Assessment and nonsurgical management of low back pain: a narrative review
Article information
Abstract
Low back pain (LBP) is a common condition that affects people of all ages and income levels worldwide. The etiology of LBP may be mechanical, neuropathic, systemic, referred visceral, or secondary to other causes. Despite numerous studies, the diagnosis and management of LBP remain challenging due to the complex biomechanics of the spine and confounding factors, such as trivial degenerative imaging findings irrelevant to symptoms and psychological and emotional factors. However, it is imperative to identify the crucial signs (“red flags”) indicating a serious underlying condition. While many recent guidelines emphasize non-pharmacologic management approaches, such as education, reassurance, and physical and psychological care, as the first option, LBP patients in many countries, including South Korea, are prescribed medications. Multidisciplinary rehabilitation combined with prudent use of medications is required in patients unresponsive to first-line therapy. The development of practical guidelines apposite for South Korea is needed with multidisciplinary discussion.
INTRODUCTION
Despite considerable progress in the diagnosis and treatment of low back pain (LBP), distinguishing among its many possible etiologies, and thus determining the most appropriate management approach, remains challenging [1–3]. Therefore, while this review does not provide standards for the management of LBP, it does present evidence-based information on its diagnosis and nonsurgical treatment. Nonetheless, it should be noted that our review is based on studies with varying levels of evidence, some of which addressed highly specific aspects of LBP. Our findings should therefore be interpreted with caution.
EPIDEMIOLOGY AND NATURAL HISTORY
LBP affects people of all ages and socioeconomic classes worldwide [4–6]. Previous studies have documented that 50% to 80% of people will experience LBP at least once in their lives, with a point prevalence of 15% to 30% and annual incidence of 15% to 45% [7–9]. Although LBP can develop in all age groups, it is most common in the working population, i.e., adults aged 35 to 55 years [2,5], with the overall prevalence and severity of LBP tending to increase over time [10,11]. LBP is the leading cause of pain and occupational disability, thus burdening patients, their families and society as a whole [1,12,13], including economically, where the medical costs associated with LBP have substantially increased [14]. In South Korea, the prevalence of LBP is 17.1% [15] and the socioeconomic cost in 2015 was approximately 6.6 billion USD. In fact, since 2010, LBP has ranked second among conditions imposing socioeconomic burden, after self-harm [16,17]. LBP was also ranked first in 2015, with the exception of indirect costs caused by productivity loss.
LBP can be classified as acute (< 4 weeks), subacute (4 to 12 weeks), or chronic (> 12 weeks), depending on the duration of symptoms. It is generally considered to be a self-limiting condition because most patients with acute LBP recover with no residual symptoms or functional loss, regardless of treatment [2,12]. A previous systematic review documented substantial improvement in patients with LBP during the first 6 weeks [18]. However, some authors reported that approximately 33% of patients with LBP experienced a recurrence within 1 year in a systematic review, and 30% to 40% of patients with LBP might experience chronic LBP [19,20]. Psychological factors, including depression, anxiety, and occupational stress, are thought to play a larger role in the transition to chronicity than organic pathologies [13,14,21]. Chronic LBP should be distinguished from acute LBP because it differs in expected course, cause, prognosis, and treatment; management and assessment of the prognosis are also more difficult.
