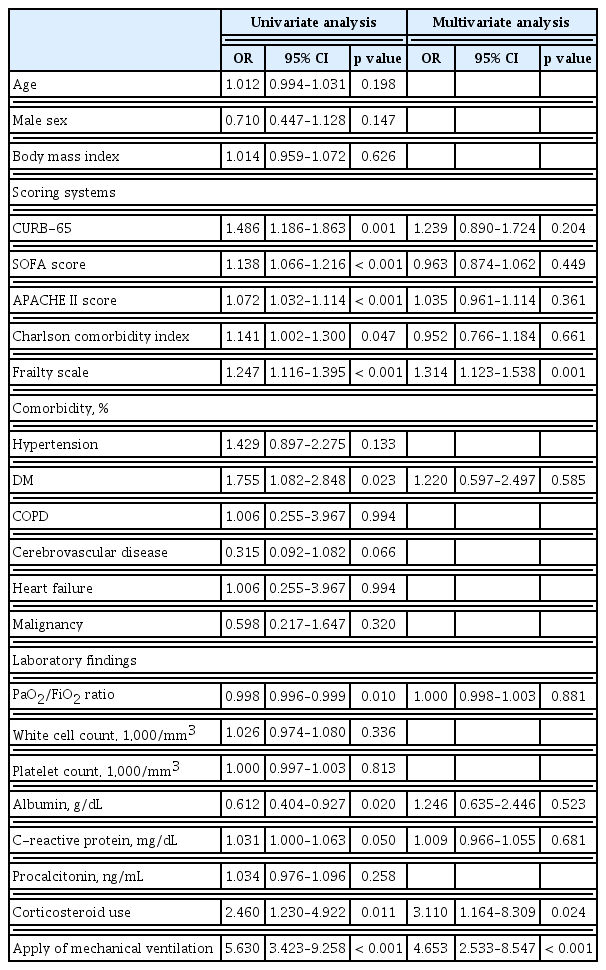
The results of the multivariate analysis of factors associated with infection are shown in Table 3. After adjusting for confounders, independent predictors of infection included the high frailty scale (OR, 1.314; 95% CI, 1.123 to 1.538; p = 0.001), corticosteroid use (OR, 3.110; 95% CI, 1.164 to 8.309; p = 0.024) and application of mechanical ventilation (OR, 4.653; 95% CI, 2.533 to 8.547; p < 0.001).
Pathogens of secondary infection and difference before and after infection
In the infected group, 73 patients (70.2%) had HAP and/or VAP, 36 patients (34.6%) had BSI and/or CLABSI, and 17 patients (16.3%) had CAUTI. The time of diagnosis of secondary infection was 13.0 days (IQR, 9.0 to 21.0) from symptom onset and 7.0 days (IQR, 4.0 to 16.0) from hospital admission (Supplementary Fig. 1). Multidrug-resistant (MDR) pathogens were identified in 51.0% (53/104) of the infected group.
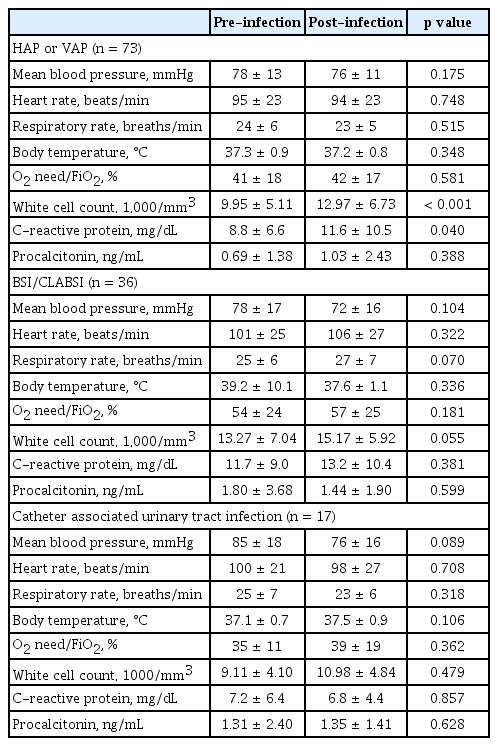
Fig. 2 shows the pathogens associated with each infection. The HAP and/or VAP pathogens were as follows: Acinetobacter baumannii (23/73, 31.5%), Klebsiella species (16/73, 21.9%), Streptococcus species (14/73, 19.2%), Haemophilus influenzae (10/73, 13.7%), Pseudomonas aeruginosa (6/73, 8.2%), Escherichia coli (3/73, 4.1%), other gram-negative bacteria (3/73, 4.1%), and Stenotrophomonas maltophilia (2/73, 2.7%). The time of diagnosis of infection for these pathogens was 12.0 days (IQR, 8.5 to 16.5) from symptom onset and 5.0 days (IQR, 4.0 to 11.5) from hospital admission (Supplementary Table 5). There were no significant differences in the vital signs before and after infection, but white blood cell (WBC) and CRP levels were significantly elevated (Table 4). Chest radiography showed ground glass opacity (51/73, 69.9%), consolidation (40/73, 54.8%), and aggravation (60/73, 82.2%). The MDR pathogen was seen in 46.6% (34/73) of cases with HAP/VAP, and the most common strain was multidrug-resistant Acinetobacter baumannii (MRAB) (28.8%, 21/73) (Supplementary Table 6).
