Diabetes screening in South Korea: a new estimate of the number needed to screen to detect diabetes
Article information
Abstract
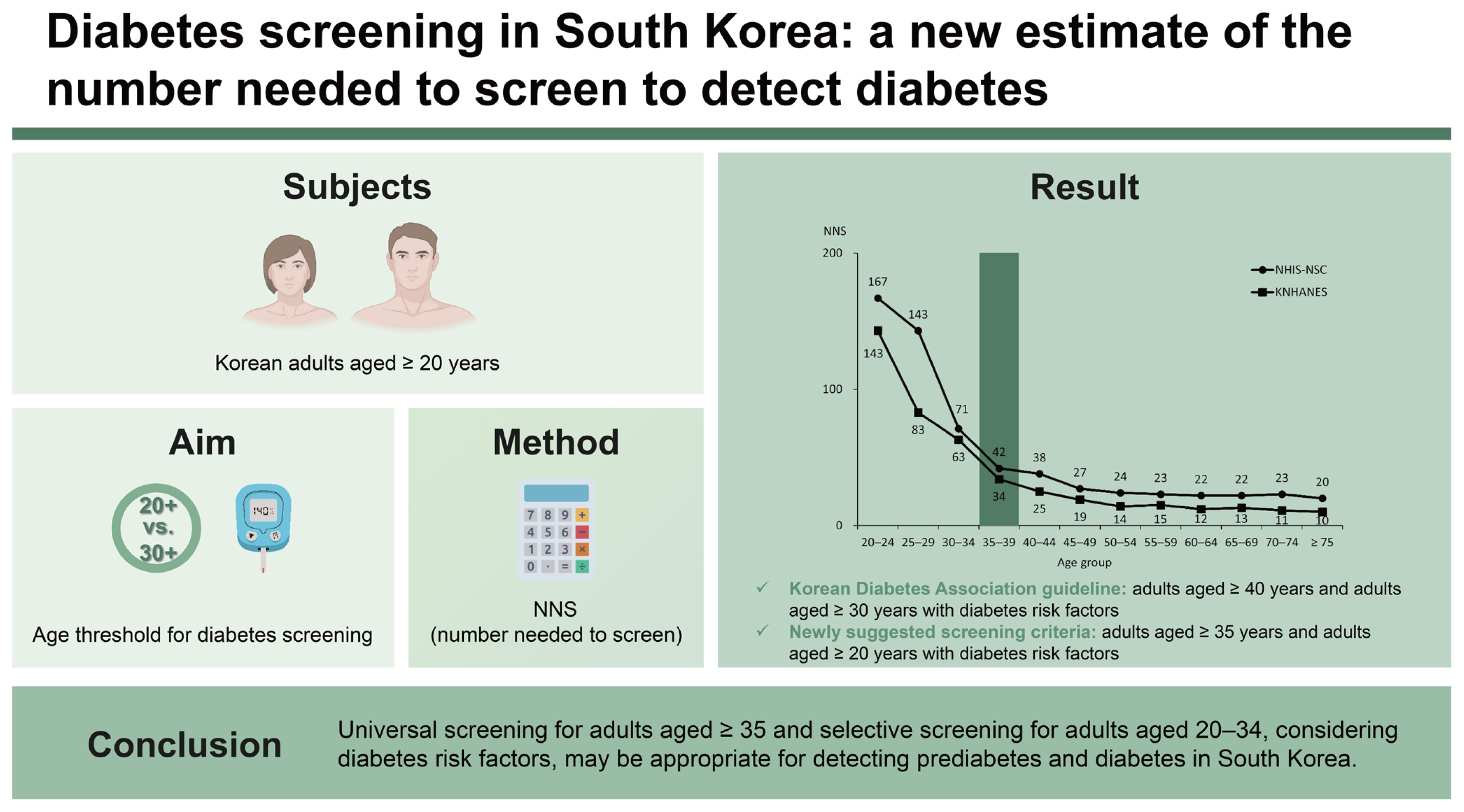
Background/Aims
The Korean Diabetes Association (KDA) guidelines recommend adults aged ≥ 40 years and adults aged ≥ 30 years with diabetes risk factors for diabetes screening. This study aimed to determine the age threshold for diabetes screening in Korean adults.
