Outcomes according to treatment modalities as a bridge to curative surgery for malignant obstruction of the proximal colon: stent versus stoma
Article information
Abstract
Background/Aims
The optimal treatment for acute malignant obstruction of the proximal colon (MOPC, proximal to the splenic flexure) remains challenging. Emergency resection, the traditional modality for MOPC, has shown significantly high mortality and morbidity rates, according to recent studies. This study aimed to investigate the clinical outcomes of stent vs stoma as a bridge to curative surgery for MOPC.
Methods
This retrospective cohort study included 72 patients who underwent endoscopic placement of a self-expanding metallic stent (SEMS) or loop ileostomy for MOPC at six referral centers between January 2011 and July 2021. Clinical and pathological characteristics, procedure-related complications, and long-term mortality rates after curative surgery were analyzed.
Results
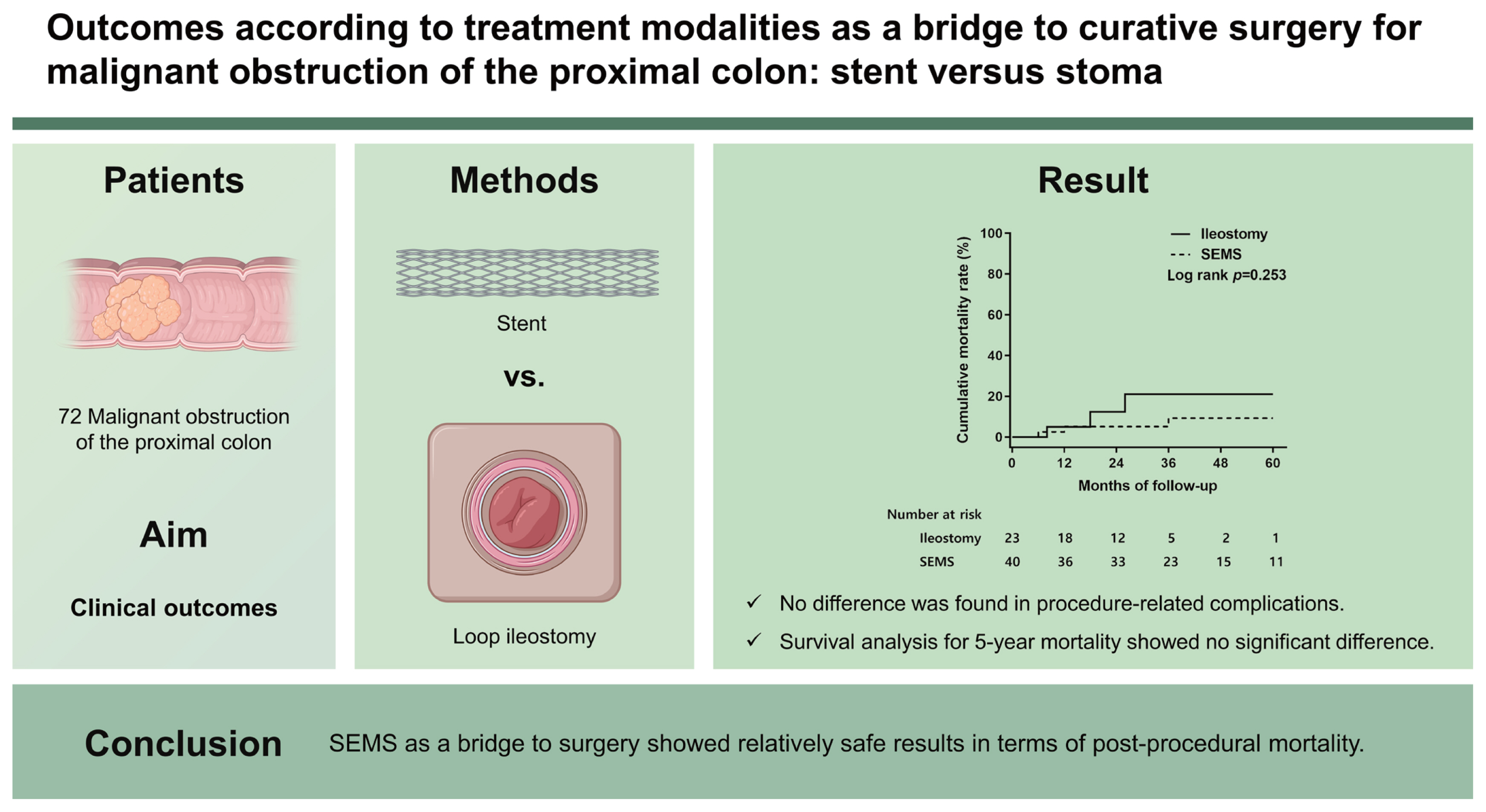
During a mean follow-up period of 32 months, 30 patients (41.7%) underwent ileostomy preferentially for more proximal cancer, complete obstruction, and advanced tumor stage compared to the SEMS group. No difference was found in procedure-related complications, but five deaths were observed after ileostomy. Survival analysis for 5-year mortality after curative surgery showed no significant difference between the bridge modalities (log-rank p = 0.253).