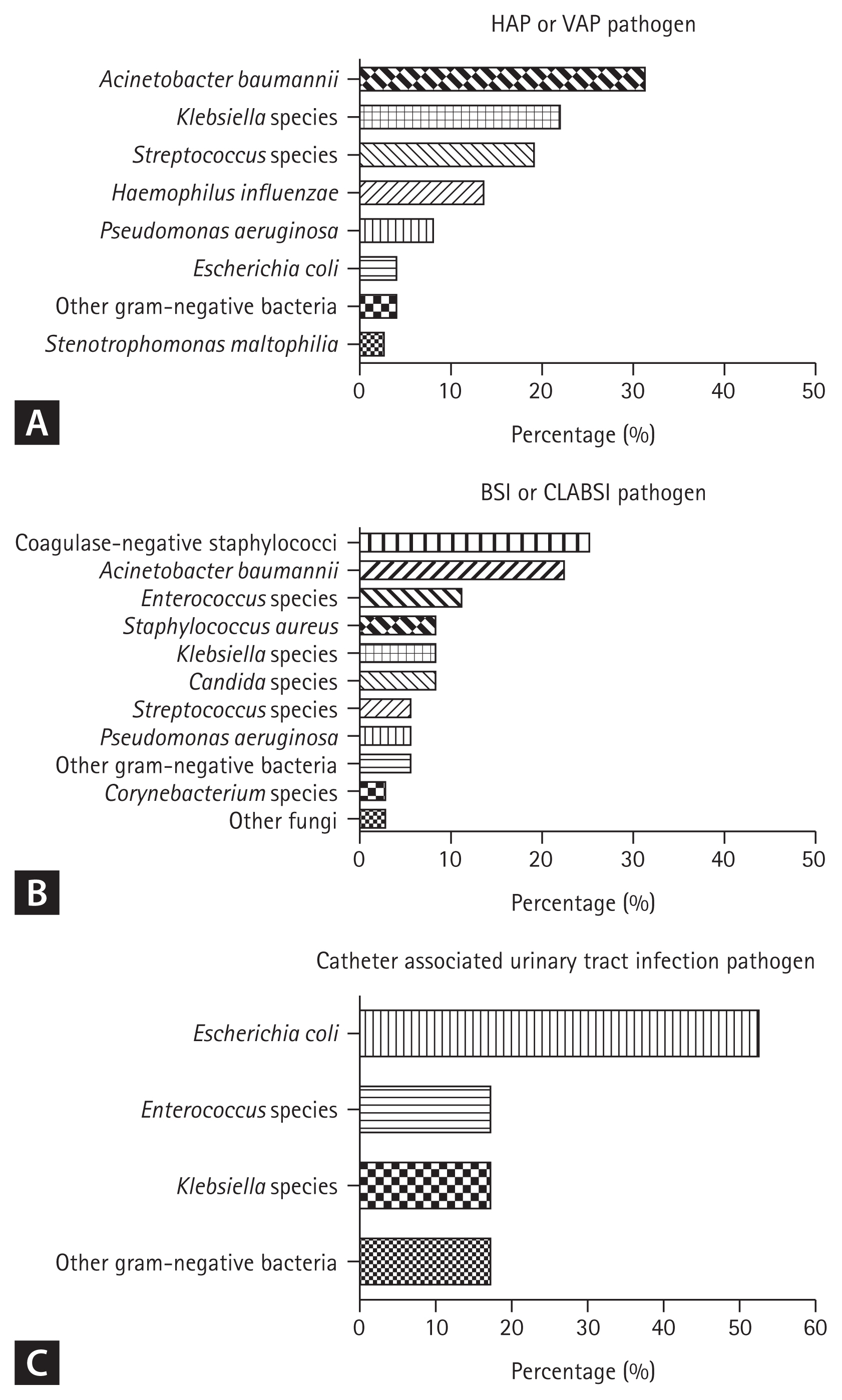
Identified pathogens for each secondary infection, (A) pathogen of hospital-acquired pneumonia (HAP) or ventilator-associated pneumonia (VAP), (B) pathogen of bloodstream infection (BSI) or central line-associated bloodstream infection (CLABSI), (C) pathogen of catheter-associated urinary tract infection.