Methods
This study was based on the analyses of Korean adults aged ≥ 20 years using the Korea National Health and Nutrition Examination Survey (KNHANES) and the National Health Insurance Service-National Sample Cohort (NHIS-NSC). To evaluate screening effectiveness, we calculated the number needed to screen (NNS).
Results
NNS to detect diabetes decreased from 63 to 34 in the KNHANES and from 71 to 42 in the NHIS-NSC between the ages of 30–34 and 35–39. When universal screening was applied to adults aged ≥ 35, the NNS was similar to that of adults aged ≥ 40. Compared to the KDA guidelines, the rate of missed screening positive in adults aged ≥ 20 decreased from 4.0% to 0.2% when the newly suggested screening criteria were applied.
Conclusions
Universal screening for adults aged ≥ 35 and selective screening for adults aged 20 to 34, considering diabetes risk factors, may be appropriate for detecting prediabetes and diabetes in South Korea.
INTRODUCTION
In recent decades, diabetes mellitus has become a major health threat worldwide. The global prevalence of diabetes in those aged 20 to 79 years has reached pandemic proportions in 2019 and has increased from 9.3% in 2019 to 10.5% in 2021 [1]. In South Korea, the prevalence of diabetes increased from 10.3% in 2011 to 13.9% in 2020 [2]. However, approximately one-third of people with diabetes aged ≥ 30 years are unaware of diabetes, and diabetes awareness has decreased from 72.4% in 2007–2009 to 67.8% in 2016–2018. Approximately one million people are estimated to live without knowing that they have diabetes [3]. In addition, the prevalence of prediabetes, an early stage in the hyperglycemic continuum, was 26.9% in 2018 [4].
Prevention of diabetic complications is a key issue in maintaining the quality of life of people with diabetes. Diabetic complications result from an abnormal metabolic environment caused by chronic hyperglycemia [5]. The duration of diabetes and degree of hyperglycemia affect the risk of diabetic complications [6–8]. Early detection and management of prediabetes and diabetes can delay the development and progression of diabetes and diabetic complications. However, diabetes is often asymptomatic in its early stages and remains undiagnosed for many years, and diabetic complications can often occur before the clinical diagnosis of type 2 diabetes [9,10]. Thus, the early identification of prediabetes and diabetes is the first step in diabetes care.
In 2021, the Korean Diabetes Association (KDA) recommended annual prediabetes and diabetes screening for adults aged ≥ 40 years and adults aged ≥ 30 years with diabetes risk factors such as overweight or obesity [11]. However, the age thresholds for screening individuals were determined based on the guidelines of other countries, and the evidence for screening for Koreans was limited [12]. In addition, in South Korea, the incidence of diabetes among adults aged 20 to 39 years has increased [13]. Therefore, this study aimed to determine the age thresholds for diabetes screening to provide evidence for screening guidelines.
METHODS
Data source
This cross-sectional study was conducted using two nationally representative datasets: the Korea National Health and Nutrition Examination Survey (KNHANES) in 2016 to 2020 and the National Health Insurance Service-National Sample Cohort (NHIS-NSC) in 2012 to 2017. The KNHANES is a national surveillance system in South Korea that has produced nationwide statistics regarding the health and nutritional status of the Korean population since 1998 [14]. The NHIS-NSC, conducted by the NHIS, is a population-based cohort comprising 2.2% of all eligible Koreans. The NHIS-NSC contains information on sociodemographic characteristics, hospital claims (defined by the International Classification of Diseases, 10th Revision, Anatomical Therapeutic Chemical [ATC] codes), and information on national health examinations [15].