Conclusions
In this study, SEMS as a bridge to surgery showed relatively safe results in terms of post-procedural mortality. However, these results should be considered when performing ileostomy in patients with more advanced malignant obstruction.
INTRODUCTION
Approximately 15% of patients with colorectal cancer (CRC) present with acute luminal obstruction as their first symptom [1,2]. Acute malignant obstruction of the proximal colon (MOPC, proximal to the splenic flexure) constitutes 35% to 54% of all obstructing CRCs and is a life-threatening condition that requires emergency intervention [3,4]. The treatment strategy has been based on emergency resection and primary anastomosis; however, recent studies have shown significantly higher postoperative mortality (9% to 10%) with this method compared to elective surgery [5–7]. Therefore, the use of proper procedures for bowel decompression as a bridge to surgery (BTS) is important for acute malignant proximal colonic obstruction, as it is on the left-sided colon cancer [7,8]. The self-expanding metallic stent (SEMS) and transient diverting loop ileostomy are options for BTS in MOPC [7,9]. Previous retrospective studies have shown that stent insertion can be performed successfully in MOPC, with comparable clinical success and complication rates to those of left-sided colon cancers [10]. A recently updated clinical guideline recommended the consideration of SEMS insertion as a bridge to curative resection as well as in a palliative setting for MOPC [11]. SEMS may control the acute malignant colonic obstruction properly; however, it has several limitations, including technical difficulty, perforation, re-obstruction, and stent migration [12,13]. In particular, SEMS insertion should be done more cautiously to prevent perforation and the possibility of intraperitoneal dissemination of carcinoma, when a distant metastasis is not initially detected [14]. Comparative studies regarding the effectiveness and safety of SEMS versus loop ileostomy as a BTS are scarce, particularly in Korea [10,15]. Thus, we aimed to compare the clinical outcomes of SEMS and diverting loop ileostomy to evaluate the feasibility for bowel decompression in acute MOPC.
METHODS
Patients
We retrospectively evaluated the medical records of all consecutive patients who underwent colonic stenting and loop ileostomy as a BTS for acute MOPC in six Korean referral centers between January 2011 and July 2021. Patients with distant metastasis who were not eligible for curative surgery at the time of diagnosis were excluded from this study. Data collected were the patients’ clinical and pathological characteristics, including age at diagnosis, sex, location of the obstructive lesion (transverse colon [T-colon] vs. ascending colon [A-colon]/hepatic flexure [H-flexure]), and predicted tumor stage (T2 and T3 vs. T4) using computed tomography (CT) scan. Acute MOPC was diagnosed based on clinical symptoms, physical examination, plain abdominal radiography, and CT scan. We investigated the American Society of Anesthesiologists (ASA) classification to assess co-morbidity in patients. In addition, body mass index (BMI) and the coexistence of hypertension (HTN) and diabetes mellitus (DM) were also assessed.
