The prognostic value of comprehensive geriatric assessment on the management of older patients with small cell lung cancer
Article information
Abstract
Background/Aims
The prognostic value of a comprehensive geriatric assessment (CGA) for the management of older small cell lung cancer (SCLC) patients remains to be established.
Methods
A retrospective cohort enrolled 21 SCLC patients over 65 years from March 2018 to 2019 at the Yonsei Cancer Center. The CGA included the following instruments: frailty, body mass index, sarcopenia (circumference of arm and calf, Timed Up and Go test, grip strength), comorbidity, polypharmacy, activities of daily living (ADL), Instrumental ADL, nutrition, depression, and cognitive function. The correlations of oncological and geriatric variables with overall survival (OS) were determined. The log-rank test with Cox model and Kaplan-Meier method were used for the analysis.
Results
The median age was 75 years (range, 67 to 85). All patients had the Eastern Cooperative Oncology Group performance status 0–2. The median survival was 9.93 months (range, 1.53 to 36.30). Among CGA parameters, ADL and nutritional status had significant differences in OS in univariate analysis. In multivariate analysis, only nutritional status was independently associated with survival (hazard ratio, 0.17; 95% confidence interval, 0.05 to 0.57). Median OS for low nutritional status was 5.63 months and the normal nutrition group was 15.5 months (p = 0.004).
Conclusions
Pre-treatment nutritional status measured by CGA appears to be a predictor of OS in older SCLC patients. However, for further generalization of the implication of CGA in SCLC, a larger scale study with prospective design is strongly needed.
INTRODUCTION
As life expectancy increases, the number of older cancer patients is also expected to gradually increase. Lung cancer, in particular, is the leading cause of cancer-related deaths in older population. Small cell lung cancer (SCLC) is a neuroendocrine tumor, accounting for approximately 15% of all lung cancers [1]. Unlike the recent development of various treatment that immunotherapy or target therapy for non-SCLC, it is true that there is no significant change in treatment for SCLC. Moreover, the median age at diagnosis of SCLC is over 65 years old, which is a cancer that mainly occurs in smokers and older populations. Although chemotherapy and radiation therapy for SCLC is very burdensome for older patients, older patients with SCLC are underestimated when making treatment decisions in the clinical setting. A comprehensive assessment of the overall health status and full support and management for the older SCLC patients is very important. This is a different concept from palliative care and requires an independent approach by geriatric professionals.
Older cancer patients are a very heterogeneous group of patients from younger patients in terms of the concept of ‘frailty,’ which includes a decreased physiological reserve resulting in reduced resilience and adaptive capacity and increased vulnerability to stressors [2]. It includes many other comorbidities, as well as cognitive impairment, and disability. This frailty itself has a negative effect on treatment tolerance, which in turn affects the prognosis. Therefore, a comprehensive approach for older cancer patients including frailty assessment is essential [3]. Several studies have investigated the effects of comprehensive geriatric assessment (CGA) on overall survival (OS), in-hospital mortality, hospitalization, adverse events, quality of life, and treatment allocation, suggesting that the CGA more accurately determines the health status of older cancer patients compared to the Eastern Cooperative Oncology Group performance status (ECOG PS) which is commonly used function measuring score in the current clinical setting [4,5]. In other words, successful treatment of older cancer patients requires overall management of physical, cognitive, emotional, nutritional status, and medication history. For this reason, a formal geriatric assessment has been developed for older adults [6–8]. However, studies specifically examining the relationship between CGA and OS in older SCLC patients are lacking. Therefore, this study aimed to estimate the prognostic role of various CGA domains on OS in older patients with SCLC. This will enable appropriate predictions and interventions to determine whether to treat chemotherapy and manage it during chemotherapy in older SCLC patients.