The study protocol was reviewed and approved by the Institutional Review Board of Ajou University Hospital (approval no. AJOUIRB-EXP-2022-280) and Soongsil University (approval no. SSU-202003-HR-201-01). The requirement for informed consent was waived because all participants were de-identified.
Study population
For analysis of the KNHANES, we identified 27,348 adults aged ≥ 20 years without a diagnosis of diabetes in KNHANES VII (2016 to 2018) and KNHANES VIII (2019 to 2020). Diagnosed diabetes was defined as a self-reported previous diagnosis of diabetes by a medical doctor or taking glucose-lowering drugs or insulin. After excluding participants with a history of pregnancy or missing values, 24,822 participants were included in the final analysis. For the analysis of the NHIS-NSC, we included adults aged ≥ 20 years who underwent national health examinations during the 2012 to 2017 period. Among 677,769 participants, we identified 417,768 who were not diagnosed with diabetes (defined as a self-reported previous diagnosis of diabetes) or were not prescribed glucose-lowering drugs or insulin (defined as ATC code A10) after excluding participants with missing values.
Positive test result for diabetes
A positive diabetes test result was defined as fasting plasma glucose ≥ 126 mg/dL and/or glycated hemoglobin (HbA1c) ≥ 6.5% for analysis of the KNHANES and fasting plasma glucose ≥ 126 mg/dL for analysis of the NHIS-NSC. A positive prediabetes test result was defined as fasting plasma glucose 100 to 125 mg/dL and/or HbA1c 5.7% to 6.4% for analysis of the KNHANES and fasting plasma glucose 100 to 125 mg/dL for analysis of the NHIS-NSC.
Risk factors for diabetes
The KDA defines diabetes risk factors to be considered for prediabetes and diabetes screening as follows: overweight or obesity, family history of diabetes, history of impaired fasting glucose and/or impaired glucose tolerance, gestational diabetes, hypertension, dyslipidemia, insulin resistance (polycystic ovary syndrome, acanthosis nigricans, etc.), history of cardiovascular disease (CVD), and use of drugs (glucocorticoids, atypical antipsychotics, etc.) [16]. Among the risk factors, we considered five (obesity, abdominal obesity, family history of diabetes, hypertension, and dyslipidemia) that were available in both the KNHANES and NHIS-NSC. We changed the definition from overweight or obesity (defined as body mass index [BMI] ≥ 23 kg/m2) to obesity (BMI ≥ 25 kg/m2). Obesity was categorized as class I (defined as 25 ≤ BMI < 30 kg/m2), class II (30 ≤ BMI < 35 kg/m2), and class III (BMI ≥ 35 kg/m2), according to the guidelines of the Korean Society for the Study of Obesity [17]. Abdominal obesity (defined as waist circumference ≥ 90 cm in men and ≥ 85 cm in women) was an additional risk factor. Data on family history of diabetes were obtained using self-reports of the diabetes history of parents and siblings. Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or the current use of antihypertensive medications. Dyslipidemia was defined as high-density lipoprotein cholesterol (HDL-C) level < 35 mg/dL and/or triglyceride level ≥ 250 mg/dL. However, we used the revised definition of HDL-C < 40 mg/dL and/or triglyceride ≥ 200 mg/dL based on the Korean Society of Lipid and Atherosclerosis guideline [18]. Information regarding CVD was available in both the KNHANES and NHIS-NSC but not diabetes risk factors, considering the low prevalence in young adults.
Statistical analyses
All categorical variables were presented as frequencies and percentages. Normally distributed data are presented as mean ± standard error in the KNHANES. To account for strata cluster and weight inherent to KNHANES, the SAS procedures SURVEYFREQ and SURVEYMEANS were used. We evaluated the screening effectiveness using the number needed to screen (NNS). NNS is defined as the number of people who need to be screened for disease detection [19]. It was calculated as the reciprocal of the rate of positive test results (e.g., a positive test rate of 5% translates to an NNS of 20 = 1/0.05). We calculated the NNS according to 5-year age groups from 20 to 24 years up to 75 years and above. We calculated the number and rate of screening-eligible individuals, screening-positive individuals, and missed screening-positive individuals. We evaluated the screening effectiveness according to diabetes risk factors in adults aged 20 to 34 years. All statistical analyses were performed using SAS 9.4 and SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC, USA).