The BSI and CLABSI pathogens were as follows: coagulase-negative staphylococci (9/36, 25.0%), A. baumannii (8/36, 22.2%), Enterococcus species (4/36, 11.1%), Staphylococcus aureus (3/36, 8.3%), Klebsiella species (3/36, 8.3%), Candida species (3/36, 8.3%), Streptococcus species (2/36, 5.6%), P. aeruginosa (2/36, 5.6%), other gram-negative bacteria (2/36, 5.6%), Corynebacterium species (1/36, 2.8%) and other fungi (1/36, 2.8%). The patients’ lines at the time of BSI or CLABSI infection were C-line (35/36, 97.2%), Hemocath (7/36, 19.4%), A-line (34/36, 94.4%), ECMO line (4/36, 11.1%). The time of diagnosis of infection for these pathogens was 21.5 days (IQR, 14.3 to 31.0) from symptom onset and 17.0 days (IQR, 8.8 to 26.5) from hospital admission (Supplementary Table 5). Before and after infection, respiratory rate and WBC levels were slightly increased (p = 0.070, p = 0.055, respectively), but there was no statistically significant difference (Table 4). The MDR pathogen was seen in 38.9% (14/36) of cases with BSI/CLABSI, and the most common strain was extended-spectrum beta-lactamase (ESBL) (+) bacteria (16.7%, 6/36) (Supplementary Table 6).
The CAUTI pathogens were as follows: E. coli (9/17, 52.9%), Enterococcus species (3/17, 17.6%), Klebsiella species (3/17, 17.6%), and other gram-negative bacteria (3/17, 17.6%). The time of diagnosis of infection was 14.0 days (IQR, 3.5 to 25.0) from symptom onset and 11.0 days (IQR, 0.0 to 20.0) from hospital admission (Supplementary Table 5). There were no statistically significant differences in vital signs or laboratory data before and after infection (Table 4). The MDR pathogen was seen in 52.9% (9/17) cases with CAUTI, and the most common strain was ESBL (+) bacteria (35.3%, 6/17) (Supplementary Table 6).
DISCUSSION
In this multicenter cohort study, secondary infection was identified in 29.9% of patients with severe COVID-19. The score to evaluate the severity of patients in the infected group was higher than that in the noninfected group at the time of admission, and with a longer duration of fever. The use of vasopressors, corticosteroids, CRRT, mechanical ventilation, and ECMO during hospitalization was higher in the infected group. There was no difference in 28-day mortality, but in-hospital mortality was higher and hospital length of stay was longer in the infected group. High frailty scales, steroid use, and the application of mechanical ventilation were associated with the occurrence of secondary infections.
In previous studies, secondary infections occurred in 3.6% to 46% of hospitalized COVID-19 patients [2,19–25]. In this study, secondary infection was identified in 29.9% of patients with severe COVID-19, and the outcomes were compared. Grasselli et al. [22] showed that hospital-acquired infection occurred in 46% of critically ill patients with COVID-19; VAP was the most common infection, found in 51%; BSI was found in 35%, and urinary tract infection (UTI) in 8% [22]. Huang et al. [24] reported that secondary infection as a complication was found in 10% of COVID-19 patients, and this study included all patients diagnosed with COVID-19. Ripa et al. [19] showed that secondary infection was diagnosed in 9.3% of hospitalized COVID-19 patients. Vijay et al. [25] reported that 3.6% of hospitalized COVID-19 patients developed secondary bacterial or fungal infections. The incidence of secondary infection varies, and the country, type of hospital, and severity of the enrolled patients may have affected the results. In the study by Langford et al. [18], bacterial infections occurred in 5.9% (95% CI, 3.8% to 8.0%) of all hospitalized patients and 8.1% (95% CI, 2.3% to 13.8%) of critically ill patients. Bacterial infections were more common in patients with higher severity. In a study by Sogaard et al. [26], hospital-acquired bacterial and fungal infections were more frequent among intensive care unit (ICU) patients than other patients (36.6% vs. 1.7%). Therefore, it seems that the incidence of secondary infection in severe and critically ill COVID-19 patients is higher than that in general COVID-19 patients. In a previous study of critically ill patients, secondary infections were common (about 51%) and the risk of infection was associated with the duration of ICU stay [27]. In a study of 55 ICUs from eight developing countries, device-associated nosocomial infection was seen in 14.7% patients, of which 41% were VAP, 30% CLABSI, and 29% CAUTI [28]. The percentage infection rate in critically ill COVID-19 patients was similar to that in critically ill patients with other illnesses, but there is a study that showed that the risk of healthcare-associated infections increases in situations such as a COVID-19 surge [29]. Therefore, it is necessary to pay attention to the occurrence of secondary infection in critically ill COVID-19 patients.