CLINICAL PRESENTATION AND EVALUATION
Etiologies
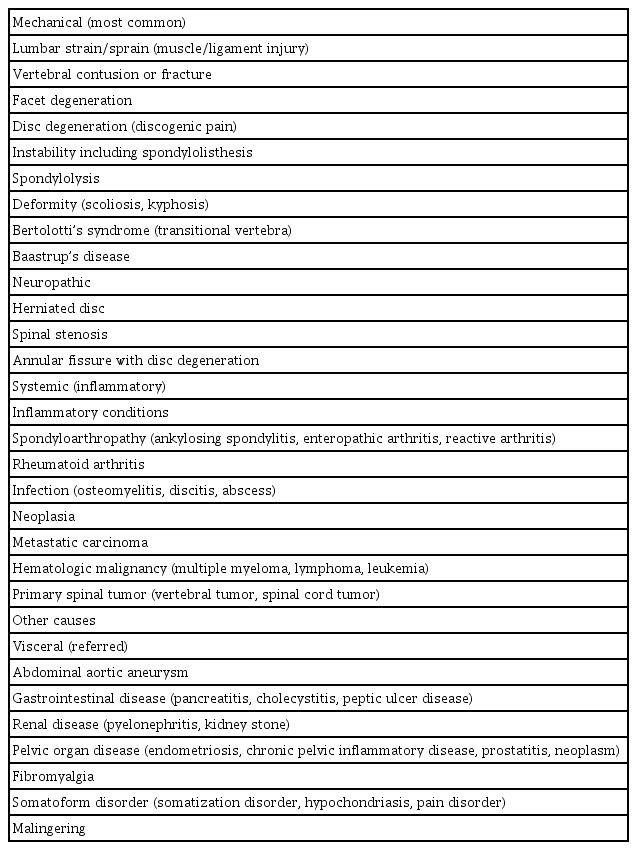
The etiologies of LBP can be classified as mechanical, neuropathic, systemic (inflammatory), and secondary to other causes (Table 1) [13,22–24]. Mechanical LBP, resulting from damage to bony structures, intervertebral discs, and ligamentous structures, is the most common cause. Neuropathic pain refers to pain originating from irritation or compression of the nerve root, and typically presents as “sharp” or “burning” pain radiating to the lower extremities, frequently below the knee [13,14]. Mechanical pain may be “aching” or “dull” and referred to the buttocks and upper thighs. Systemic conditions, including inflammatory or neoplastic conditions involving the lumbar spine, can also cause LBP. Morning pain, pain that wakes the sufferer, and pain that improves with exercise suggest an inflammatory etiology [11,23]. Pain stimuli from the abdominal or pelvic organs can be referred to the lumbar area, mimicking LBP. However, the specific etiology of LBP is rarely identified because of the complex biomechanics of the spine and confounding psychological, emotional, and occupational factors [11,12]. In fact, an exact diagnosis of LBP is made in only 15% to 20% of patients [3,25].
Medical history and physical examination
Patients with LBP often report radiating or referred pain in the lower extremities or neurological symptoms, such as motor weakness or changes in bladder or bowel habits. Obtaining a complete medical history should thus be the first step in evaluating a patient with LBP, including pain description (location, onset, duration, severity, consistency, and aggravating or relieving factors), associated symptoms (radiating pain, motor weakness or sensory change in the lower extremities, bladder or bowel symptoms, fever, and unexplained weight loss), previous medical history (malignancy, infection, osteoporosis, previous fractures, psychological history, and family history), and other details (body mass index, physical activity, socioeconomic status, occupation, psychosocial factors, and smoking status).
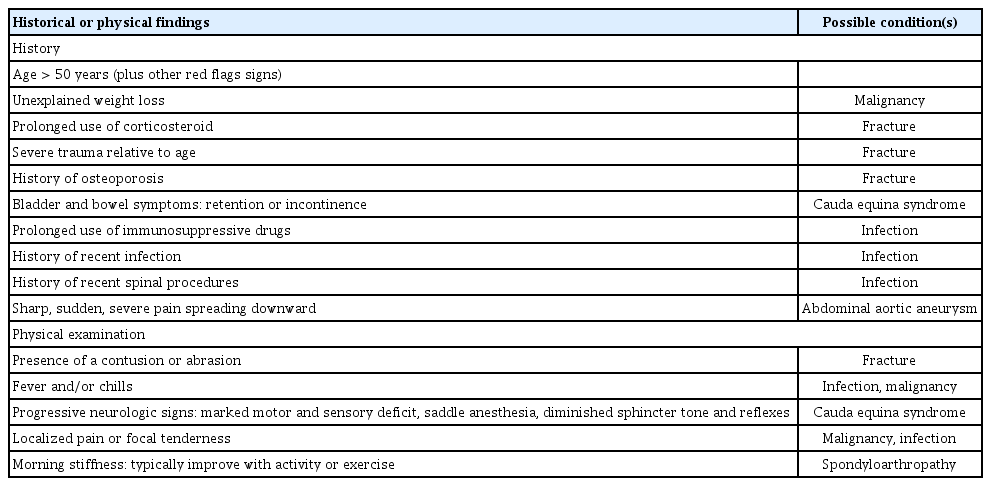
The physical examination consists of inspection and palpation of the lumbosacral structures to evaluate tenderness, muscle spasm, range of motion of the trunk, and alignment; assessment of the lower extremities to check motor power, sensory, and reflexes; the straight leg raise test; and rectal examination, if necessary. Information acquired during history-taking and the physical examination can reveal crucial signs (“red flags”) indicating the need for further evaluation and management rather than empirical treatment (Table 2) [2,13,26].
