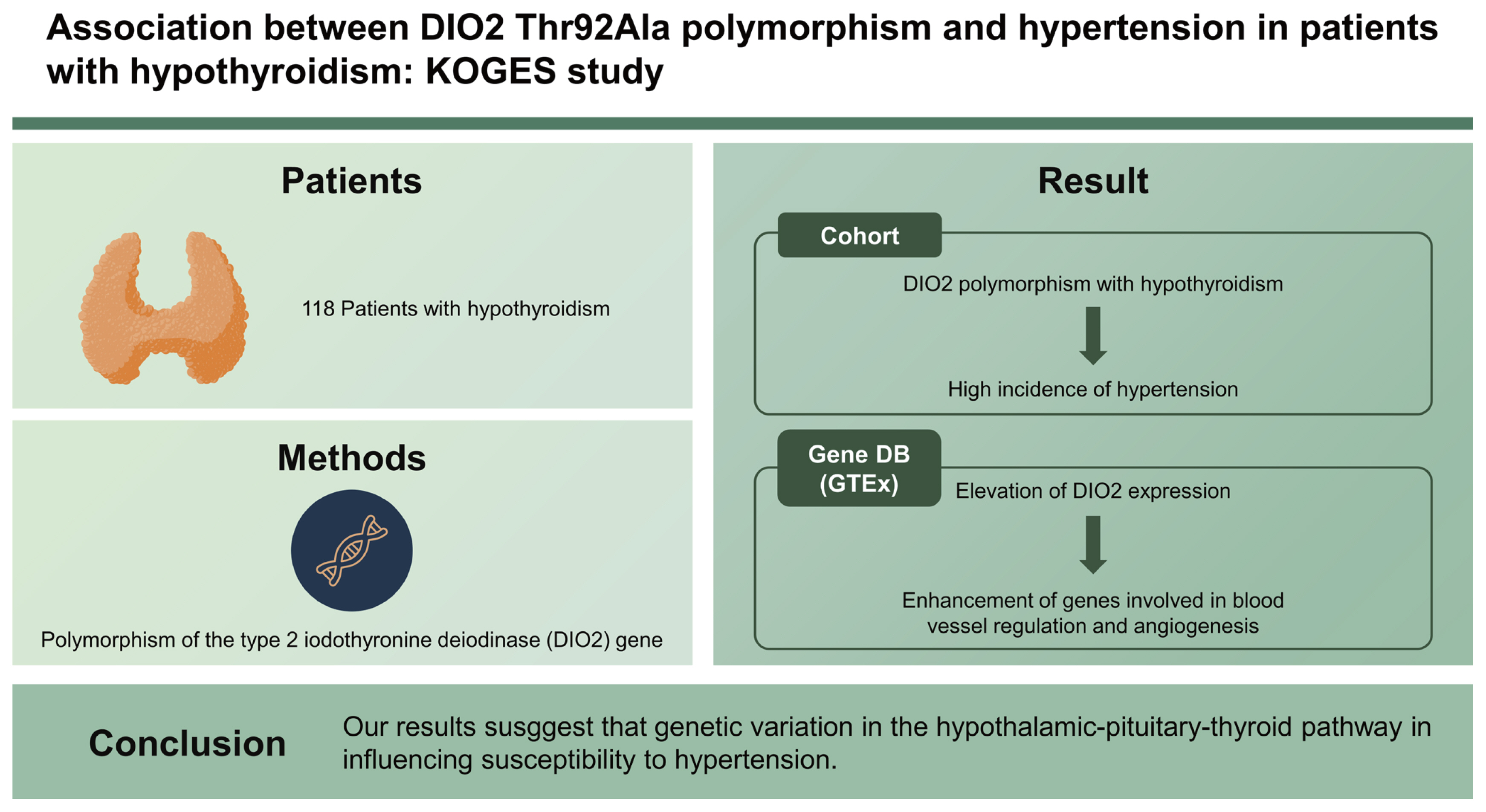
Association between DIO2 Thr92Ala polymorphism and hypertension in patients with hypothyroidism: Korean Genome and Epidemiology Study
Article information
Abstract
Background/Aims
Recent evidence has identified the significance of type 2 iodothyronine deiodinase (DIO2) in various diseases. However, the role of DIO2 polymorphism in metabolic parameters in patients with hypothyroidism is not fully understood.
Methods
We assessed the polymorphism of the DIO2 gene and various clinical parameters in 118 patients who were diagnosed with hypothyroidism from the Ansan-Anseong cohort of the Korean Genome and Epidemiology Study. Furthermore, we systematically analyzed Genotype-Tissue Expression (GTEx) data.