Total obstruction was defined as the existence of continuing nausea, vomiting, abdominal distention, decreased or absent bowel sounds, or the inability to pass any stool or gas via the anus [9]. Subtotal obstruction was defined in this study as when an obstructive lesion was confirmed on plain abdominal radiography or CT scan, but none of the above symptoms were present. The study protocol was reviewed and approved by the Institutional Review Boards of Ulsan University Hospital (2021-07-010), Inje University Haeundae Paik Hospital (2021-08-025-001), Pusan National University Hospital (2108-007-105), Inje University Busan Paik Hospital (2022-02-026), Gyeongsang National University Changwon Hospital (2022-01-015-023), and Dong-A University Hospital (22-029). Written informed consent by the patients was waived due to a retrospective nature of our study.
Procedures
When identifying an acute MOPC, specialized gastroenterologists and colorectal surgeons selected either SEMS or diverting loop ileostomy as a BTS, considering the location and severity of the obstructing lesion, availability of SEMS, and risk of perforation. In cases of SEMS, the location and etiology of acute bowel obstruction are revealed by colonoscopy after bowel cleaning with a simple enema. The guidewire was positioned under fluoroscopy, and suitable stents were placed according to the standard method (Fig. 1) [16]. All the SEMSs used were uncovered (BONASTENT, Seoul, Korea; or HANAROSTENT, Seoul, Korea) and had a diameter of 24 mm and length of 60, 80, 100, or 120 mm.

Colonoscopy and radiography at the time of self-expandable metal stent insertion for acute malignant obstruction on the proximal ascending colon. (A) Computed tomography shows inhomogeneous colonic wall thickening at the proximal ascending colon and upstream bowel distension (white triangles). (B) Colonoscopic view of endoscopic cannulation for self-expandable metal stent insertion. (C) Fluoroscopic view of guidewire insertion under a colonoscopic assistant. (D) Colonosocpic view after full expansion of the metal stent at the obstructive tumor. (E) Fluoroscopic view after full expansion of the metal stent with the narrowed part of the middle portion (white triangles).
