The role of discharge checklist in guideline-directed medical therapy for heart failure patients
Article information
Abstract
Background/Aims
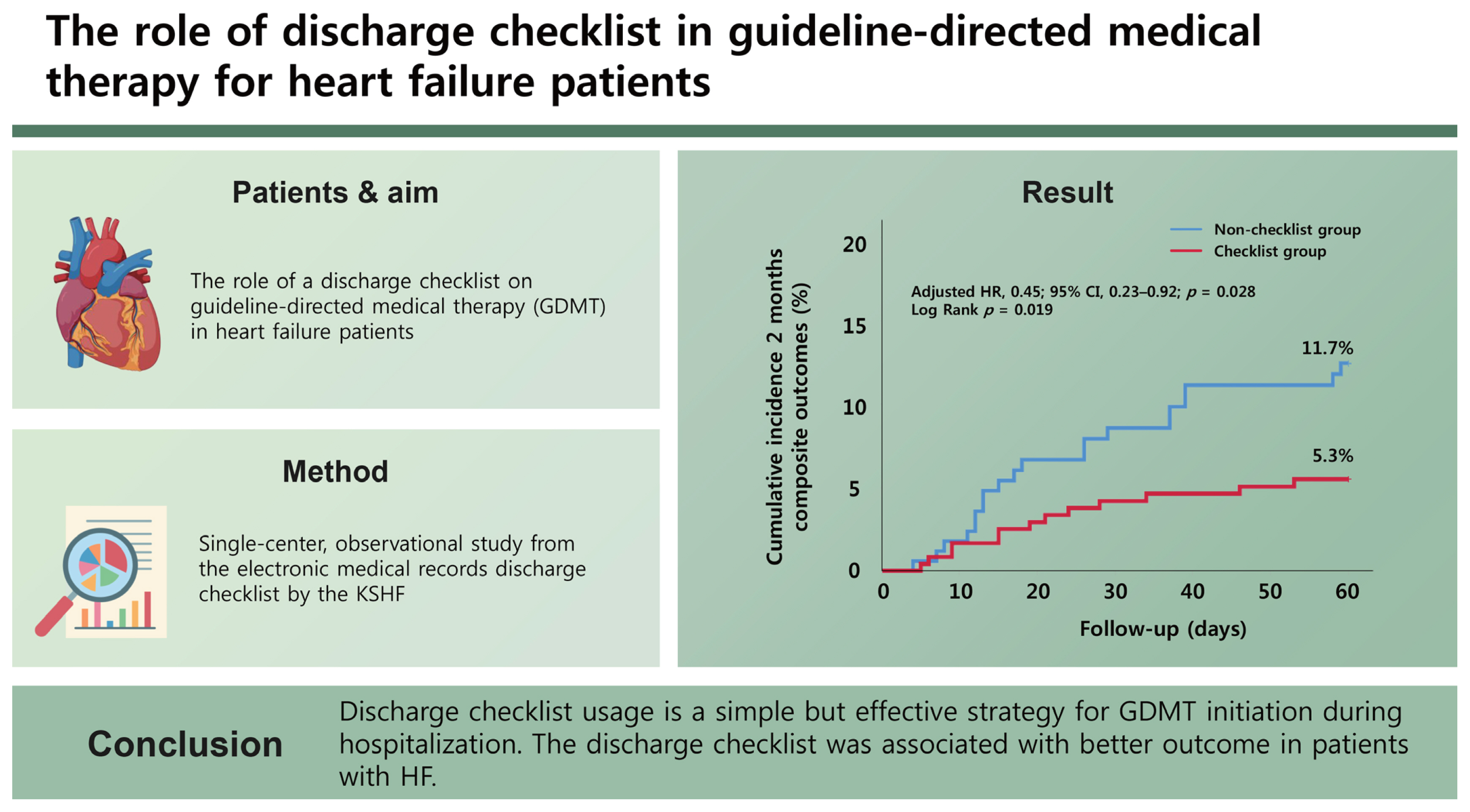
Initiation of guideline-directed medical therapy (GDMT) during hospitalization is recommended for patients with heart failure (HF). However, GDMT is underutilized in real-world practice. This study evaluated the role of a discharge checklist on GDMT.
Methods
This was a single-center, observational study. The study included all patients hospitalized for HF between 2021 and 2022. The clinical data were retrieved from the electronic medical records and discharge checklist published by the Korean Society of Heart Failure. The adequacy of GDMT prescriptions was evaluated in three ways: the total number of GDMT drug classes and two types of adequacy scores. The primary endpoint was the incidence of all-cause mortality or rehospitalization due to HF within 2 months of discharge.