Results
A total of 118 participants with hypothyroidism were recruited; 32 (27.1%) were homozygous for the Thr allele, 86 (73.9%) were homozygous for the Ala allele or heterozygous. Patients with hypothyroidism with DIO2 polymorphism without hypertension at baseline had higher incidence of hypertension compared to patients without DIO2 polymorphism. Analysis of the GTEx database revealed that elevation of DIO2 expression is associated with enhancement of genes involved in blood vessel regulation and angiogenesis.
Conclusions
Commonly inherited variation in the DIO2 gene is associated with high blood pressure and prevalence of hypertension in patients with hypothyroidism. Our results suggest that genetic variation in the hypothalamic-pituitary-thyroid pathway in influencing susceptibility to hypertension.
INTRODUCTION
The association of essential hypertension (HTN) and serum thyroid-stimulating hormone (TSH) concentrations in various animal models is not known [1–3]. Recently, it has been reported that the overexpression of thyrotropin-releasing hormone (TRH) precursor in the third ventricle of the central nervous system of normal rats induces a prolonged elevation of arterial blood pressure (BP) along with an increase in the diencephalic TRH content in a dose-dependent manner. These effects were specifically reversed by an antisense treatment, indicating that the extrahypothalamic TRH system effectively participates in cardiovascular regulation in rats [4]. Thyroid hormone also has significant effects on the peripheral vascular system, including increased blood volume due to decreasing systemic vascular resistance and increasing left ventricular contractility [5]. Patients with hypothyroidism are known to have increased systemic vascular resistance, arterial stiffness, and reduced cardiac output, resulting in diastolic HTN in approximately 30% of patients and increasing the risk of cardiovascular diseases [6]. In fact, many research groups also have described the association of thyroid function and maintenance of BP in numerous clinical studies [7–10].
Accumulating evidence suggests that genetic variations in the hypothalamic-pituitary-thyroid axis, including those in type 2 iodothyronine deiodinases (DIO2), influence susceptibility to HTN. The DIO2 gene is known to catalyze the conversion of intracellular inactive thyroid hormone (thyroxine [T4]) to active thyroid hormone (triiodothyronine [T3]) for use in local tissues [11]. A genome-wide linkage analysis identified a common nonsynonymous DIO2 single nucleotide polymorphism (SNP), resulting in a Thr92Ala substitution, which has been observed in healthy individuals [12]. Recent studies have revealed the effects of DIO2 genetic polymorphisms on HTN [13,14]. The estimated odds ratio for HTN in Ala92 allele carriers compared with Thr92 homozygotes is increased, and the common Ala92 allele of DIO2 increases the risk for development of HTN among euthyroid adults [14]. DIO2 is also expressed in human aortic and coronary artery smooth muscle cells [15] and the implication of the FoxO1–DIO2 axis in hypertrophic growth of cardiomyocytes [16] suggesting a role for DIO2 in mediating vascular tone and BP. However, in patients with type 2 diabetes mellitus (T2DM), the DIO2 Thr92Ala polymorphism is associated with lower insulin sensitivity, but it is not a major determinant of BP levels or HTN [17]. These results suggest that the effects of DIO2 polymorphism on BP differs according to comorbidity, and the effects of DIO2 polymorphism on HTN are controversial [14,18].
Hypothyroidism is an important disease in which the levels of iodothyronine deiodinases change [19]. As a result of low thyroid hormone levels, the activity of DIO2 is accelerated in almost all tissues, increasing the whole-body fractional conversion of T4 to T3, which helps preserve serum levels of T3 in hypothyroidism [20]. Since the activity of the DIO system in various tissues may change in hypothyroidism status, genetic background in genes, including thyroid hormone synthesis and DIO2 polymorphism, has been reported to play an important role in drug response [21]. Recent studies have suggested that patients with hypothyroidism who possess the DIO2 polymorphism have beneficial drug response [22,23]; however, the association between DIO2 polymorphism and HTN has not been identified in patients with hypothyroidism taking thyroid hormone medications.
Thus, we investigated whether polymorphisms in the DIO2 gene contribute to BP and HTN using community-acquired cohort data. We also evaluated whether DIO2 Thr92Ala (rs225014) was associated with newly diagnosed HTN in patients with hypothyroidism during the 48-month follow-up period.