Imaging
The benefit of routine imaging studies is limited for most patients with LBP, given the self-limiting natural history of the pain [11]. Furthermore, since degenerative findings are frequently seen in asymptomatic individuals, imaging is likely to reveal unrelated minor abnormalities [13,27]. Several studies have shown that the early imaging of LBP has no benefit in terms of pain or function [28]. In addition, the diagnosis of nonspecific degenerative diseases may cause patients to perceive themselves as sick or disabled, which may lead to a decrease in their activities in daily life, resulting in a delay in recovery and worsening of symptoms. Thus, imaging is recommended only for patients with “red flags” or persistent symptoms after 6 weeks of sufficient conservative treatment [11,29]. However, since plain radiographs are inexpensive and easy to perform, even in primary care clinics in the South Korean healthcare environment, their use is typically not limited.
Anteroposterior (AP) and lateral standing plain radiographs are the initial imaging modalities of choice [30]. Additional dynamic flexion-extension radiographs can also be obtained to evaluate dynamic instability [31]. AP and lateral radiographs in the supine position should be considered in patients with suspected fractures. An oblique view can reveal abnormalities of the pars interarticularis [32]. These images can aid assessment of bone structures and the alignment of the spinal column.
Magnetic resonance imaging (MRI) is the most sensitive diagnostic tool, and is performed in patients with subtle spinal pathologies not detectable on radiographs, such as fractures, infections, and tumors. However, as the specificity of MRI is poor [11], care should be taken not to correlate symptoms with insignificant findings.
Computed tomography (CT) is also used in the assessment of LBP because, unlike other imaging modalities, it can provide optimal images of the bony architecture [33]. It is particularly helpful for evaluating bone-related pathologies, such as fractures, spondylolysis, facet joint osteoarthritis, and primary bone tumors [34]. It is also useful in patients with previously inserted instrumentation, as an alternative to MRI [12]. However, while CT can identify root compressive lesions, such as herniated disc and spinal stenosis, it has lower sensitivity than MRI and may lead to an incorrect diagnosis [34,35]. Moreover, the radiation dose should be considered, especially in younger patients.
NONSURGICAL TREATMENT
Nonsurgical treatment options for LBP include pharmacological and non-pharmacological therapies. As it is difficult to determine the cause of LBP in most patients, management remains challenging despite numerous treatment options. Moreover, despite the extensive literature on the management of LBP, decisive evidence supporting one modality over others is lacking. Therefore, the management strategy should be tailored to each patient and effective treatment may require a combined approach.
Education and counseling
A core component in the management of LBP is education and counseling by the treating physician [2,12,36]. Several high-quality trials and guideline reviews have demonstrated the effectiveness of educating and reassuring patients [37]. Patients should be informed of the natural history, expected course, and often favorable outcome of LBP [38,39]. It should be emphasized that the patient does not have severe disease, that symptoms will improve within several months, and that it is important to stay active, as a low level of physical activity is associated with the development of chronic LBP [40,41]. Lifestyle changes, such as smoking cessation, weight management, regular walking, and avoidance of pain-provoking activities, should also be encouraged [11,12,41]. For patients in Asian countries, sedentary behavior such as sitting or lying on the floor can be detrimental to lumbar spine health.
Physiotherapy
Physiotherapy includes exercises (strengthening, stretching, Pilates, Tai Chi, yoga), manual therapy (manipulation, mobilization, massage), and other local treatment modalities (heat, ice, ultrasound, transcutaneous electrical nerve stimulation [TENS]) [37,42].