Results
Overall, the checklist was completed by 244 patients (checklist group) and was not completed in 171 patients (non-checklist group). The baseline characteristics were comparable between two groups. At discharge, a higher proportion of patients in the checklist group received GDMT than in the non-checklist group (67.6% vs. 50.9%, p = 0.001). The incidence of primary endpoint was lower in the checklist group compared to the non-checklist group (5.3% vs. 11.7%, p = 0.018). The use of the discharge checklist was associated with significantly lower risk of death and rehospitalization in the multivariable analysis (hazard ratio, 0.45; 95% confidence interval, 0.23–0.92; p = 0.028).
Conclusions
Discharge checklist usage is a simple but effective strategy for GDMT initiation during hospitalization. The discharge checklist was associated with better outcome in patients with HF.
INTRODUCTION
Heart failure (HF) is a clinical syndrome that results from structural or functional impairment of ventricular filling or ejection [1]. It is associated with a high morbidity and mortality burden [2,3]. There are estimated to be 64 million people suffering from HF, and the number is increasing worldwide [2]. The prevalence of HF morbidity in Asia is approximately 1% to 6%, which is higher than in Western countries [4]. According to the Korean Acute Heart Failure Registry (KorAHF), the in-hospital mortality rate for acute HF is approximately 5%, and the probability of death after 1 year of discharge reaches 18% [5].
Guideline-directed medical therapy (GDMT) is essential with a proven benefit of mortality and morbidity reduction in patients with HF with reduced left ventricular ejection fraction (HFrEF) [6]. GDMT includes the following drug therapies: renin-angiotensin system inhibitors (RASI) consisting of angiotensin receptor blockers (ARB) or angiotensin-converting enzyme inhibitors (ACEI), or angiotensin receptor-neprilysin inhibitor (ARNI), in addition to beta-blockers [7], and mineralocorticoid- receptor-antagonists (MRA) [8,9]. Additionally, recent guidelines include sodium-glucose cotransporter-2 (SGLT2) inhibitors in GDMT [8], although SGLT2 inhibitors are currently only covered by insurance in patients with diabetes mellitus [3,10].
GDMT is, however, underutilized in real-world practice in patients with HF. The European HF pilot survey showed that more than 70% of patients with acutely decompensated HF were treated with the GDMT (RASI, beta-blockers, and MRA) upon discharge [11]. However, the GDMT prescription rate in Asia-Pacific regions, including Korea, was only approximately 50% [12]. Therefore, a promotion strategy to enhance GDMT adherence during hospitalization is necessary. The discharge checklist may also be a solution for enhancing the initiation of GDMT by clinicians during hospitalization. Basoor et al. [13] demonstrated in a small non-randomized study that utilizing a simple HF checklist sheet was associated with better physician-prescribed HF treatment and reduced 30-day readmission rates in 48 patients with HF. In another study by Allain et al. [14] regarding the usefulness of a discharge checklist, they found that a simple checklist improved comorbidity management and referral programs for patient follow-up in 139 elderly patients.
The Korean Society of Heart Failure has provided a discharge checklist for the public (khfs.or.kr/news/news_01.php?boardid=ksnotice&mode=view&idx=27&sk=). This checklist aims to ensure that all the standard treatment drugs are prescribed. However, the effect of using such a discharge checklist on GDMT prescriptions in the real world has not been studied. We aimed to evaluate the role of using the discharge checklist in the management of patients with HF. This study hypothesized that there would be a significantly better quality of care for patients whose discharge checklists were completed by the physician during hospitalization. Specifically, we hypothesized that patients with HF whose discharge checklists were completed might have a higher probability of being issued GDMT prescriptions, decreased incidence of loss to follow-up, and better clinical outcomes than those in the non-completed group.
METHODS
Study population
We retrospectively included all patients hospitalized for HF between March 2021 and February 2022 in the cardiology wards of Seoul National University Hospital. HF was diagnosed by cardiologists, internists, or residents, based on a standard definition. Exclusion criteria included patients who died or were transferred to other departments during hospitalization. The protocols of this observational study were approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB No. 2202-095-1301). All the study protocols complied with the ethical guidelines of the 2013 Declaration of Helsinki. The requirement for informed consent from the IRB was waived.