METHODS
Study participants
The study participants were enrolled from the Ansan-Anseong cohort, a population-based cohort included in the Korean Genome and Epidemiology Study (KoGES). A total of 7,213 cohort members aged 40 to 69 years were assessed biennially with scheduled on-site follow-up visits at baseline (2001 to 2002) [24–27]. We defined patients with hypothyroidism on a questionnaire basis. To include the patients diagnosed as hypothyroidism, we reviewed the record of medication of thyroid hormone and diagnosis of thyroid disease. In the questionnaire, patients were asked to indicate the presence or absence of a history of thyroid cancer or thyroid disease. Moreover, it was necessary to check whether or not thyroid hormone medications were taken. We excluded thyroid cancer patients because most of them suppress their TSH levels to lower than normal to prevent cancer recurrence, and the TSH concentration differs depending on the pathological results of the cancer. Finally, we defined patients with hypothyroidism as patients diagnosed with thyroid disease who are taking thyroid hormones. A total of 224 people responded to the questionnaire that they had thyroid disease and prescribed thyroid hormone. After excluding participants without record of thyroid hormone medication (n = 106), total of 118 patients with hypothyroidism were diagnosed as hypothyroidism patients. The purpose of the study was to analyze the clinical parameters, including BP and HTN, in relation to DIO2 polymorphism.
Study design
The DIO2 Thr92Ala (rs225014) polymorphism was selected for further investigation of its potential involvement in BP and associated diverse clinical parameters of this study population. Further information and the study design for this ongoing prospective cohort are available in a previous report [28]. All participants participated voluntarily, and this study was reviewed and approved by the Institutional Review Board of Chungnam National University Hospital (CNUH-2018-03-029). Each participant signed an informed consent form, approved by the Human Subjects Committee, before the baseline health examination and during every visit. A comprehensive set of questionnaires, laboratory tests, and clinical measurements were completed at the baseline visit.
Assessment of hypertension
BP was measured according to the World Health Organization-International Society of Hypertension (WHO-ISH) guidelines 21 using a mercury sphygmomanometer by a trained technician. Three BP readings were taken on either arm at 30-second intervals. Participants assumed the supine position for 5 minutes, and caffeine substances and smoking were restricted for 30 minutes before the measurements. The BP presented in this study was the average of three measurements. HTN was defined as a systolic BP of ≥ 140 mmHg or a diastolic BP of ≥ 90 mmHg according to the WHO-ISH guidelines [29–31] or those who were receiving antihypertensive therapy at the time of the examination.
Clinical factors and biochemical parameters
The participants’ information was collected using a standardized questionnaire. For more detailed information of the KoGES (Anseong-Ansan) cohort questionnaire, we confirmed through previous cohort profile publication for this cohort [25]. Clinical factors, including cigarette smoking, alcohol consumption, and menopausal status, were investigated. Participants were questioned about cigarette smoking (current, past, or never) and alcohol consumption (units per week). Height, body weight, and waist circumference were measured while wearing light clothes and no shoes. Height was measured using a stadiometer to the nearest 0.1 cm, and weight was measured using a metric scale to the nearest 0.01 kg. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Blood samples were collected after > 8 hours of fasting, and plasma concentrations of total cholesterol, high-density lipoprotein cholesterol (HDL-C), glycated hemoglobin (HbA1c), fasting glucose, and fasting insulin were measured using biochemical assays performed in a central laboratory (Seoul Clinical Laboratories, Seoul, Korea) [32].
Genotyping and single nucleotide polymorphism analysis
Genomic DNA samples were isolated from whole blood and genotyped using a Genome-Wide Human SNP Array 5.0 (Affymetrix Inc., Santa Clara, CA, USA). Bayesian robust linear modeling using the Mahalanobis distance genotyping algorithm was employed for genotype calling. The DIO2 Thr92Ala polymorphism was initially investigated based on the KoGES [32].
Gene Ontology and pathway-enrichment analysis with the GTEx database
To analyze DIO2 related genes and pathways in artery-aorta and heart-left ventricle, we utilized GTEx database. The RNAseq data (transcripts per million [TPM] and read count) were downloaded from GTEx database (https://www.gtexportal.org/). Using tissue TPM data, upper and lower 25% groups were classified according to the expression of DIO2. Differentially expressed gene analysis (DEA) through DESeq2 analysis of R program was performed using read count data. Gene set (Kyoto Encyclopedia of Genes and Genomes [KEGG] and Gene Ontology [GO]) required for gene set analysis were downloaded from EnrichR (https://maayanlab.cloud/Enrichr/). Gene set analysis was performed by inputting the DEA result and the gene set to Platform For Integrative Analysis of Omics data (PIANO) of the R program (R Foundation for Statistical Computing, Vienna, Austria).