Loop ileostomy was performed using a routine surgical approach [17]. After the abdominal wall incision at the ileostomy site, the tension-free loop of the distal ileum was pulled out of the abdominal wall to create a stoma. The sutures were then placed for mature and evert loop ileostomy. Curative resection can be performed in eligible patients after decompression of the bowel. The time of surgery was determined according to the patients’ general condition and co-morbidities and the degree of edematous bowel at the time of BTS. The surgical method was either right hemicolectomy (RHC) or extended RHC, depending on the location of the tumor. Investigation of complications and short-term outcomes associated with SEMS insertion or diverting ileostomy was performed. The result of curative resection after BTS was also analyzed for the corresponding patients to evaluate the long-term effect of the two bridge modalities.
Statistical analysis
Categorical variables were compared using the chi-square or Fisher’s exact test, and continuous variables were compared using the Student’s t test. All results were considered statistically significant at two-sided p values < 0.05. The cumulative probability of mortality was evaluated using the Kaplan-Meier method. All analyses were performed using the IBM SPSS software version 24.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Patients and procedure-related outcomes
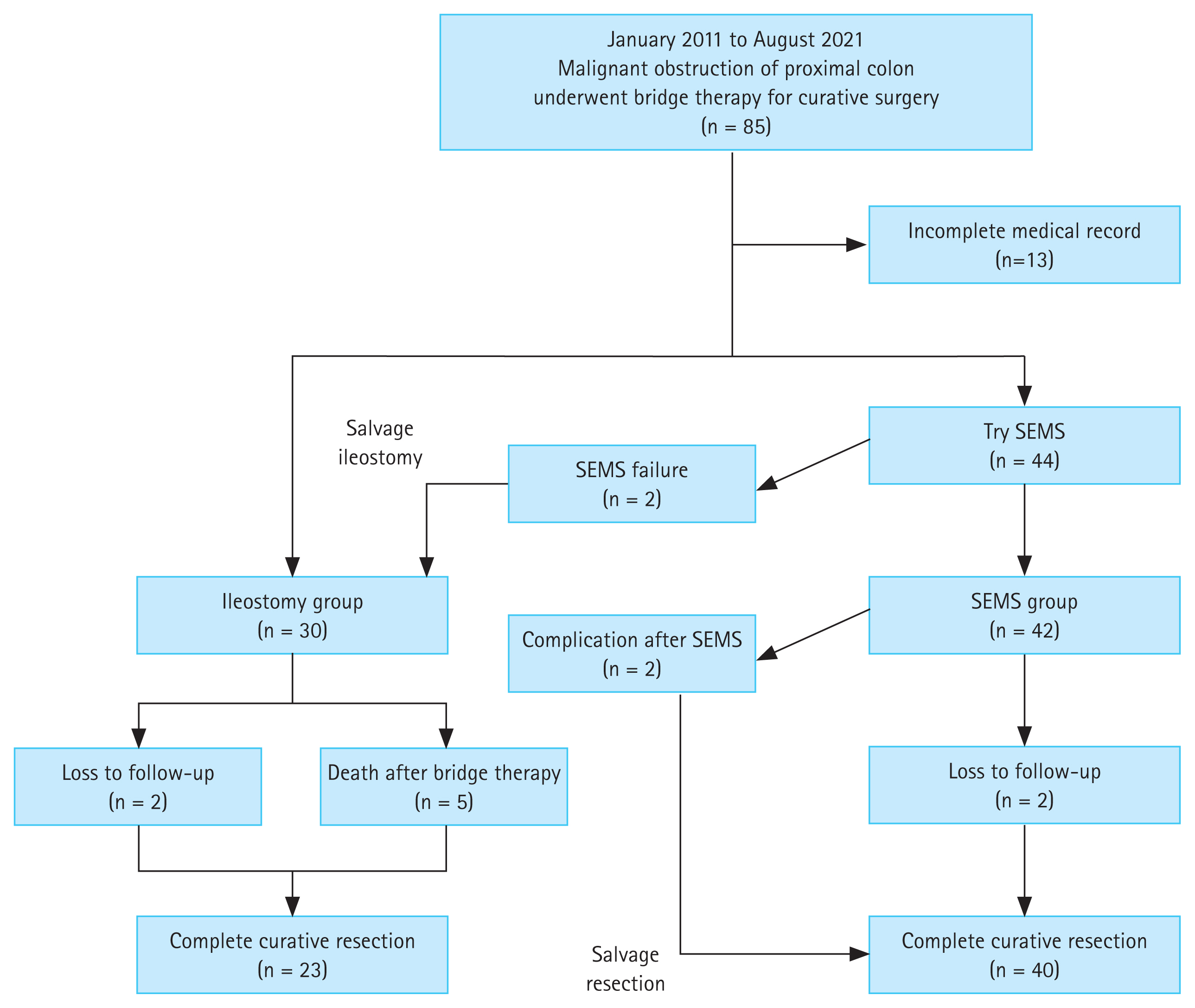
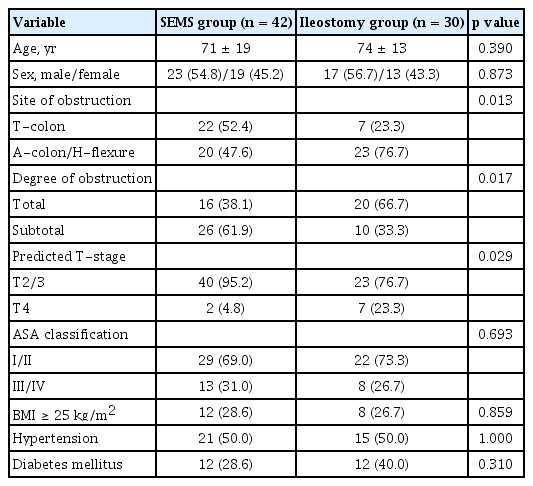
The clinical flow charts of the study population are presented in Fig. 2. This study included 72 patients (mean age ± standard deviation, 72 ± 16 years), and their demographic, clinical, and pathological characteristics are summarized in Table 1. As a BTS strategy, SEMS insertion was significantly higher in patients whose obstructing tumors were located in the distal colon (T-colon vs. A-colon/H-flexure, p = 0.013). Clinically complete luminal obstruction was a major reason for choosing an ileostomy for emergency decompression (p = 0.017). Ileostomy was preferred even when the main tumor status was predicted to be T4 on a CT scan at the time of diagnosis (p = 0.029). Following the BTS procedure (Table 2), bowel decompression was achieved within 24 hours in 53 of 72 (73.6%) patients. In addition, we investigated the ASA classification, BMI, and the coexistence of HTN or DM to assess co-morbidity in patients. As a result, no significant difference was found between the SEMS group and the ileostomy group in the relevant factors.