Clinical variables
The clinical data of the enrolled patients were retrieved from the electronic medical records and discharge checklist published by the Korean Society of Heart Failure. Data from electronic medical records included baseline demographic and clinical characteristics and prescribed medication (types and doses). The baseline characteristics included sex, age, height, weight, body mass index, and history of previous HF. We obtained the following clinical information from the medical records: etiology, aggravating factors, comorbidities, vital signs at admission and discharge, discharge medication list, discharge date, readmission date, and follow-up data. Additionally, we collected the following information from the discharge checklist: HF phenotype, etiology, aggravating factors, type of medication at discharge, and discharge date. Discharge checklist have been filled up from one day before discharge to the discharge day. For those whose discharge checklists were not filled, we collected all clinical information by reviewing the electronic medical records only. We generated two kinds of GDMT adequacy scores based on the recent guidelines according to the type of GDMT, GDMT dose, and heart rate [10]. We referred the format of the adequacy score to the study by DeFilippis and Fiuzat [15]. The difference between the two types is adequacy score type 1 was based on beta-blocker heart rate, and adequacy score type 2 was based on beta-blocker dose (Supplementary Table 1). We collected mortality data from the National Statistical Office through the medical information protection office in the hospital.
Endpoints
The primary endpoint in this study was the composite of readmission or all-cause mortality within 2 months of follow-up. In contrast, the secondary endpoints included each readmission event, all-cause mortality, loss to follow-up within 2 months, number of GDMT prescriptions, and GDMT adequacy score.
Statistical analysis
Demographic and baseline characteristics are presented using descriptive statistics for continuous variables, which are presented as mean ± standard deviation (SD) or median and interquartile range. Categorical variables, which are expressed as frequencies (n) and percentages (%). Univariate analysis was used for comparison between groups and to determine the factors associated with outcomes. The unpaired Student’s t-test or Mann-Whitney U test was used to compare continuous variables; in contrast, the chi-square test or Fisher’s exact test was used to compare the proportions.
Data analyses were conducted using the SPSS statistical software (version 26; IBM Corp, Armonk, NY, USA). Survival analysis was performed using Kaplan-Meier estimation, and Kaplan-Meier curves were used to plot the time-to-event distribution of the primary endpoint. Furthermore, variables were examined using multivariable Cox proportional hazards regression analysis to estimate the composite outcome hazard ratio (HR). A statistical p-value below 0.05 was considered statistically significant.
RESULTS
Baseline characteristics of patients with HF based on the discharge checklists completed
Of the 464 patients enrolled, 25 patients were excluded because of in-hospital death, and 24 were excluded because they were transferred to other departments. In total, 415 patients were included in the analysis. The discharge checklist was completed for 244 patients (58.8%, the checklist group) and not completed for 171 patients (41.2%, the non-checklist group). The flowchart of the study is shown in Figure 1.

Discharge checklist study flow chart. TAVI, transcatheter aortic valve implantation; CAG, coronary angiography; ICD, implantable cardiac defibrillator; LVAD, left ventricular assisting device; CAGB, coronary artery bypass graft.