Statistical analyses
All data are expressed as mean ± standard deviation for continuous variables and as numbers (%) for categorical variables. A paired t test and chi-squared test were used to compare simple differences in continuous and categorical variables, respectively, among the groups. All analyses were performed using the SPSS version 24.0 (IBM Corp., Armonk, NY, USA). A p < 0.05 was considered statistically significant.
RESULTS
General baseline characteristics of patients with hypothyroidism and the Thr92Ala DIO2 genotype
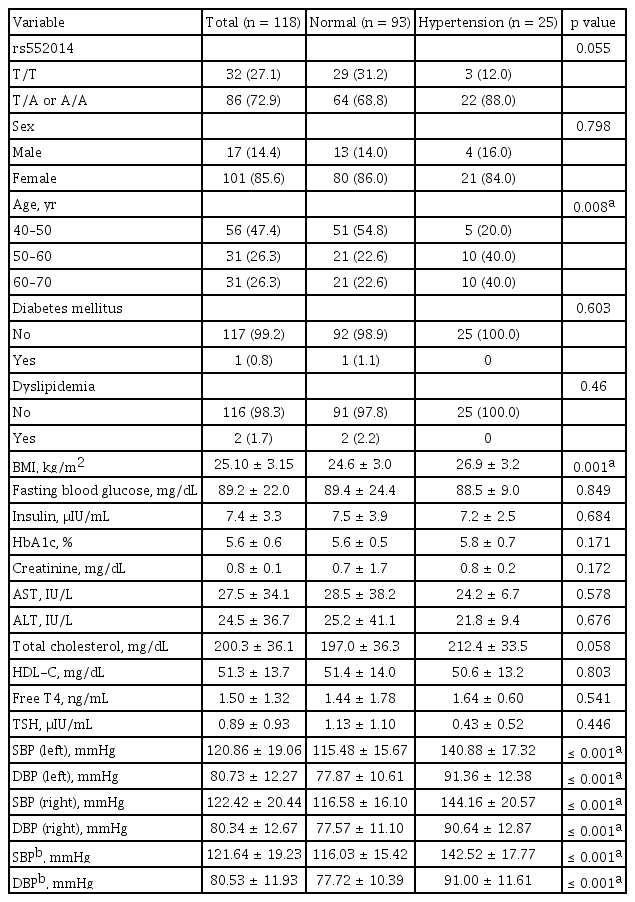
The baseline characteristics of 118 participants with complete Thr92Ala DIO2 genotypes are shown in Table 1. The genotypes of the DIO2 Thr92Ala polymorphism present in the cohort included Thr/Thr (T/T) (n = 32), and Thr/Ala (T/A) or Ala/Ala (A/A) (n = 86) (Supplementary Table 1). In relation to DIO2 Thr92Ala polymorphism, there was no significant difference in sex, age, BMI, menopausal status, alcohol status, smoking status, and diagnosis of diabetes mellitus, dyslipidemia, or HTN among the three groups at baseline (Supplementary Table 1). Particularly, the mean systolic BP (T/T, 114.50 mmHg; T/A or A/A, 124.30 mmHg) and diastolic BP (T/T, 74.94 mmHg; T/A or A/A, 82.62 mmHg) were significantly increased in patients with hypothyroidism with DIO2 Thr92Ala polymorphism; however, the BPs were in the normal range (Supplementary Table 1). Next, we investigated the association between the DIO2 Thr92Ala genotype and biochemical parameters, including serum TSH level and free T4 levels, and there were no statistically significant differences between the two groups (Supplementary Table 1).
DIO2 polymorphism status and biochemical parameters between the normal blood pressure and hypertension groups in patients with hypothyroidism (n = 118)
We classified participants into two groups, the normal BP group and the HTN group, and investigated the association between DIO2 polymorphism status and biochemical parameters. The HTN group tended to reveal higher DIO2 polymorphism (T/A or A/A, 88%) compared to the normal BP group (T/A or A/A, 68.8%); however, there was no significance (p = 0.055) (Table 1). The HTN group had significantly higher age group (40–50 years, 54.8%; 50–60 years, 22.6%; and 60–70 years, 22.6%) compared to the normal BP group (40–50 years, 20%; 50–60 years, 40%; and 60–70 years, 40%) (p = 0.008) (Table 1). Additionally, the HTN group in patients with hypothyroidism showed significantly higher BMI (26.9 ± 3.2 vs. 24.6 ± 3.0), compared to the normal BP group (p = 0.001) (Table 1). However, there were no significant differences in sex, diagnosis of diabetes mellitus, diagnosis of dyslipidemia, fasting glucose level, Insulin, HbA1c level, creatinine level, hepatic function, total choles terol level, and HDL-C level (Table 1). These results suggest that DIO2 polymorphism may be a contributing factor to the development of HTN in patients with hypothyroidism.