METHODS
Patients
This retrospective cohort study enrolled 291 patients aged over 65 years diagnosed with cancer at Yonsei Cancer Center between March 2018 and March 2019. A CGA test was performed on patients diagnosed with cancer and scheduled for chemotherapy. That is, patients for whom chemotherapy was not planned were not enrolled. Of these, 21 patients were diagnosed with SCLC and completed CGA prior to initiation of treatment. The interval between the date of CGA after diagnosis of SCLC was approximately one month. Their survival was followed until August 2021. Apart from the CGA, ECOG PS was also measured. We aim to analyze the CGA domain associated with OS in older SCLC patients. Ethical approval for this study was obtained from the Institutional Review Board (IRB) of Yonsei University’s Health System (IRB number: 4-2021-1348) and adhered to the tenets of the Declaration of Helsinki. Informed consent was waived by the IRB of Yonsei University’s Health System for this study as all the data was obtained from medical records.
Statistical analysis
The Cox regression test was used for univariate and multivariate analyses using the Cox proportional hazards model to evaluate the prognostic value of CGA domains. The log-rank test was used to evaluate survival differences between the groups according to CGA scores. Cumulative survival rates were calculated by the Kaplan-Meier method. Confidence intervals (CIs) at the 95% level were calculated, and statistical significance was considered with p values less than 0.05. All analyses were performed using SPSS for Windows version 25 (IBM Co., Armonk, NY, USA).
Comprehensive geriatric assessment
All participants underwent the CGA by trained geriatric nurses before the starting of chemotherapy. The CGA consisted of the following domains: basic items, physical activity, functional status, frailty, nutritional status, cognition, and depression. Basic items consisted of medical history including comorbidity, medications being taken, Timed Up and Go (TUG) test, grip strength, and lifestyle habits such as smoking. Comorbidity was evaluated by Charlson’s comorbidity index (CCI) [6], summing up the data regarding myocardial infarction, congestive heart failure, peripheral vascular disease, cerebral vascular disease, dementia, chronic pulmonary disease, rheumatologic disease, peptic ulcer disease, mild liver disease, diabetes, and so on. Since we only enrolled the SCLC patients, all patients scored 6 points in CCI, and 9 points were set as cut-offs for analysis [6,9]. The number of medications was analyzed by dividing five drugs as a cut-off value [10]. The clinical significance of sarcopenia is increasingly recognized as a component of cancer cachexia syndrome, and with this increasing awareness, a recent international consensus has established sarcopenia as the major diagnostic criterion for cancer cachexia [11]. In general, to evaluate sarcopenia, skeletal muscle mass is measured by computed tomography (CT) scan. However, not all patients had CT scans at a specific spinal level, so for ease of evaluation, arm and calf circumferences were measured. This has already been verified through other studies as an index to evaluate sarcopenia [12–14]. Since muscle mass itself may not indicate reliable muscle function, we further measured the TUG test and grip strength. In the TUG test, the patient was observed and timed while standing up from the chair, walking 3 m, turns walking back, and sitting down again [15]. Patients who were able to complete the task in less than 12.6 seconds were considered to have good mobility [16]. The cut-off value of