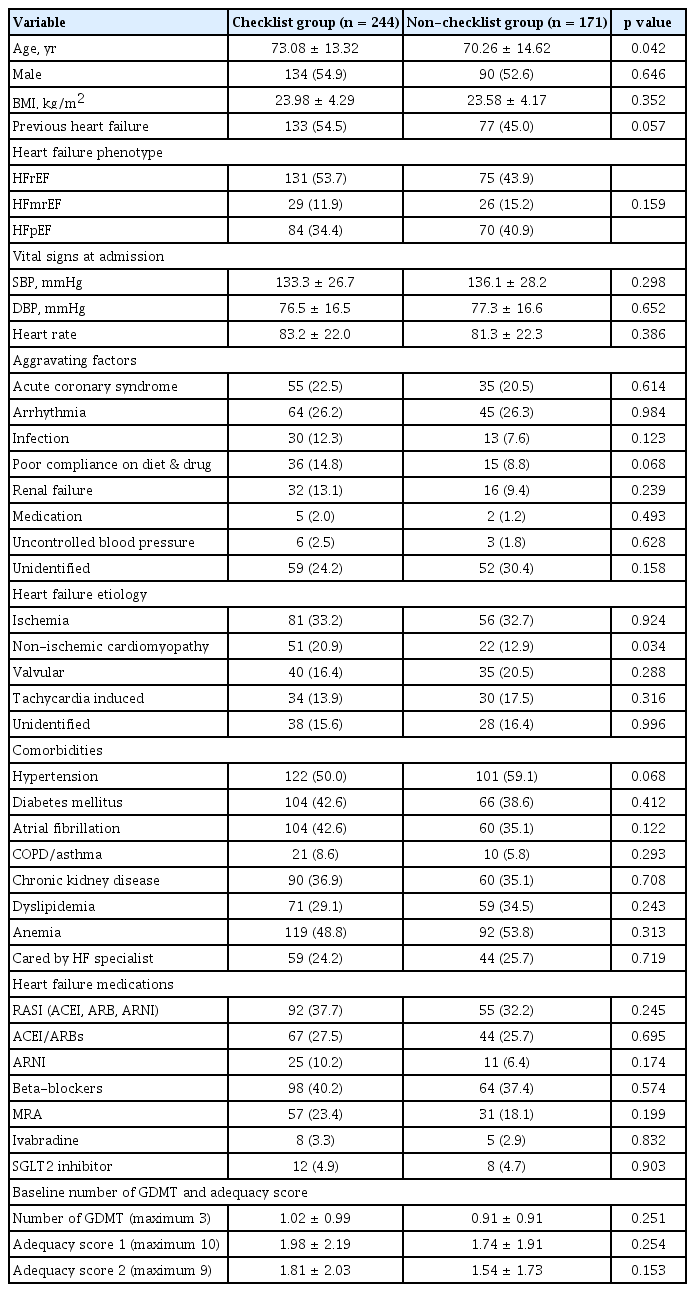
The baseline characteristics of the discharge checklist are shown in Table 1. Male predominance (p = 0.646) and HF phenotypes (HFrEF, heart failure with mildly reduced ejection fraction [HFmrEF], and heart failure with preserved ejection fraction [HFpEF]) were comparable between the two groups (p = 0.159). Patients in the checklist group were significantly older than those in the non-checklist group (73.08 ± 13.32 years vs. 70.26 ± 14.62 years, p = 0.042). The proportion of patients with a previous diagnosis of HF was comparable between two groups (p = 0.057). The primary etiology of HF in both groups was ischemia (33.2% vs. 32.7%; p = 0.924). The incidence of non-ischemic cardiomyopathy was higher in the checklist group (20.9% vs. 12.9%, p = 0.034). The aggravating factors, comorbidities, and vital signs at the time of admission were comparable between two groups.
The GDMT prescription rate according to the discharge checklist completion status
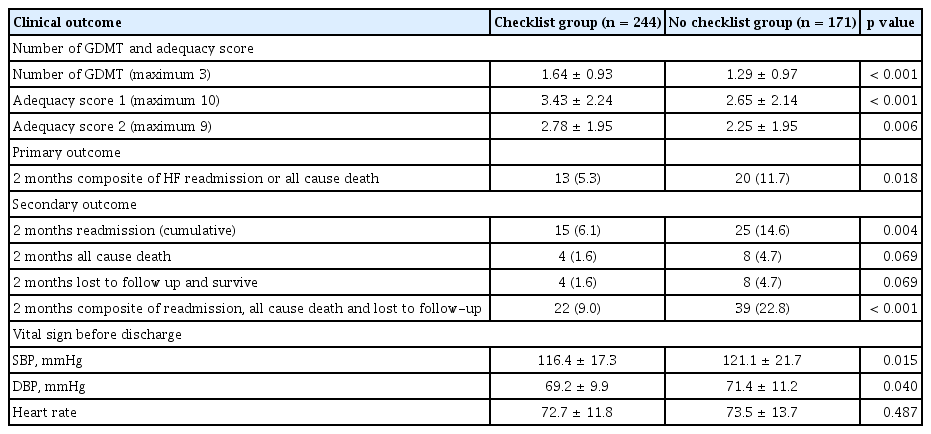
As described in Table 1, the number of GDMT prescriptions at the time of admission was comparable between the two groups. However, the number of GDMT prescriptions at discharge was higher in the checklist group than in the non-checklist cohort (mean ± SD; 1.64 ± 0.93 vs. 1.29 ± 0.97, p < 0.001) (Table 2, Fig. 2). Adequacy scores of type 1, with a maximum of 10, were higher in the checklist group than in the non-checklist group (mean ± SD; 3.43 ± 2.24 vs. 2.65 ± 2.14, p < 0.001). Adequacy score type 2, with a maximum of 9, was also higher in the checklist group than in the non-checklist group (mean ± SD; 2.78 ± 1.95 vs. 2.25 ± 1.95, p = 0.006). The detailed prescription patterns according to the discharge checklist completion status are shown in Supplementary Table 2. A higher proportion of patients were prescribed GDMT in the checklist group, especially beta-blockers (67.6% vs. 50.9%, p = 0.001). There was a marginally significant difference between the two groups for RASI, including ACEI, ARB, and ARNI (54.9% vs. 45.6%, p = 0.062).