Exercises are prescribed to improve coordination and strengthen the muscles supporting the spine and trunk. A study of patients with acute, subacute, and chronic LBP reported no difference in the effects of various exercise protocols, including motor control exercise (MCE), supervised exercise therapy, and directional exercise [37]. Although whether exercise therapy is better than usual care in terms of pain and functional outcome in patients with acute and subacute LBP is unclear [43], another study recommended exercise therapy as the first-line treatment option for patients with chronic LBP [39]. MCE consists of exercises that strengthen the core muscles supporting the spine in a neutral position [37,44]. In a previous meta-analysis of studies with short- to long-term follow-up of patients with chronic and recurrent LBP, MCE improved pain intensity and function compared with minimal intervention or general exercise [45]. Supervised exercise therapy involves patient-tailored exercises or activities performed under the guidance of healthcare professionals, including MCE, directional exercises, and strengthening exercises [44]. Although there is a paucity of evidence regarding the clinical benefit of early supervised exercise therapy in patients with recent-onset LBP, it may be effective for patients with a slow recovery or risk of developing chronic LBP [46]. Moreover, the potential positive effects of supervised exercise on general health should not be ignored [44]. Directional exercise refers to repeated spinal movements in a specific direction, and directional preference to repeated spinal movement or sustained position in a specific direction that decreases pain intensity. Although a previous systematic review found mixed results, some studies have shown that directional exercise matched with directional preference is more likely to yield a positive outcome, whereas unmatched exercise has negative outcomes [47,48]. Therefore, directional preference exercise can be considered for patients with LBP characterized by a directional preference. However, it is important to avoid directional exercise when there is a possibility of spinal fracture. Pilates, Tai Chi, and yoga can serve as adjunctive treatments for LBP, although previous results have been inconsistent and there is no clear scientific evidence supporting their utility for LBP [49–51]. In general, the exercise protocol should be selected according to the patient’s symptoms, preferences, and response to therapy.
Manual therapy, such as spinal manipulation (SM) and massage, can also be considered for LBP. SM is performed manually by chiropractors, physiotherapists, and other healthcare providers. The aim is to restore joint or spinal motion and function to within normal range [52]. Previous studies have found that SM combined with usual care in patients with acute LBP is associated with a small but significant short-term effect on pain intensity or function [53–55]. When combined with another active treatment, SM may also provide pain relief and improved function in patients with chronic LBP [56,57]. Although the results of previous reports were inconclusive, SM may be prescribed as a second- line or add-on modality [39]. In patients with subacute to chronic LBP, massage may provide better short-term pain relief than sham and other alternative therapies (SM, TENS, physical therapy, exercises, or education) [58]. As its effects are often small, massage can be considered as an adjunctive treatment for acute and chronic LBP, with only minor adverse effects.
In the management of acute LBP, superficial heat provides better pain relief than placebo, acetaminophen, and ibuprofen [59,60]. The combination of superficial heat therapy and exercise was shown to be more effective than exercise alone for acute LBP [61], but its efficacy in patients with chronic LBP is unclear. There is insufficient evidence regarding the effectiveness of other local treatment modalities, such as ultrasound, TENS, inferential therapy, traction, and short-wave diathermy [37,62].
As mentioned above, psychological factors are one of the most common causes of LBP and play a significant role in the development of chronic LBP. Thus, various psychological therapies, including progressive relaxation therapy, cognitive behavioral therapy, and mindfulness-based stress reduction, have been suggested for the management of chronic LBP [2,37]. For persistent LBP unresponsive to other conventional treatments, multidisciplinary rehabilitation, consisting of combined physical, educational, and psychological management, is also recommended [37]. In a previous systematic review, these biopsychosocial interventions were shown to be more effective than usual care and physical therapy in terms of pain and function in patients with chronic LBP [63].