handgrip strength was based on the consensus of the Asian Working Group for Sarcopenia (AWGS): 26.0 kg for male and 18.0 kg for female patients, respectively [17]. It is necessary to define the operational definition of sarcopenia by developing an index that combines the arm and calf circumferences, TUG test results, and grip strength values. However, in this study, the effect of only each domain on the OS were analyzed. Additionally, body mass index (BMI) was also measured. The level of frailty was assessed by the FRAIL scale, which was translated into Korean from Morley et al.’s FRAIL scale [18,19]. This self-reported five-item tool includes fatigue (F), resistance (R), ambulation (A), illnesses (I), and loss of weight (L). The total score (0–5) can be categorized into three: robust (0), pre-frail (1–2), frail (3–5). Functional status was evaluated by assessing activities of daily living (ADL) [20] and Korean Instrumental Activities of Daily Living (IADL) [21]. Six items of ADL included dressing, eating, walking on a corridor, toilet use, bathing, fecal, and urinary continence. Older adults with at least one dependency in ADL category were considered as ADL dependent. IADL included eight items which were shopping, housekeeping, ability to handle finances, ability to prepare food, traveling via car or public transportation, doing laundry, ability to use the telephone, and medication use. Older adults with at least one dependency in IADL category were assessed as IADL dependent. Nutritional status was assessed by the Mini Nutritional Assessment short-form (MNA-SF) [22]. It consists of six questions: decreased food intake, weight change, exercise capacity, medical history, cognitive impairment, and BMI. The MNA-SF score (range 0–14) of over 12 was evaluated as normal nutritional status, a score of 8–11 as a risk of malnutrition, and a score of 0–7 as a stage of malnutrition. Cognitive function was assessed using the Korean version of the Mini-Mental Status Examination for Dementia Screening (MMSE-DS). This 30-item tool indicated decrease in cognitive capacity with lower score [23]. A score below 17 denotes impairment, a score from 17 to 24 denotes mild impairment, and a score of 25 or more denotes normal in cognitive capacity [24]. The Korean version Short-Form of the Geriatric Depression Scale (GDSSF-K) was used to assess depression. This 15-item tool (range, 0–15) indicated the risk of depression when the score is more than 5 points [25].
RESULTS
Patients characteristics
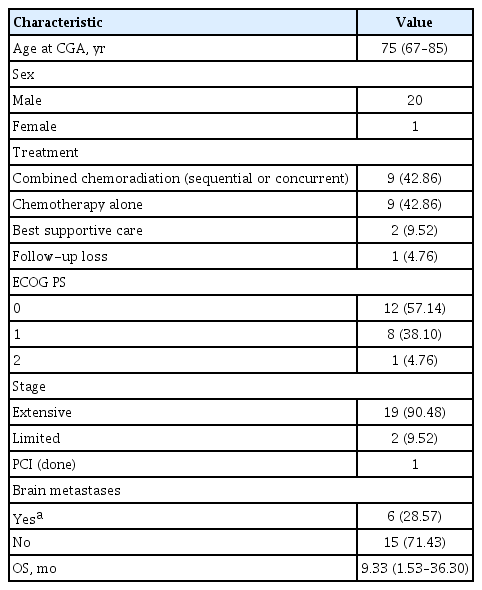
Of the 21 older SCLC patients, 20 were males, and one was female (Table 1). The median age of the patients was 75 years old (range, 67 to 85). Nineteen patients were initially at the extensive stage; two patients were at the limited stage. Of the 21 patients, 20 (95.24 %) patients had ECOG PS 0 or 1. Treatment of SCLC was classified as combined chemoradiation (sequential or concurrent), chemotherapy alone, and best supportive care. The recommended chemotherapy for SCLC in this study was carboplatin-etoposide. Nine patients underwent combined chemoradiation therapy including radiation to the lung, and only one of them received radiation to the metastatic lesion of the pelvic bone. Nine patients received chemotherapy alone, one patient was not treated due to spondylitis and the other patient was due to a urinary tract infection. Only one patient received prophylactic cranial irradiation. Six patients with brain metastases underwent the whole brain radiation therapy, and 15 patients were without brain metastases. The median OS was 9.3 months (range, 1.53 to 36.30) (Table 1).