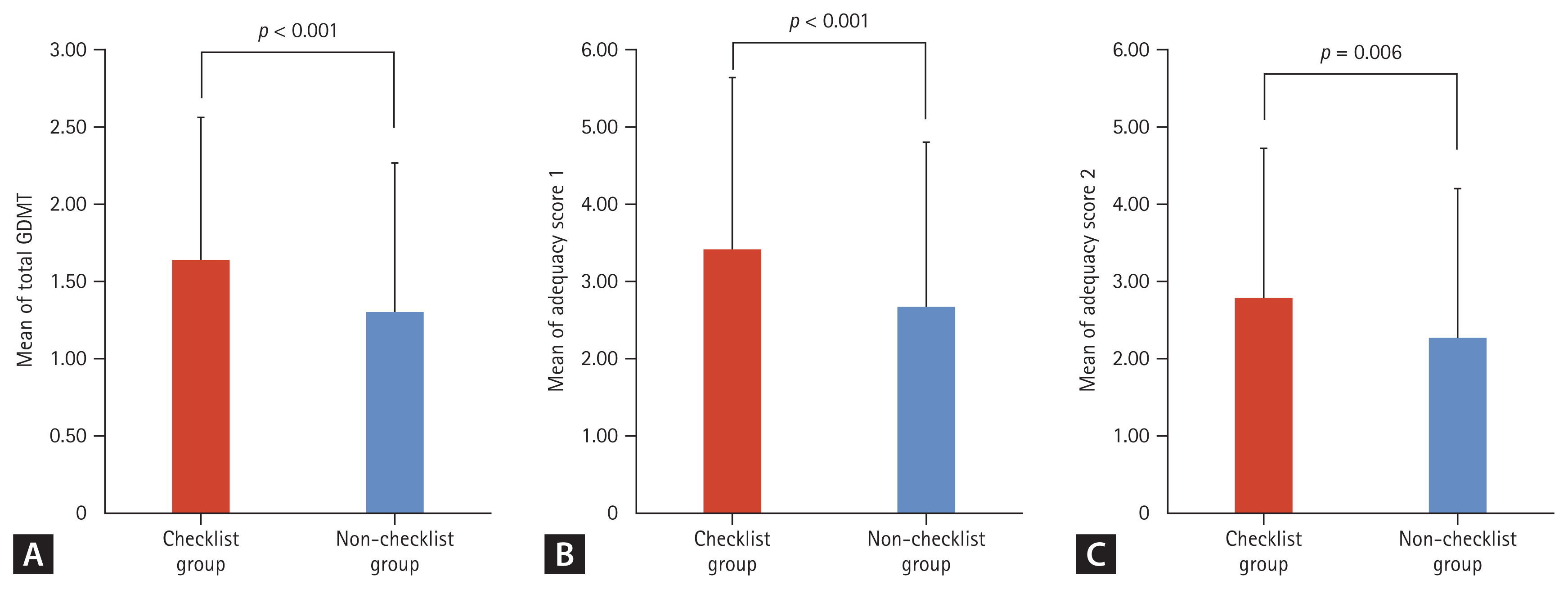
The effect of using a discharge checklist on guideline-directed medical therapy (GDMT) prescription and adequacy score. (A) The mean of total GDMT prescription between the checklist and non-checklist groups. (B) The mean of adequacy score 1 between the checklist and non-checklist groups. (C) The mean of adequacy score 2 between the checklist and non-checklist groups. Data are presented as mean and standard deviation.