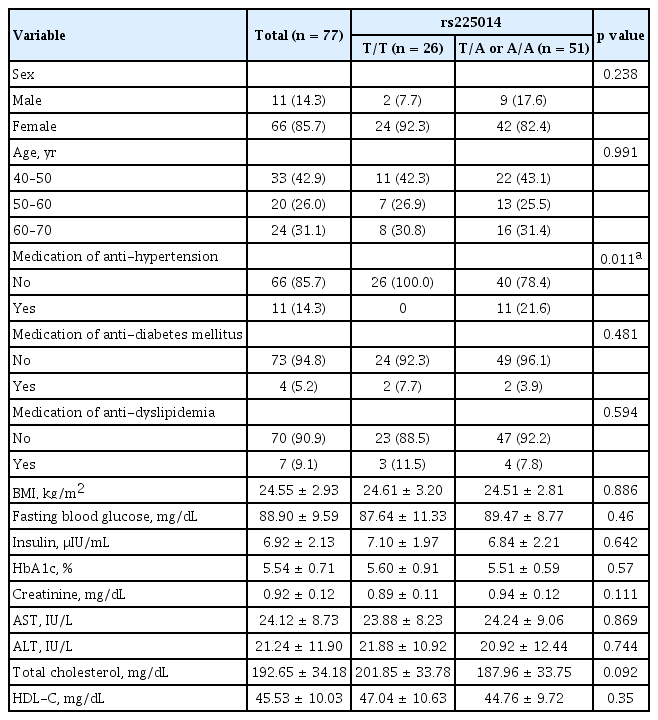
Association between DIO2 Thr92Ala genotype and diagnosis of hypertension in participants without hypertension after the 48-month follow-up period
To investigate the effect of the DIO2 Thr92Ala polymorphism on HTN in patients with hypothyroidism, we investigated the incidence of newly diagnosed HTN after the 48-month follow-up period in participants without HTN at baseline (Table 2). In total, 77 participants had normal baseline BP based on BP measurement. There was no event of HTN in the T/T group; however, 11 (21.6%) patients were diagnosed with HTN in the DIO2 polymorphism group (p = 0.011). At baseline, there were no significant differences in other clinical parameters, including BMI, age, waist circumference, fasting blood glucose level, HbA1c level, and diagnosis of HTN or dyslipidemia (Table 2). Collectively, our data showed that the prevalence of newly diagnosed HTN was significantly increased in participants with DIO2 Thr92Ala polymorphism in patients with hypothyroidism with normal BP at baseline after the 48-month follow-up period.
GTEx gene-set-enrichment analysis of blood vessels, in relation to DIO2 expression
To gain further insight into the relationship between HTN and DIO2 expression in human blood vessels, gene-enrichment analysis was performed with aorta-artery and left ventricle expression data from the GTEx database, with reference to levels of DIO2 expression. Of the 432 artery-aorta samples in the GTEx database, samples with DIO2 expression levels in the highest quartile (n = 108) and the lowest quartile (n = 108) were used for GO pathway analyses (Fig. 1A and 1B). In these samples, 5367 genes were upregulated in the high-DIO2 group compared with the low DIO2 group (Fig. 1C). In a GO-Biological Process (GO-BP) analysis, among the significantly upregulated processes were regulation of angiotensin levels in blood, regulation of endothelial cell proliferation, and positive regulation of angiogenesis (Fig. 1D). In addition, genes involved in mitochondrial respiration chain complex biogenesis and fatty acid oxidation were downregulated in the artery-aorta of individuals with higher DIO2 expression (Fig. 1E). Analysis of expression data from left ventricle revealed the similar results (Fig. 2). In a GO-BP analysis, among the significantly upregulated processes were regulation of angiogenesis, regulation of endothelial cell proliferation, and positive regulation of blood vessel endothelial cell migration (Fig. 2D), and downregulated processes were mitochondrial adenosine triphosphate (ATP) synthesis, fatty acid beta-oxidation, and mitochondrial ATP synthesis coupled electron transport (Fig. 2E). These results indicate that DIO2 expression in human blood vessels is associated with BP regulation and vessel development with metabolic regulation involving processes such as the tricarboxylic acid cycle and lipid metabolism.