Baseline CGA results
Results regarding the relationship between the CGA domains and OS are summarized in Table 2. The effect on OS according to ECOG PS 0, 1, and 2 did not show any significant difference in univariate analysis. The degree of frailty also did not affect OS. The median arm circumference of the patients was 24 cm and the median calf circumference was 30.5 cm, which also did not affect OS. Seven patients with BMI over 25 kg/m2 were defined as obese, six patients with BMI over 23 kg/m2 were overweight, and eight patients with BMI less than 23 kg/m2. Differences by BMI were also insignificant. There were eight patients with normal TUG test, five patients with prolonged TUG test, and eight patients who did not perform the test. The TUG test also did not affect OS. A minimum score of 6 points on CCI was the baseline score for patients diagnosed with SCLC, and 10 patients with a total score of over 9 points, including other chronic diseases, did not affect OS. Ten patients were taking more than five drugs, and this also did not affect OS. There were six patients with current smokers, 14 patients with ex-smokers, and one patient with never-smoker. Smoking history did not affect OS. Eleven patients with a depression-related GDS score of over 5 and 11 patients with IADL dependency were measured, but none of them significantly affected OS. The MMSE defined cognitive impairment based on a score of less than 24, with only one patient scoring less than 24 and all other patients being normal. Therefore, MMSE did not affect survival. Finally, in univariate analysis, ADL and nutritional status significantly affected the survival of SCLC patients (p = 0.017, p = 0.025, respectively). There were seven patients with ADL dependency, 11 patients with normal nutritional status with an MNA-SF score of over 12, eight patients with 8–11 points, and two patients with 0–7 points. It showed that the better the nutritional status, the longer the survival. In multivariate analysis, adjusted for ECOG PS, sex, and age, only nutritional status had a significant effect on OS. Those with an MNA-SF score of over 12 had significantly longer survival than those with an MNA-SF score of less than 12 (median survival 5.63 vs. 15.50; hazard ratio, 0.17; 95% CI, 0.05 to 0.57; p = 0.004) (Fig. 1).