Complications associated with SEMS insertion or ileostomy occurred in four patients (5.6%). There was no statistical difference between the ileostomy and SEMS groups in terms of early decompression (p = 0.175) or procedure-related complications. Curative surgery was possible in 63 patients whose general conditions were suitable post-BTS. When the tumor-node-metastasis (TNM) stage was confirmed after radical surgery, all patients were in stage II or III, and no difference was identified according to the BTS method (p = 0.355). RHC or extended RHC was performed in all cases, and five surgery-related complications occurred (four in the SEMS group and one in the ileostomy group, respectively, p = 0.644). The baseline characteristics of 63 patients who underwent curative surgery are also summarized in Supplementary Table 1.
Among those considered unfit to undergo curative resection (Table 3), three out of nine patients were considered too elderly (> 90 years), five died after BTS (up to 5 months), and one was lost to follow-up. Of the five patients who died before the curative surgery, two had T-colon cancer, and three had obstructive lesions in the A-colon. In the two patients with T-colon cancer, ileostomy was performed in both. The causes of death were pneumonia or sepsis, respectively. In the three patients with A-colon obstruction, the tumor was large at the time of presentation, and ileostomy was performed for decompression in all patients; however, it did not work effectively, and pneumonia or sepsis progressed and resulted in death.
Survival analysis for long-term mortality
During the follow-up period of up to 60 months, six deaths (three in the SEMS group and three in the ileostomy group) were recorded among 63 patients who underwent RHC or extended RHC after successful decompression. Two died within 12 months in the course of postoperative care (one each in the SEMS and ileostomy groups). Notably, both patients had postoperative ileus as a complication after curative surgery (Table 2). The remaining four patients, whose main causes of death were recurrence and progression of cancer, survived up to 36 months (Fig. 3). Survival analysis for long-term mortality after curative surgery showed no significant difference between the bridge modalities up to 60 months (log-rank p = 0.253). The 1- and 3-year cumulative survival rates for SEMS versus ileostomy groups were 94.9% versus 95.0% and 90.7% versus 78.9%, respectively.
DISCUSSION
According to this study, the overall short-term mortality rate was 6.9%, which was higher than the result of a recent population-based analysis (SEMS, n = 44 and ileostomy, n = 42; mortality 2.4% vs. 2.4%, respectively) [7]. SEMS insertion showed more favorable outcomes as a BTS in terms of post-procedural mortality, but long-term death rates after curative surgery for primary cancer showed no difference according to the BTS modalities. The main factor for the better short-term clinical outcome in the SEMS group was that all five patients who died early after BTS underwent ileostomy. Although it is not certain due to the nature of the retrospective study, the reason that SEMS was not inserted in all five patients is probably due to the risk of perforation.
We analyzed the causes of the six deaths after curative resection, especially for the two patients that died before 12 months. One underwent ileostomy immediately after stent insertion failure. The patient recovered after ileostomy, completed curative resection, and was undergoing chemotherapy but died of sepsis due to sudden bowel infarction 6 months after surgery. Due to the nature of the retrospective study, it is difficult to conclude how the stent failure situation affected the prognosis of this patient. The second patient was in the ileostomy group, and it was possible to complete RHC after ileostomy. However, the patient died of pneumonia after surgery, and the reason recovery was difficult after surgery is thought to be due to the patient’s age (92 years). The remaining four patients (two each from the SEMS and ileostomy groups) who survived after radical surgery but died within 3 years all died from cancer progression.
Procedures for decompression in MOPC had been considered less common than those for distal obstruction. A previous population-based analysis of MOPC reported that acute primary resection was still a major strategy for acute MOPC, which was performed in 95% of the whole study population, with a primary anastomosis rate of 86%. A decompressing BTS, including SEMS insertion and diverting loop ileostomy, was performed in only 5% of the study population [7]. According to the results of that study, mortality was significantly lower after a bridging strategy than after primary resection. A recent systematic review also revealed that colonic stenting, compared with primary surgery, caused lower mortality (0% vs. 10.8%, p = 0.009), less major morbidity (0.8% vs. 23.9%, p = 0.049), and lower risk of anastomotic leakage (0% vs. 9.1%) in patients with MOPC [3].