Factors related to secondary infection include age, positive end-expiratory pressure, treatment with broad-spectrum antibiotics at admission, anti-inflammatory treatment, baseline lymphocyte count, baseline PaO2/FiO2 ratio, and ICU admission in the first 48 hours after hospital admission [19,22,30]. In this study, the occurrence of secondary infection was associated with the frailty scale, use of corticosteroids and application of mechanical ventilation. The use of anti-inflammatory agents may [30–32] or may not [22,33] increase the risk of infection. However, the use of steroids [34,35] and increased severity [36] of the patient may increase the likelihood of a concomitant infection; therefore, it is necessary to carefully examine whether additional infections occur in patients.
Our study provided a detailed description of secondary infections in severe to critically ill COVID-19 patients. In this study, the common pathogens of HAP and VAP were A. baumannii, Klebsiella species, and Streptococcus species, that of BSI or CLABSI were coagulase-negative staphylococci and A. baumannii, and that of CAUTI was E. coli. Various infectious pathogens were identified in other studies. VAP is one of the common complications in critically ill COVID-19 patients. In a study of critically ill COVID-19 patients by Meawed et al. [37], the bacterial pathogens commonly found in VAP were pandrug-resistant Klebsiella pneumonia (41.1%) and MRAB (27.4%). The commonly identified strain in the coVAPid cohort study was P. aeruginosa (24.9%), and MDR pathogen was found in 20.7% of the cases [38]. A meta-analysis of Ippolito et al. [39] showed various pathogens in VAP, and the MDR pathogen was identified in 1.4–67% of the cases. Grasselli et al. [22] reported that in critically ill COVID-19 patients, the common pathogens for VAP were S. aureus and P. aeruginosa, the common pathogens for BSI and CLASI were Enterococcus species and Enterobacter species, and the common pathogens for UTI were E. coli, similar to our results. The most common pathogens of respiratory tract infection in other studies are S. aureus [40], Mycoplasma species [18,41], and Klebsiella species [42]. The most common pathogens of BSI or CLABSI in other studies are S. aureus [40], coagulase-negative staphylococci [19,30,43], enterococcus species [30,44,45], A. baumannii [21,32], P. aeruginosa [32]. A study by Fakih et al. [46] compared CLABSI and CAUTI before and after the COVID-19 pandemic. There was an increase in coagulase-negative Staphylococcus and Candida species among CLABSI pathogens after the COVID-19 pandemic, but there was no significant difference in CAUTI pathogens before and after the pandemic [46]. The type of pathogen and drug resistance are affected by the type of hospital and country. Therefore, we believe that identifying the pathogens of each country and hospital and using appropriate antibiotics will help improve patient prognosis.
Changes in vital signs and laboratory data before and after infection have rarely been studied. In previous correspondence, mean blood pressure (91.8 ± 12.0 mmHg vs. 85.3 ± 12.5 mmHg, p = 0.002) decreased slightly, but other vital signs were not significantly different. Chest radiography worsened in 58.1% of the infected group [47]. In this study, the vital signs before and after infection were directly compared, and there was no significant difference. However, chest radiography worsened after HAP or VAP, and the WBC count and CRP increased after infection. Therefore, it seems that the WBC count and CRP levels are good indicators for detecting the occurrence of secondary infections.
This study had several limitations. First, the study group included only patients in tertiary or referral hospitals capable of critical care. This may have affected the results, as it included patients with worsening conditions who had been transferred from other hospitals or from living treatment centers. Second, data were collected from the electronic health record database instead of manually reviewed medical records; thus, excluding possible levels of detail. Third, we were operating on COVID-19 quarantine beds in a ward where intensive treatment is possible. Therefore, it was impossible to analyse the differences in strain rates according to the wards and ICUs. Fourth, we classified the pathogen by setting diagnostic criteria for secondary infection. However, it may have been difficult to completely differentiate between colonization and pathogens.
In conclusion, secondary infection was confirmed in 29.9% of patients with severe COVID-19, and HAP or VAP was the most common infection. In-hospital mortality was more than doubled in the group of patients with secondary infections. Patients with a high frailty scale, the use of steroids and application of mechanical ventilation are associated with a high risk of secondary infection. Therefore, we believe that rapid diagnosis of infection and appropriate prevention and treatment in this patient population can help improve patient prognosis.
KEY MESSAGE
1. Secondary infection was confirmed in 29.9% of patients with severe coronavirus disease 2019 (COVID-19).
2. Hospital acquired pneumonia or ventilator-associated pneumonia was the most common secondary infection and the in-hospital mortality was more than doubled in patients with secondary infection.
3. Patients with a high frailty scale, use of steroids and application of mechanical ventilation are associated with a high risk of secondary infection.
Notes
No potential conflict of interest relevant to this article was reported.