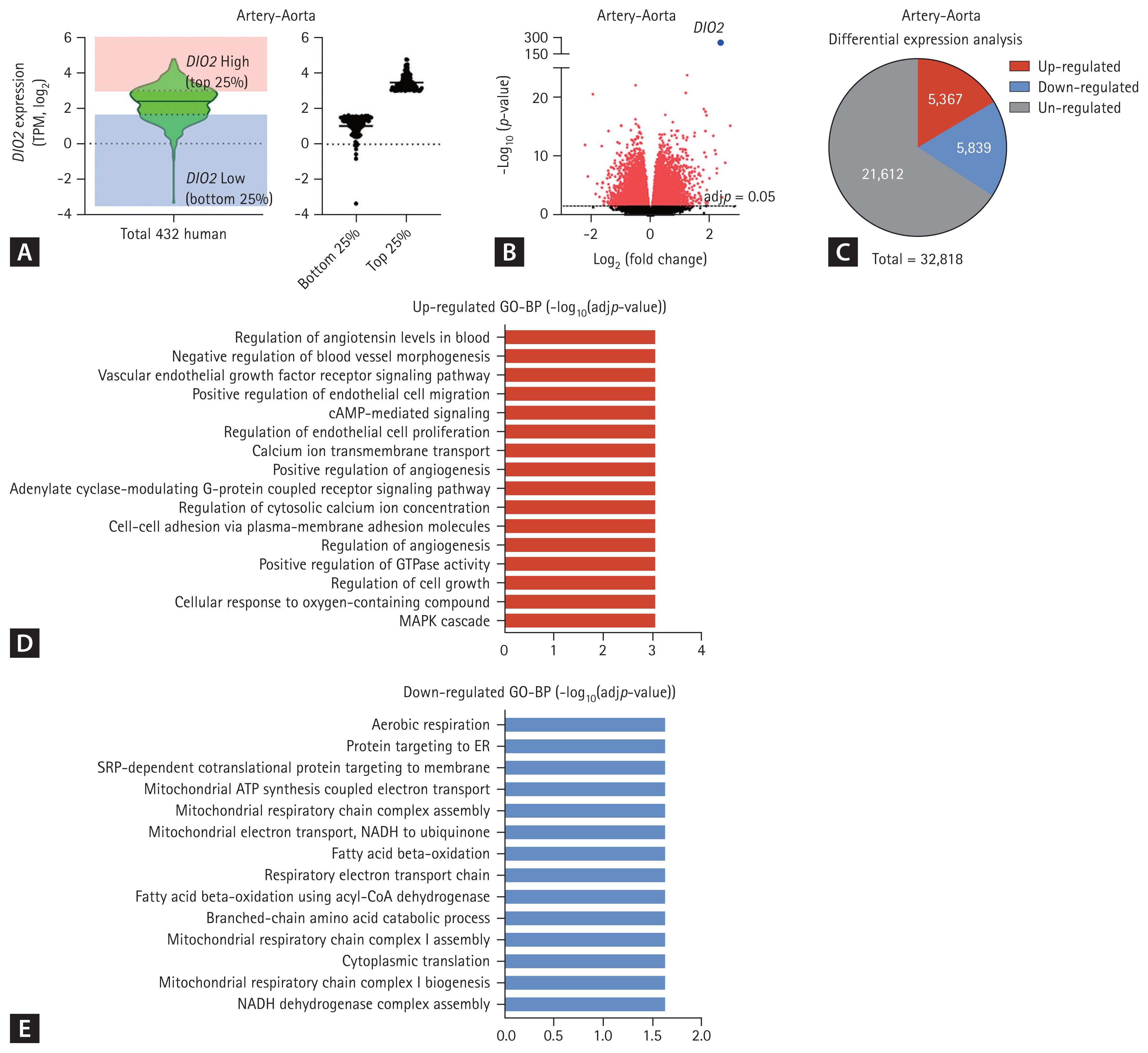
Upregulation of physiological pathways in artery-aorta with high expression of type 2 iodothyronine deiodinase (DIO2) in the Genotype-Tissue Expression database. (A) DIO2 expression levels in 432 human artery-aorta samples in the University of California Santa Cruz (UCSC) database. (B) Volcano plot of differentially expressed gene (DEG) between DIO2 top 25% group and DIO2 bottom 25% group. (C) Numbers of DEGs in a comparison of the samples in the highest quartile for DIO2 expression and those in the lowest quartile. (D) Upregulated biological processes in the Gene Ontology (GO) annotation associated with increased DIO2 expression. (E) Down-regulated biological processes in the GO annotation associated with increased DIO2 expression. TPM, transcripts per million; GO-BP, GO-Biological Process; MAPK, mitogen-activated protein kinase; cAMP, cyclic adenosine monophosphate; ER, endoplasmic reticulum; ATP, adenosine triphosphate; NADH, nicotinamide adenine dinucleotide hydrogen; CoA, coenzyme A.