The reasons for non-implementation of GDMT recorded in the discharge checklist were assayed. In case of RASI, including ACEI and ARB, the reasons included ejection fraction of more than 40% (33.3%), followed by the use of ARNI (22.4%), elevated serum creatinine (19.2%), and hypotension (9.0%). In case of ARNI, the main reason was an ejection fraction of more than 40% (35.5%), followed by taking ACE/ARB medication within 4 weeks (21.5%), elevated serum creatinine (13.1%), and hypotension (7.0%). In case of beta-blockers, ejection fraction of more than 40% (40.0%) was the most common cause, followed by hypotension and bradycardia (15.6%). Lastly, the reasons for suboptimal MRA were due to an ejection fraction of more than 35% (41.9%), elevated serum creatinine (19.6%), and hypotension (9.5%) (Supplemental Fig. 1).
Clinical parameters and study outcomes
The systolic and diastolic blood pressure readings at the point of discharge were lower in the checklist group than in the non-checklist group (116.4 ± 17.3 mmHg vs. 121.1 ± 21.7 mmHg, p = 0.015 and 69.2 ± 9.9 mmHg vs. 71.4 ± 11.2 mmHg, p = 0.040) (Table 2). The heart rates of the patients were comparable between the two groups.
During follow-up for 2 months, HF readmission or allcause mortality occurred in 13 patients (5.3%) in the checklist group and 20 patients (11.7%) in the non-checklist group (p = 0.018, Table 2). The checklist group showed a significantly decreased incidence of composite outcome endpoints than the non-checklist group in the Kaplan-Meier analysis (Fig. 3).

Kaplan-Meier curves for primary composite outcomes for 2 months. The cumulative incidences of primary composite outcome for 2 months were compared between checklist group and non-checklist group in patients with heart failure. Covariates for adjusted HR were as follows: age, gender, anemia, atrial fibrillation, diabetes mellitus, heart failure etiology, left ventricular ejection fraction, chronic respiratory disease, and chronic kidney disease. HR, hazard ratio; CI, confidence interval.
We then confirmed the impact of the discharge checklist by univariable and multivariable analyses using Cox proportional hazard regression analysis (Table 3). In the univariable analysis, the completion of the discharge checklist was significantly associated with decreased incidence of events, whereas anemia and reduced EF were associated with increased incidence of events. And three parameters above still showed significant association with cardiovascular events. Remarkably, the completion of the discharge checklist was associated with a reduced incidence of events with an HR of 0.45 (95% confidence interval, 0.23–0.92; p = 0.028) (Table 3).
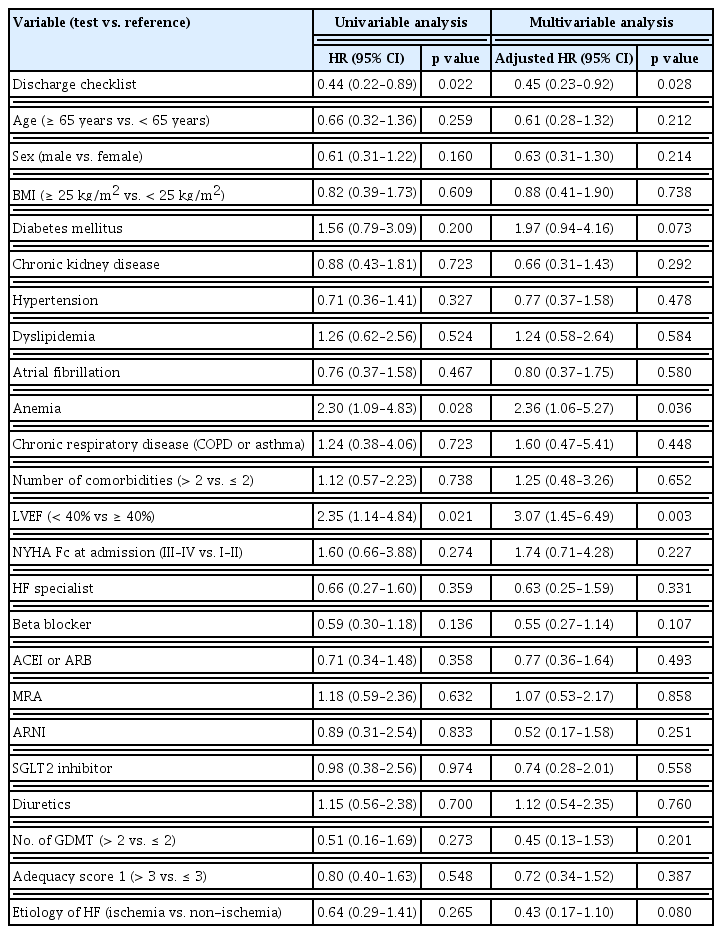
Univariable and multivariable analyses using cox proportional hazard regression analysis for the primary outcome
Individual clinical outcomes, including readmission events, all-cause mortality, and loss to follow-up within 2 months, were all lower in the checklist group. Overall, 15 (6.1%) the cumulative of 2-month readmission in the checklist group were lower than 25 (14.6%) in the non-checklist group (p = 0.004). Moreover, the readmission rate due to HF aggravation was lower in the checklist group than in the non-checklist group (3.7% vs. 8.2%, p = 0.049). The mortality outcome and follow-up loss were similar and marginally lower in the checklist group (1.6% vs. 4.7%, p = 0.069) (Supplemental Fig. 2).