RESULTS
In the KNHANES, there were 24,822 adults aged ≥ 20 years without diagnosed diabetes, representing 35,151,173 Korean adults (Supplementary Table 1). In the NHIS-NSC, there were 417,768 adults aged ≥ 20 years without diagnosed diabetes.
When classified by age group at 5-year intervals, the NNS to detect diabetes decreased from 63 to 34 between the ages of 30–34 years and 35–39 years in the KNHANES. In the NHIS-NSC, the NNS decreased from 71 to 42 in the same age range, and the greatest declines were noted between the ages of 25–29 year and 30–34 years (from 143 to 71) (Fig. 1 and Supplementary Table 2). In detecting prediabetes and diabetes, the NNS was approximately 10 in those aged 20 to 24 years, which continued to decrease with age (Supplementary Table 3).
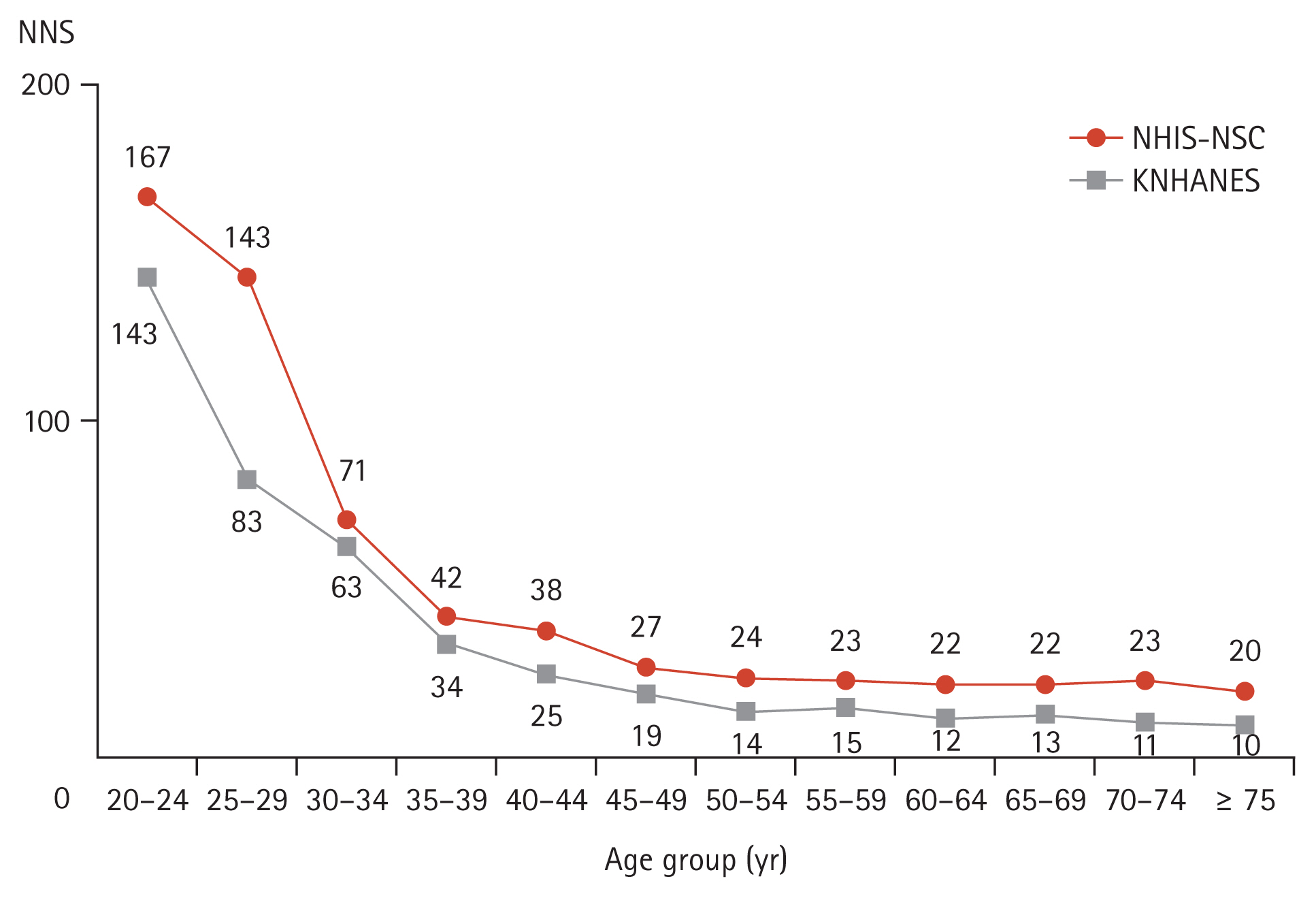
Number needed to screen to detect diabetes according to age group. NNS, number needed to screen; NHIS-NSC, National Health Insurance Service-National Sample Cohort; KNHANES, Korea National Health and Nutrition Examination Survey.
When universal screening was applied by age, in the KNHANES, the NNS slightly increased from 15 (rate of positive test = 6.8%) in adults aged ≥ 40 years (age threshold of the KDA recommendation for universal screening) to 20 (rate of positive test = 4.9%) in adults aged ≥ 20 years (Table 1). In the NHIS-NSC, the NNS increased from 26 to 33 (rate of positive test, 3.8% to 3.0%) in the same age groups (data not shown). When universal screening was applied to adults aged ≥ 35 years, the NNS was similar to that of adults aged ≥ 40 years (Table 1).