Medications
Although there are various classes of medications for LBP, pharmacological therapy does not necessarily provide better outcomes than non-pharmacological management [1]. Indeed, several guidelines recommend non-pharmacological interventions as first-line treatment and pharmacological therapy only in unresponsive patients [37]. However, medications are frequently prescribed during the first visit, either for pain relief or patient satisfaction.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are the first-line and most commonly used medications for LBP [2,14]. They are more effective than placebo for improving symptoms in acute LBP [64] and can also provide short-term relief of chronic LBP [65,66]. There are two types of NSAIDs: non-selective NSAIDs, which inhibit both cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2), and selective COX- 2 inhibitors. There is no evidence of a difference between them in terms of pain relief in LBP [67], and the response to a particular agent can vary among patients. However, NSAIDs are also associated with several adverse effects, such as gastrointestinal, renal, and cardiovascular complications [68]. A selective COX-2 inhibitor was developed to reduce gastrointestinal side effects (e.g., gastric ulcer, perforation, bleeding, and obstruction). Since all NSAIDs at therapeutic doses differ in their selectivity for COX-1 and COX-2, physicians should keep in mind that NSAIDs with greater COX-2 than COX-1 selectivity generally have a lower risk of gastrointestinal adverse events than those with lower COX-2 selectivity. By contrast, both non-selective and COX-2 selective NSAIDs have been associated with an increased risk of cardiovascular complications, such as hypertension, myocardial infarction, stroke, and heart failure, especially at high doses [69]. Since the risk of adverse events is often dose- and duration-dependent, NSAIDs should be used at the lowest effective dose and for the shortest duration [68,70,71]. In addition, their co-administration with aspirin or other antiplatelet/anticoagulation drugs should be avoided if possible, due to the risk of adverse drug interactions [68]. Therefore, the specific NSAID should be chosen based on the recommended dose/schedule, as well as the patient’s clinical history (e.g., gastrointestinal and cardiovascular risk factors), response to each drug, and preference. A previous study found no evidence that acetaminophen is superior to placebo [65]. However, because it is inexpensive and safe, it is a reasonable option for patients with contraindications to other medications and might also be useful because of its opioid-sparing and synergistic effects [72].
Tramadol, a centrally acting non-narcotic analgesic, is commonly used to treat LBP. Given its positive effect on pain and function and less potential for abuse than narcotic drugs, it is a useful option, especially for patients with chronic LBP [2,37].
Skeletal muscle relaxants (SMRs), a class of heterogeneous drugs with different chemical structures and mechanisms of action, can be classified as antispastic (e.g., baclofen and dantrolene) or antispasmodic (e.g., carisoprodol, chlorzoxazone, and cyclobenzaprine) [73]. Antispastic agents act on upper motor symptoms by resisting exaggerated reflexes and reducing muscle rigidity, with the mechanism varying among agents [74]. Antispasmodic agents can be prescribed for peripheral muscle pain and spasm, but their precise mechanism of action is unclear; however, it likely includes sedative effects on the central nervous system [74]. SMRs were shown to be more efficacious than placebo in acute LBP [75] and may offer additional benefits when used in combination with NSAIDs [76]. Although the interobserver reliability of muscle spasm on physical examination is relatively low [76], SMRs could theoretically be effective in acute LBP associated with paraspinal muscle spasm. However, there is no evidence of their efficacy for treating chronic LBP [37]. Potential side effects, including drowsiness, somnolence, and dizziness, should also be considered. Therefore, when prescribing these drugs their benefits and risks should be weighed.
Given their minimal benefits and potential risks, the routine use of opioids is not recommended [39]. Nonetheless, opioids are one of the most frequently prescribed medications for LBP worldwide, including in South Korea, and their use in acute LBP has been advocated despite their unclear efficacy [77,78]. In patients with chronic LBP, opioids may provide short-term pain relief, but their efficacy is generally considered clinically insignificant and evidence of long-term effectiveness is lacking [79–81]. Adverse effects of opioids include dizziness, drowsiness, nausea, vomiting, constipation, abuse and misuse potential, and overdose-related death [12,78]. There is a guideline for prescribing opioids for chronic pain in South Korea [82], which could be applied to patients with LBP. In brief, opioid prescriptions should be limited to a short period of time or restricted to patients in whom conventional medications have failed.