In addition, subgroup analyses were performed to investigate the consistency of the effect of the discharge checklist among different subpopulations. The forest plot diagram of subgroup analyses depicts that the effect of using the discharge checklist was consistently better than not using the checklist on advanced age, male, non-obese, with diabetes, GDMT number below the median, and adequacy score below the median (Fig. 4). No interaction was observed between the primary endpoint and other variables in the subgroup analysis.
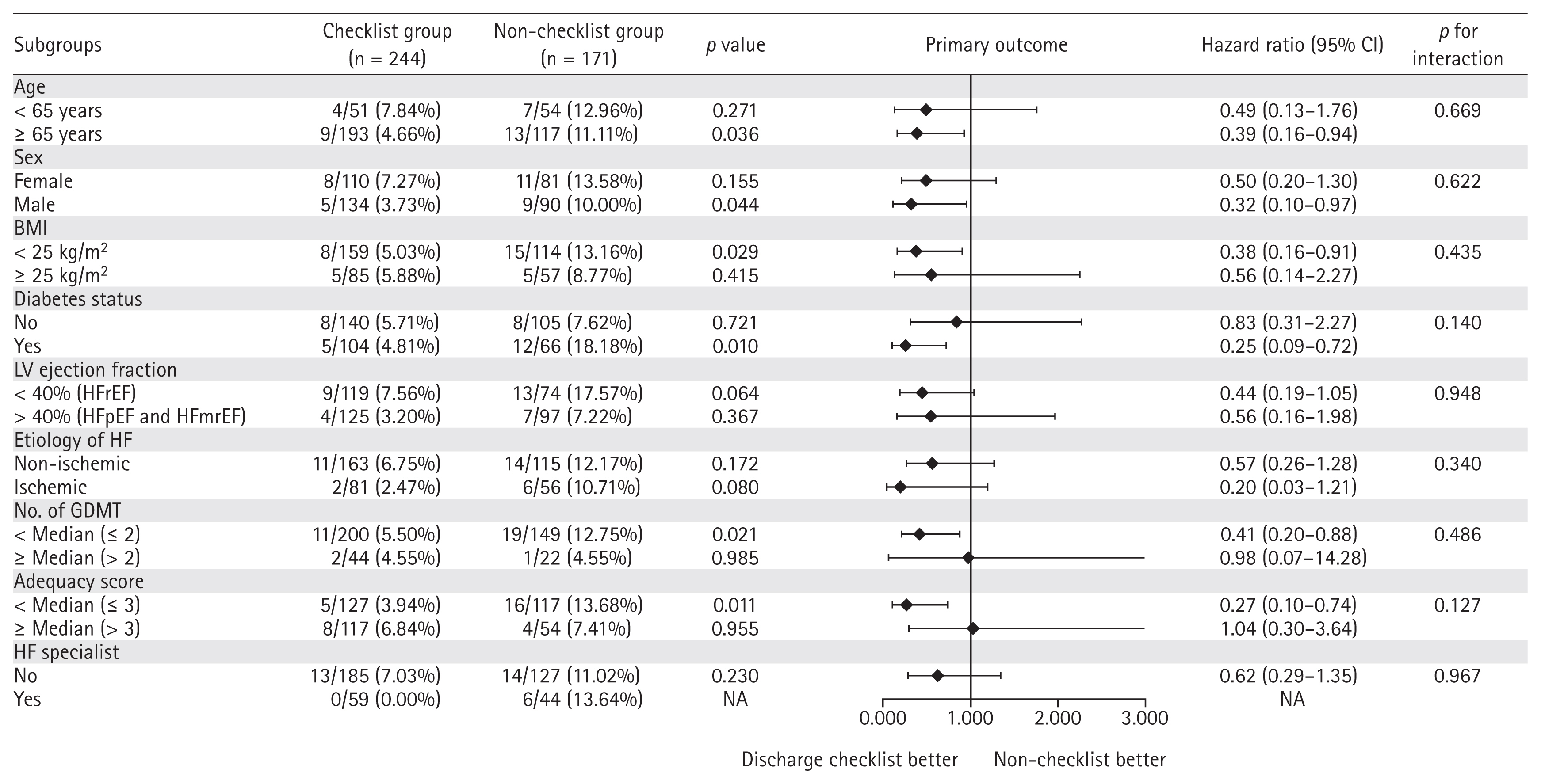
Forest plot of subgroup analysis for composite outcomes. For subgroups that were defined according to age, sex, BMI, diabetes status, LV ejection fraction, etiology of HF, number of GDMT, adequacy score, and the presence of HF specialist were used for the interaction test. CI, confidence interval; BMI, body mass index; LV, left ventricle; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFmrEF, heart failure with mildly reduced ejection fraction; HF, heart failure; NA, not applicable; GDMT, guideline-directed medical therapy.