However, stent placement for obstructing proximal colon cancer has not been commonly used in the past years, which is probably due to fear of stent-related complications and uncertainty about the oncologic long-term outcomes. The premature closure of two Dutch randomized controlled trials comparing stents with emergency surgery indicated a high incidence of stent-related complications [18,19]. It has also been suggested that stent insertion in obstructive colon cancer capable of curative resection may have a negative effect on the oncological outcome [19,20]. However, the meta-analysis results published in 2018 showed that stent insertion as a BTS in an acute malignant left-sided colonic obstruction did not differ in 5-year overall survival and 5-year disease-free survival compared to emergency resection [21]. In addition, the results of a multicenter randomized controlled trial recently published with 5-year survival results showed that stent insertion with BTS showed no difference in oncological outcome at both 3- and 5-year follow-up compared to emergency surgery, including both enterostomy and bowel resection [22,23]. Since the above studies were for left-sided colonic obstruction, it cannot be directly compared to the case with proximal colons as in this study. Moreover, whether stent insertion is associated with oncologically poor outcomes in proximal colonic obstruction is not well known. Due to the small number of patients in this study, it is not appropriate to draw conclusions comparing oncological outcomes; however, according to this study alone, tumor recurrence after curative resection occurred in four patients (two patients in the stent group and two patients in the ileostomy group), and there was no difference in the results. Of course, it is an obvious limitation that the baseline characteristics between the two groups differed enough to affect the oncological outcome. However, it cannot be ignored that there is no significant difference between the two groups in TNM stage results after curative resection. If BTS for acute obstruction was safely completed and curative resection was subsequently performed, TNM stage after curative resection is thought as the most important factor affecting the long-term oncological outcome. Those factors showed no significant difference in both SEMS and ileostomy groups in this study. A larger prospective study will be needed in the future.
Furthermore, the long distance from the anus and tortuosity of the bowel make proximal stenting considerably more difficult than in the distal colon. However, another report found no difference between proximal and distal obstructions of colorectal malignancy in terms of technical and clinical success rates [10]. Our study showed that SEMS implantation within the proximal colon achieved 95.2% (40/42), comparable to the results of previous studies [9,15]. Salvage ileostomy could be performed immediately in the two patients who failed SEMS insertion in the first attempt, and short-term survival was not affected.
In this study, all five patients who died after BTS underwent diverting ileostomy. In case of very severe obstruction at the time of diagnosis, with a subsequent high risk of perforation, it may have been inappropriate to attempt an SEMS procedure that required colonoscopy to enter the proximal area. In other words, it can be interpreted that the patient’s underlying condition was a major contributing factor to mortality after BTS, not the problem between BTS and ileostomy. When curative surgery was possible after BTS, the mortality risk for up to 5 years showed no difference among the two BTS groups. Given that the sample size of the study and the number of deaths were small, there was a limit to interpreting the statistical significance of the results of this study. Therefore, we do not seek to draw definitive conclusions here as to which method is superior for acute MOPC. However, as this is an important issue that many researchers and clinicians are curious about, we have tried to show the data we obtained as it is, and we expect that further research will be conducted in the future.
In conclusion, SEMS as a BTS showed relatively safe results in terms of post-procedural mortality. However, these results should be considered when performing ileostomy in patients with more advanced MOPC. At least, the results of this study showed that stent insertion did not adversely affect the long-term oncologic outcome of MOPC compared to ileostomy. An individualized approach is necessary considering the advantages and disadvantages of SEMS and diverting loop ileostomy as a bridge strategy for curative surgery.
KEY MESSAGE
1. Stent insertion as a bridge to curative surgery for an acute malignant obstruction of the proximal colon showed a relatively more favorable result in terms of post-procedural mortality.
2. Survival analysis for long-term mortality up to 60 months after curative surgery showed no significant difference between the bridge modalities: stent versus stoma.
Notes
No potential conflict of interest relevant to this article was reported.