Antidepressants serve as an alternative or adjuvant option for the management of LBP, especially in patients with comorbid mood disorders [12,83,84]. Because mood and LBP are closely related [13,85], antidepressants can provide significant pain relief, especially in patients with chronic LBP, as documented in previous studies and guidelines [2,75,86,87]. Two classes of antidepressants are frequently used in the management of LBP: tricyclic antidepressants (TCA) and serotonin and norepinephrine reuptake inhibitor (SNRI) antidepressants [37,88]. TCA can be considered for patients with LBP and sleep disturbances, which are often associated with mood disorders [89]. The SNRI duloxetine was shown to provide pain relief and functional improvement in the management of LBP [90–92]. However, antidepressants can be associated with nausea, dizziness, fatigue, and somnolence [83].
Anticonvulsant agents, such as pregabalin and gabapentin, are generally used to control neuropathic pain. In a prospective randomized trial, pregabalin combined with celecoxib was more effective than monotherapy for chronic LBP [93]. However, the evidence supporting a beneficial effect of these medications for patients with LBP or sciatica is insufficient [94–96].
Topical analgesics refer to agents rubbed (e.g., cream or gel) or stuck (e.g., patches or plasters) on the skin. This class of agents includes topical NSAIDs, salicylate-containing rubefacients, capsaicin, and lidocaine [97]. They are used to treat acute or chronic painful conditions, including LBP. A 5% lidocaine patch was reported to be effective for pain relief in patients with acute, subacute, and chronic LBP [98,99]. Topical capsicum is recommended on a short-term basis for chronic LBP, including neuropathic LBP associated with radiculopathy [99,100]. However, patients should be closely monitored for the development of common side effects of topical agents, including localized erythema, rash, or a burning sensation [101].
As there are no definitive recommendations or guidelines regarding the pharmacological management of LBP, clinicians should be familiar with the fundamental principles and indications of each drug and consider patient-tailored pharmaceutical therapy.
Intervention
LBP arising from organic causes can be classified as somatic pain (facet joint, sacroiliac joint, myofascial, and discogenic pain) or radicular pain (disc herniation, annulus tear, and spinal stenosis), depending on its origin. Although the etiology of LBP is often multifactorial, damage to the facet joint (zygapophyseal joint) and sacroiliac joint is a major cause [102]. Facet joint pain can be managed by therapeutic interventions such as medial branch block (MBB), facet joint injection (FJI), and radiofrequency ablation (RFA) [103,104]. MBB, which blocks the medial branch of the dorsal ramus innervating the facet joints, can be performed for diagnostic purposes to identify target lesions before RFA, and for therapeutic purposes to provide long-lasting pain relief [105]. Miyakoshi et al. [106] suggested conventional MBB with a large volume of local anesthetics to block all three branches (medial, lateral, and intermediate) of the dorsal ramus and thus reduce LBP originating from both the facet joints and myofascial structures. In FJI, the anesthetic agents are injected intra-articularly, while in RFA the innervating nerves are denatured to stop pain signals from injured joints reaching the brain. Pain arising from the sacroiliac joint can be managed with intra-articular injections and RFA. Caudal epidural injection can exert both short- and long-term effects on chronic LBP (with or without radiculopathy) caused by discogenic sources, spinal stenosis, or post-spinal surgery syndrome [107].
LIMITATIONS
This review had several limitations. First, it was a narrative rather than systematic review or meta-analysis. Therefore, bias in the literature search or selection cannot be ruled out. In addition, the treatment modalities were not graded according to the level of evidence. Second, LBP is an extremely broad comprehensive category, such that its mechanisms, etiologies, examinations, and management protocols remain controversial. Third, given the lack of conclusive evidence supporting any one treatment option unconditionally, we cannot provide definitive recommendations for the management of LBP. Despite these limitations, this review provided an overview of the LBP management process and can thus act as a guide for physicians involved in the treatment of this common condition.
CONCLUSIONS
Despite a plethora of studies, trials, and clinical guidelines regarding the nonsurgical management of LBP, gaps remain between their recommendations and actual practice, depending on the health system and economic status of a given country, as well as patient behaviors and demographics. In South Korea, the lack of apposite guidelines often results in inappropriate imaging or drug prescriptions and subsequent substantial and unnecessary medical costs. Multidisciplinary discussions with clinicians from various departments, researchers, pharmaceutical companies, and lawmakers are required to devise practical guidelines. Ultimately, it is essential to comply with the basic principles of care, taking into account the benefits and potential risks of treatment.
Notes
No potential conflict of interest relevant to this article was reported.