Upregulation of physiological pathways in left ventricle with high expression of type 2 iodothyronine deiodinase (DIO2) in the Genotype-Tissue Expression database. (A) DIO2 expression levels in 432 human left ventricle samples in the University of California Santa Cruz (UCSC) database. (B) Volcano plot of differentially expressed gene (DEG) between DIO2 top 25% group and DIO2 bottom 25% group. (C) Numbers of differentially expressed genes in a comparison of the samples in the highest quartile for DIO2 expression and those in the lowest quartile. (D) Upregulated biological processes in the Gene Ontology (GO) annotation associated with increased DIO2 expression. (E) Downregulated biological processes in the GO annotation associated with increased DIO2 expression. TPM, transcripts per million; GO-BP, GO-Biological Process; cAMP, cyclic adenosine monophosphate; MAPK, mitogen-activated protein kinase; ATP, adenosine triphosphate; NADH, nicotinamide adenine dinucleotide hydrogen; CoA, coenzyme A.
DISCUSSION
In the current study, we identified an association between the DIO2 Thr92Ala (rs225014) polymorphism and BP in patients with hypothyroidism using the KoGES [21,22]. The analysis of newly diagnosed HTN after the 48-month follow-up period in the normal BP group revealed a significantly increased incidence of HTN in the DIO2 Thr92Ala (rs225014) polymorphism group in patients with hypothyroidism.
The heart is a major target of the thyroid hormone, and abnormal thyroid hormone levels in the fetus and neonate are associated with several cardiovascular complications. Importantly, dysregulated thyroid hormone homeostasis in adults is also associated with cardiovascular diseases [16]. DIO2 is involved in active thyroid hormone synthesis and plays a pivotal role in maintaining the levels of circulating and tissue thyroid hormones [33–35]. DIO2 is expressed in the heart atrium and ventricle by regulation through nkx2–5; its consensus binding site is virtually identical to thyroid transcription factor-1, which regulates DIO2 expression in the human thyroid [36,37]. A recent study identified that DIO2-induced thyroid hormone synthesis is associated with cardiac remodeling and contributes to stress-induced cardiomyopathy [16], suggesting the implication of DIO2 in cardiovascular structure. DIO2 polymorphism was also known to be associated with other metabolic diseases. The Thr92Ala common variants, including rs225011 and rs225015, were modestly associated with early-onset T2DM in the case-control study [13], and an association with an increase in BMI in carriers of both the beta-3 adrenergic receptor (ADRB3) Trp64Arg and the DIO2 variants has been reported [38]. However, an observational cohort study identifying that the DIO2 Thr92Ala polymorphism does not confer an increased risk of T2DM, obesity, or insulin resistance [39] suggests that the metabolic phenotyping ability of DIO2 has not been established. Our study indicates that the DIO2 Thr92Ala polymorphism in patients with hypothyroidism is closely associated with high BP. However, we did not find any significant differences in other clinical characteristics between different genotypes, including plasma glucose levels, plasma lipid levels, or BMI. These results suggest that vascular structure may be sensitively affected by a decrease in DIO2-mediated T3 production compared to other organs with low thyroid hormone status.