Diabetes screening effectiveness according to the change in the KDA guideline in the Korea National Health and Nutrition Examination Survey
Half of the adults aged 20 to 34 years were overweight or obesity, and approximately 20% had abdominal obesity (Supplementary Table 1). In adults aged 20 to 34 years who were overweight or obesity, the rate of missed screening positive was the lowest at 10.6% compared to the other risk factors (obesity, 20.0%; abdominal obesity, 17.6%; family history of diabetes, 55.6%; hypertension, 67.5%; and dyslipidemia, 43.8%). However, NNS was the highest at 48 (Table 2). When considering the risk factors of obesity and/or abdominal obesity, the rate of missed screening positive increased from 10.6% to 17.6%; however, NNS decreased from 48 to 34 compared to overweight or obesity (Table 2). Supplementary Table 4 shows the diabetes screening effectiveness according to age groups from 20–24 years and up to 35–39 years for each diabetes risk factor.
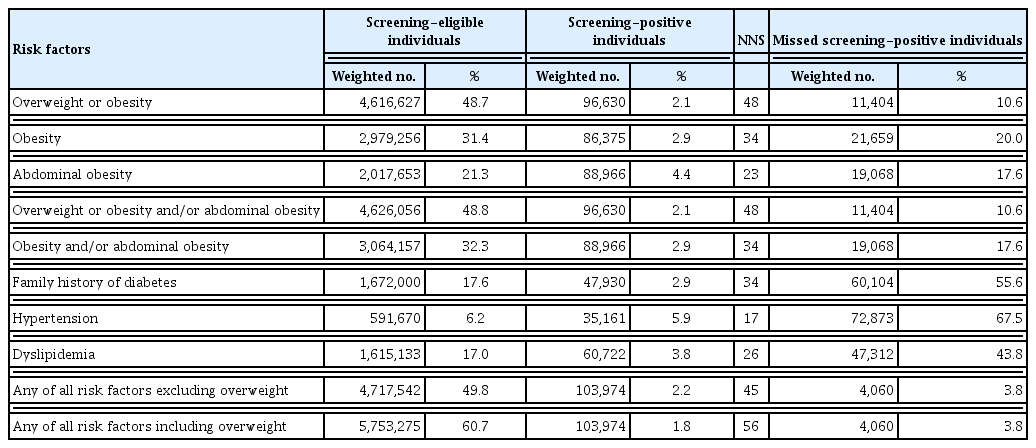
Diabetes screening effectiveness in adults aged 20 to 34 years according to diabetes risk factors in the Korea National Health and Nutrition Examination Survey
Compared to the KDA guidelines, NNS was slightly increased when applying the newly suggested screening criteria (adults aged ≥ 35 years and adults aged ≥ 20 years with diabetes risk factors). However, the rate of missed screening positive in adults aged ≥ 20 years decreased substantially from 4.0% to 0.2% in the KNHANES (Table 1).