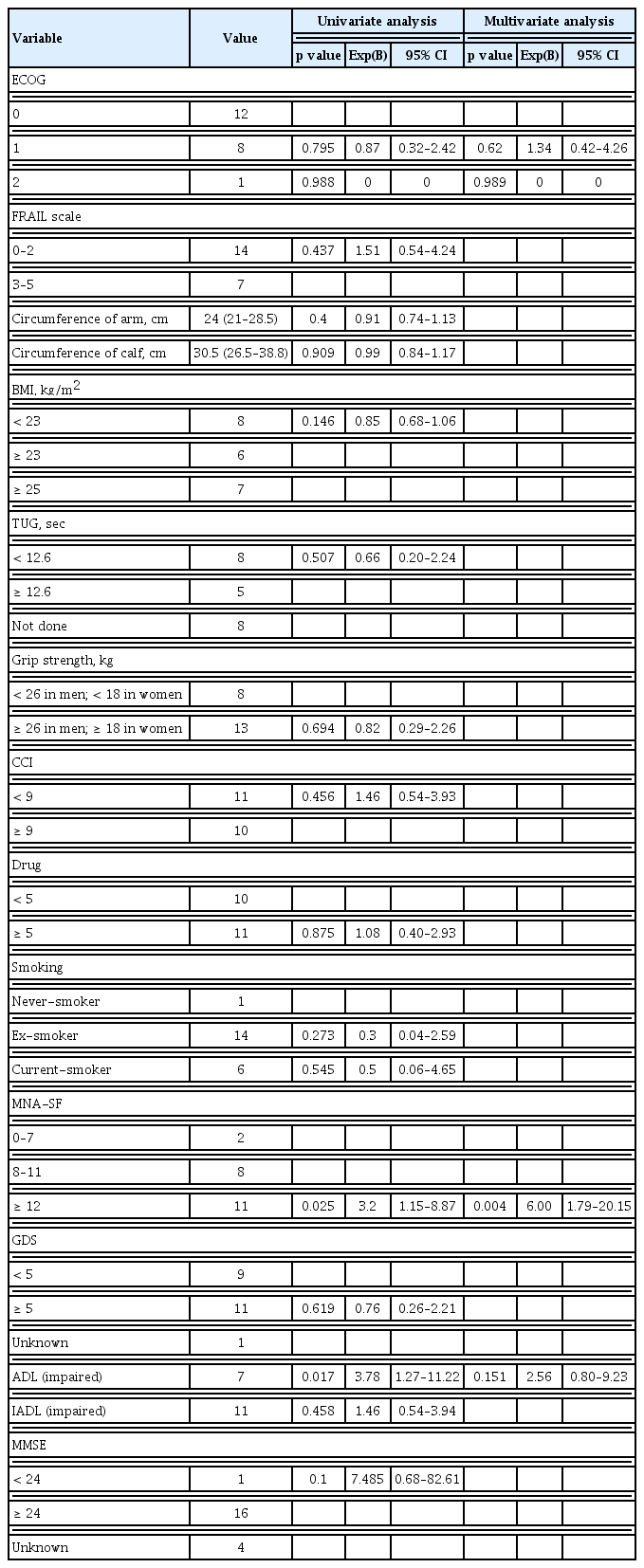
Univariate and multivariate analysis of overall survival by comprehensive geriatric assessment domains
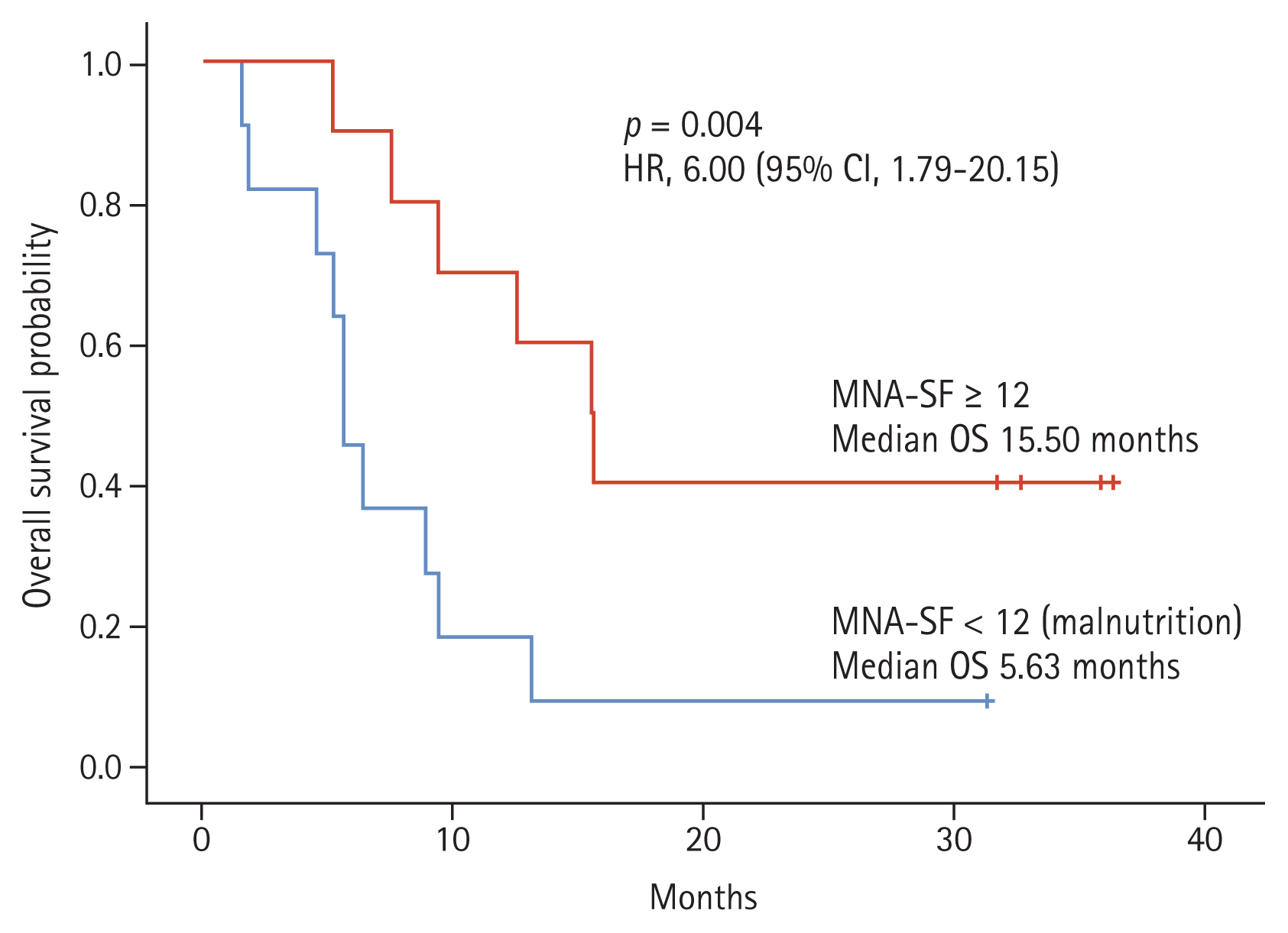
Overall survival (OS) according to nutritional status. The Mini Nutritional Assessment short-form (MNA-SF) is one of the domains of comprehensive geriatric assessment. The OS was longer in older small cell lung cancer patients with normal nutritional status or at risk of undernutrition compared to patients with malnutrition (median survival 15.50 vs. 5.63; hazard ratio [HR], 6.00; 95% confidence interval [CI], 1.79 to 20.15; p = 0.004).
DISCUSSION
CGA has an important prognostic information. This can be helpful to predict survival and life expectancy in older people, which is crucial for oncologists to make treatment decisions. CGA in older cancer patients indicates previously unknown geriatric problems in many patients (up to 50 %) and may alter treatment decisions in 10% to 20% of patients [26]. Among the domains of CGA, limitations in cognition, nutrition, function, and comorbidities have been shown to be important factors in diminishing OS in older cancer patients in several studies [27–29]. It has also been shown that comprehensive geriatric intervention for older cancer patients significantly improved OS and facilitated a return to their own home compared to conventional hospital care. We recommend some form of geriatric assessment, such as CGA, in all older cancer patients for whom to make important treatment decisions, and our study began on this basis.