A previous study has explored the influence of DIO2 polymorphisms in different tissues and disease states [40]. Accumulating evidence suggests the importance of DIO2 in vascular structure; however, the influence of DIO2 polymorphisms on BP is controversial. In healthy euthyroid adults, DIO2 polymorphism is a risk factor for the development of HTN [14]. Another study identified the association between DIO2 polymorphism and risk for diabetes mellitus and HTN using the Framingham Heart Study, a prospective, population-based cohort study of cardiovascular disease incidence and risk factors [17,41]. Among the 1,557 participants, 12.9% were homozygous for the alanine allele, and 48.1% were heterozygous; however, there was no significant association between with DIO2 polymorphism and the development of HTN [17] or T2DM risk [41]. However, that study was designed to stratify the risk for cardiovascular comorbidity and included a heterogeneous population with severe obesity, T2DM, or coronary artery disease. Another study also identified the association between DIO1 polymorphism and HTN in patients with acute myocardial infarction [42], and enrolled participants also had severe comorbidity, including obesity and T2DM. Thus, these previous studies may not have well-established study designs focusing on the role of genetic investigation of DIO2 in humans. Previously, we also used a healthy community-acquired large cohort study to identify the role of DIO2 polymorphism in humans and found that only bone mineral density was significantly different in relation to DIO2 polymorphism [32]. However, the activity of DIO2 is known to be accelerated in almost all tissues in hypothyroidism due to low thyroid hormone levels; therefore, we investigated the effect of DIO2 polymorphism on metabolic parameters in patients with hypothyroidism. The present study indicated a significant association between BP and DIO2 polymorphism in patients with hypothyroidism, and our data suggest that blood vessels are sensitive organs affected by DIO2 polymorphism in hypothyroidism. To identify our results, further functional study of the effect of loss of DIO2 in blood vessels in patients with hypothyroidism may be necessary.
Several studies have implicated the importance of DIO2 in patients with hypothyroidism; however, the effect of DIO2 polymorphism on BP in patients with hypothyroidism has not been identified. One study focused on the association between DIO1 and DIO2 variants in patients with thyroid cancer undergoing thyroidectomy and treatment with thyroxin [43]. They reported that participants with DIO2 polymorphism, rs1388378_G>T, required a higher hormonal dose; however, they did not suggest the influence of DIO2 polymorphism on disease status or other clinical parameters. The combination of polymorphisms in DIO2 (rs225014) and monocarboxylate transporter 10 (MCT10) (rs17606253) enhances hypothyroid patients’ preference for L-T4 + L-T3 replacement therapy [22], and patients with hypothyroidism with DIO2 polymorphism are also known to have improved well-being in response to combination therapy with T3 [23]. A recent study investigated the intracellular and serum T3 concentrations that are not adequately compensated for by LT4 in patients with thyroidectomy with DIO2 polymorphism, providing the evidence, in favor of L-T4 + L-T3 replacement therapy for hypothyroidism with DIO2 polymorphism [44]. We could not validate the medication dose and characteristics of drugs in patients; however, our data suggested the implication of T3 replacement therapy in patients with hypothyroidism with DIO2 polymorphism to prevent HTN, since the prescription of T4 alone was significantly higher than the prescription rate of T4 + T3 in South Korea.
The present study has several strengths. First, to the best of our knowledge, this study is the first to examine the association between DIO2 polymorphism and the diagnosis of HTN in patients with hypothyroidism, suggesting the importance of DIO2 on blood vessel integrity in patients with low thyroid hormone status. Second, we prospectively evaluated the association between diverse metabolic parameters and DIO2 polymorphism by high-throughput SNP in a cohort drawn from the general population. Third, all participants were Korean, decreasing the risk of false-positive or false-negative associations due to ethnic bias. Finally, we prospectively observed the effect of DIO2 polymorphism on the incidence of HTN in participants with normal BP at baseline, demonstrating the role of DIO2 in BP in patients with hypothyroidism.
However, the study’s limitations include a relatively small sample size due to inclusion and exclusion criteria and dropout in our study. Thus, further studies should replicate the model and include a large number of participants. Second, the diagnosis of hypothyroidism was based on questionnaires, and the cause of hypothyroidism and characteristics of medication were not evaluated. Lastly, since HTN is a heterogeneous disease, the contribution of a single gene may be limited, and this finding may not apply to other ethnic populations. Further large-scale prospective studies in other populations are warranted to validate our results.
In conclusion, DIO2 polymorphism may be significantly associated with HTN diagnosis in patients with hypothyroidism, and our data provide evidence on the role of DIO2 polymorphism in BP in hypothyroidism.
KEY MESSAGE
1. The type 2 iodothyronine deiodinase (DIO2) Thr92Ala polymorphism in participants with hypothyroidism revealed higher blood pressure compared to controls.
2. Analysis of the Genotype-Tissue Expression (GTEx) database revealed that elevation of DIO2 expression is associated with enhancement of genes involved in blood vessel regulation and angiogenesis.
3. Commonly inherited variation in the DIO2 gene is associated with high blood pressure and prevalence of hypertension in patients with hypothyroidism.
Acknowledgments
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (grant numbers: HC19C0103 and HR20C0025). This research was supported by the National Research Foundation of Korea (NRF) (grant number 2021R1C1C1011183) and the Korean Thyroid Association Young Investigator Award 2020 to Yea Eun Kang.
Notes
No potential conflict of interest relevant to this article was reported.