DISCUSSION
This study provides estimates of the NNS to detect diabetes in adults in South Korea. When NNS is estimated by 5-year age categories, the NNS noticeably begins to decrease after 35 years and continues to decline until 75 years and older. When universal screening was applied to adults aged ≥ 35 years, the NNS was similar to that of adults aged ≥ 40 years.
In 2021, the KDA recommended annual universal screening for the detection of prediabetes and diabetes in adults aged ≥ 40 years and adults aged ≥ 30 years with diabetes risk factors [11]. It was established by considering to the prevalence of diabetes by age in Korea and previous studies in other countries [12]. However, in the United States, the optimal age cut-point for opportunistic universal screening based on the NNS was suggested as 35 years old [20]. Recently, the U.S. Preventive Services Task Force recommended screening in adults aged 35 to 70 years who are overweight or obesity, which decreases the age from 40 to 35 years to consider the increase in diabetes incidence in young adults [21]. The American Diabetes Association also recommends universal screening for adults aged ≥ 35 years and adults of any age with overweight or obesity (BMI ≥ 25 or ≥ 23 kg/m2 in Asian Americans) who have one or more risk factors, such as a history of CVD, hypertension, and dyslipidemia [22]. The Diabetes Canada Clinical Practice Guidelines Expert Committee suggested that the age to begin screening is 40 years, based on the Diabetes Screening in Canada Study [23,24]. Adults at high risk on the Canadian Diabetes Risk were also included for prediabetes and diabetes screening [24].
In South Korea, the incidence of diabetes has increased from 1.3 to 1.7 per 1,000 individuals in adults aged 20 to 39 years, with increasing obesity from 2006 to 2015 [13]. People diagnosed at 20 to 39 years of age had worse status of insulin resistance and β-cell function with severe obesity than those diagnosed at older ages [25]. In addition, the proportion of diabetes awareness was 76.4% in adults aged 65 years or older but decreased to 65.8% in adults aged 30 years or older [26]. The onset of diabetes at a younger age is associated with prolonged disease exposure and increased risk of diabetic complications [27]. Thus, active diabetes screening is required in young adults. However, screening individuals unlikely to benefit can result in unnecessary test burdens and costs for the healthcare system. In this study, the evidence for screening all adults aged 20 to 34 years is unclear. Compared to screening people aged 35 to 39 years, the NNS increased markedly from 34 to 63 in the KNHANES and from 42 to 71 in the NHIS-NSC for ages 30 to 34 years. In addition, when universal screening was changed from ≥ 35 to ≥ 20 years, the NNS increased from 16 to 20. However, excluding young adults from diabetes screening would have resulted in almost 100,000 people potentially being missed. Thus, we need to screen adults aged 20 to 34 years with diabetes risk factors. When adults were overweight or obesity based on the KDA guideline, the rate of missed screening positive was the lowest, but NNS was the highest among the diabetes risk factors. In addition, approximately 50% of adults aged 20 to 34 years must be screened. However, although the rate of missed screening positive increased, adults with obesity or abdominal obesity had a relatively low NNS and screening eligibility rate. Thus, diabetes screening may be more beneficial for adults aged 20 to 34 years with obesity and/or abdominal obesity than for those who are overweight or obesity.