We analyzed retrospectively enrolled data to investigate the prognostic significance of the CGA domain and identified that low MNA-SF scores and impaired ADL were significant indicators of poor OS in patients with SCLC. In multivariate analysis, only malnutrition, independent of PS, was associated with poor OS. Our results are consistent with previous studies examining CGA parameters associated with changes in cancer treatment. In cancer treatment, malnutrition is known as a practical parameter to consider, as it is associated with therapeutic toxicity and mortality [30]. In several studies, a low BMI of less than 21 kg/m2 was related to a modification of the cancer treatment regimen [31]. According to another study, malnutrition as assessed by MNA-SF, BMI, weight loss, and low serum albumin was also related to changes in cancer treatment, mostly a decrease in treatment intensity [32]. In comparative studies of biochemical markers, anthropometric measures, and the MNA-SF was found to be strongly correlated with baseline weight loss in cancer patients and was recommended as a nutritional assessment tool. However, looking at the details of MNA-SF, it was not much different from the FRAIL scale, so the clinical effectiveness of MNA-SF may be questioned [33,34]. Since MNA-SF includes BMI, weight change in the last 3 months, and recent mental stress or acute illness, it can be considered to reflect more recent nutritional and systemic status [35]. Based on the above results, early intervention and treatment for malnutrition in older SCLC patients might be particularly important for improving OS. Malnutrition screening in older cancer patients enables preventive actions early stage of malnutrition in their cancer treatment [36]. Nutritional supplementation may be beneficial for older patients. A meta-analysis of 55 clinical trials showed the benefit of oral supplementations on OS in older patients. They reported that a reduction in mortality among malnourished older patients who took oral supplements compared to those who did not take oral supplements (odds ratio, 0.66; 95% CI, 0.49 to 0.90) [37]. In line with our findings, it appears that malnourished older cancer patients might also be able to improve their OS with oral supplementation, which requires further research.
A recent meta-analysis study has been reported that sarcopenia affects the survival rate of lung cancer patients [38]. Most of these studies performed survival analysis by measuring muscle size via CT, but our study analyzed survival by measuring arm and calf circumferences and assessing muscle function (i.e., TUG test and grip strength), respectively. Although there are some studies on the effect of arm and calf circumferences and muscle function assessment on survival, it is still insufficient [39]. We can put all of this together and define as sarcopenia. In order to analyze whether sarcopenia significantly affects the survival of SCLC patients, it is necessary to develop new indicators such as the sarcopenia index that combines each domain. In this study, independent analysis of individual domains was performed and none of them showed statistical significance. However, each domain cannot be said to be an indicator that properly reflects sarcopenia, so caution is required in interpretation.
In addition to sarcopenia, there are studies on the effects of ADL and IADL assessment on survival, but data are still lacking. In our study, the ADL dependency was found to affect survival in a univariate analysis, but not in a multivariate analysis. Each domain of CGA may have little effect on the OS of older SCLC patients, but approaching it as an integrated concept of ‘Frailty index’ can be a more important factor for OS.
Each CGA domain and Frailty index have the potential to be useful evaluation tools for predicting the prognosis of older cancer patients, but it is not easy to apply them to clinical practice. Implementation of CGA in various clinical environments can pose challenges due to the lack of specific information about CGA tools. For example, what is the exact question for a particular geriatric assessment tool, what cut-off scores are used within a particular scale, how the results should be interpreted, and whether this scale has been validated in oncology. In other words, a comprehensive evaluation tool such as an CGA analysis or a Frailty index are required, and many studies are needed to develop it. To overcome these limitations, the National Comprehensive Cancer Network (NCCN), the European Organization for Research and Treatment of Cancer (EORTC), and the International Society of Geriatric Oncology (SIOG) guidelines introduce CGA tools [40]. Our institution also designed the CGA tool by reviewing many references. In addition, this information should be easily accessible not only to geriatricians, but also to many professionals who care for geriatric patients. These are the parts we are challenging in the future. The information obtained through CGA requires the patient to intervene accordingly. For example, providing nutritional supplementation for malnourished older patients, and muscle strength enhancement and rehabilitation treatment for older patients with sarcopenia. In many cases, interventions cannot be provided to patients and the CGA is only performed, which is a limitation due to the absence of experts and difficulties in interpretation of the CGA.
Our study has several limitations. It was conducted at a single institution with a relatively small sample size of 21 patients. In addition, since only patients suitable for chemotherapy were initially enrolled in the study, only patients with relatively good cognitive function or performance status were selected, which may have resulted in statistical bias. Statistical adjustments were not made for the cancer stage and various treatment modality (concurrent chemoradiotherapy, chemotherapy alone, best supportive care). Therefore, the interpretation of our results should be cautious, and prospective studies with larger sample sizes are desirable in the future. It can also be an important prospective study in the future to analyze whether the OS has improved after intervening for malnutrition. Despite the above limitations, this is an important study to suggest the prognostic implications of CGA in older patients with SCLC.
In conclusion, geriatric assessment detects more information than routine oncological evaluation alone, such as ECOG PS. Pre-treatment MNA-SF score appears to be a predictor of OS in older SCLC patients with relatively good cognition and performance. Further studies are needed to verify the prospective role of CGA in older cancer patients.
KEY MESSAGE
1. The correlations of oncologic and geriatric variables with overall survival (OS) in older patients with small cell lung cancer (SCLC) were determined.
2. Pre-treatment nutritional status measured by comprehensive geriatric assessment (CGA) appears to be a predictor of OS in older SCLC patients.
3. Rather than survival analysis for each domain in CGA, a new index integrating each domain for survival prediction is needed.
Acknowledgments
The study is sponsored by a grant of Patient-Centered Clinical Research Coordinating Center (PACEN) funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HC20C0086).
We would like to thank the Division of Medical Oncology for assistance with patient enrollment.
Notes
No potential conflict of interest relevant to this article was reported.