Prediabetes may be an important stage for early intervention to prevent the development of diabetes and its complications. A lifestyle intervention based on a healthy diet or physical activity prevented the progression of prediabetes to diabetes [28]. The Diabetes Prevention Program trial showed that weight loss through lifestyle intervention reduced the risk of developing diabetes by 44% [29]. In addition, lifestyle interventions in prediabetes reduced the risk of diabetic complication and diabetes-related mortality [30,31]. In this study, the NNS to detect prediabetes or diabetes remained stable under 10 among adults of all ages. Thus, the early detection of prediabetes through the screening may be an effective strategy to delay the development of diabetes and its complications.
The benefits of diabetes screening in older adults remain controversial. In the United States, the proportion of deaths or regression to normoglycemia from prediabetes was higher than progression to diabetes in older adults aged > 70 years with prediabetes [32]. The U.S. Preventive Services Task Force does not recommend diabetes screening for adults aged > 70 years [21]. In South Korea, almost 20% of adults aged ≥ 65 years with prediabetes progress to diabetes. In addition, in adults aged ≥ 75 years diagnosed with prediabetes or diabetes, the risk of diabetic complications did not increase [33]. However, in the current study, we included all adults aged ≥ 20 years, including older adults, to evaluate the effectiveness of screening for diabetes. Thus, further studies on the upper age limit for diabetes screening are required.
In the current study, compared to when fasting plasma glucose used alone as the positive test definition, the rates of positive test result were higher when both fasting plasma glucose and HbA1c were used simultaneously. In general, fasting plasma glucose, 2-hour plasma glucose during a 75g oral glucose tolerance test (OGTT), or HbA1c are all needed for diagnosing prediabetes and diabetes. Among the tests, OGTT is the diagnostic standard for diabetes. However, it is difficult to use for screening purposes because of time-consuming and inconvenient to the patient. Although fasting plasma glucose and HbA1c test is a simple and time-saving test that can be performed instead of OGTT, the measurement of HbA1c is a more reliable than fasting plasma glucose. And, HbA1c is better associated with diabetic complications than fasting plasma glucose [34]. In addition, the combination of fasting plasma glucose and HbA1c may be a more sensitive and specific screening tool for early identification of high-risk individuals with early diabetes [35]. Thus, it is necessary to consider HbA1c as well as fasting plasma glucose for diabetes screening.
The current study had several limitations. First, diabetes is diagnosed based on the two or more abnormal test results using fasting plasma glucose, 2-hour plasma glucose during a 75 g OGTT, or HbA1c value [11]. However, our study considered only one positive screening test. Second, the NNS is easy to calculate and intuitively shows the effectiveness of screening for clinicians and patients. However, we were unable to consider the risk and cost of screening.
In conclusion, universal screening for adults aged ≥ 35 years and selective screening for adults aged 20 to 34 years, considering diabetes risk factors such as obesity and/or abdominal obesity, may be appropriate for detecting prediabetes and diabetes in South Korea.
KEY MESSAGE
1. To determine the age thresholds for diabetes screening, we estimated the number needed to screen (NNS).
2. When universal screening was applied to adults aged ≥ 35 years, the NNS was similar to that of adults aged ≥ 40 years.
3. NNS was slightly increased when applying the newly suggested screening criteria (adults aged ≥ 35 years and adults aged ≥ 20 years with diabetes risk factors). However, the rate of missed screening positive in adults aged ≥ 20 years decreased.
Acknowledgments
This study used NHIS-NSC data (NHIS-2022-2-228) made by National Health Insurance Service (NHIS). The authors declare no conflict of interest with NHIS.
Notes
No potential conflict of interest relevant to this article was reported.