DISCUSSION
Despite the proven benefit of GDMT in patients with HF, the implementation rate of GDMT during hospitalization observed in this study was very low, with a GDMT adequacy score of approximately 3 out of the maximal score of 10. Therefore, more effort should be made to enhance the implementation of GDMT in HF patients. This study showed that the completion of the discharge checklist during hospitalization was significantly associated with quality of care by increasing the number of GDMT prescriptions, especially beta- blockers. Furthermore, the checklist group showed better clinical outcomes, with significantly reduced readmission or all-cause mortality within 2 months after discharge. To the best of our knowledge, this is the first study to demonstrate the feasibility and benefits of a discharge checklist in real- world situations, especially in Asia.
GDMT prescription rate in some registries
GDMT initiation during hospitalization is insufficient in real-world practice. For instance, the Acute decompensated HF National Registry (ADHERE) in the United States reported that ACEI/ARB, beta-blockers, and aldosterone antagonists were prescribed in 83%, 80%, and 33% of patients, respectively [16]. Similarly, the Acute Decompensated HF Registry International-Asia Pacific (ADHERE-AP), analyzing 10 thousand patients from eight Asia Pacific countries, reported insufficient GDMT prescription at discharge time; ACEI or ARB in 63%, beta-blockers in 41%, and aldosterone antagonists in 31% of patients [17]. The KorAHF registry also showed inadequate prescription of GDMT. At discharge in the KorAHF registry, physicians prescribed ACEI or ARB, beta- blockers, and aldosterone antagonists in 69%, 52%, and 47% of the patients, respectively [12]. The prescription rate in our study was similar to that in the KorAHF registry. Seo et al. [18] analyzed the KorAHF registry data and evaluated the role of GDMT in reducing all-cause mortality in Korea. They found that GDMT prescription was associated with a 3-year risk reduction of 53% mortality in patients with HF compared to non-adherence to GDMT [18]. However, the prescription rate of GDMT (ACEI/ARB and beta-blockers) was suboptimal as 44% [18]. The GDMT prescription rate decreased due to the advanced age of patients, especially beta-blockers [18]. Considering the lower implementation rate of GDMT despite its proven benefit in the real-world situation, various efforts to enhance it are warranted for the best care of patients with HF.
The Get With the Guidelines-HF (GWTG-HF) program is an American physician’s effort to translate guidelines to improve patient care [19]. Bergethon et al. used GWTG-HF data linked to Medicare claims between 2009 and 2012 with 21,264 patients from 70 hospitals [20]. That study presented a relative reduction of 20% in rates of 30-day all-cause readmission among patients with HF and improvements in 30-day risk-adjusted readmissions between 2009 and 2012 [20]. Hospitals that used post-discharge HF management programs in the GWTG-HF program had lower relative readmission rates [20].
The role of the discharge checklist affects the GDMT prescription rate
The discharge checklist was associated with better clinical outcomes. In KorAHF registry, the 1- and 3-month post-discharge death rate of the patients hospitalized with acute HF were reported as 3.3% and 8.8%, respectively [1,21]. The 2-month post-discharge death rates of the checklist and non-checklist groups in this study were 1.6% and 4.7%, indicating that the significant difference between two groups was not caused by suboptimal treatment of the non-checklist group, but by better clinical outcome in the checklist group.
The beneficial effect of the discharge checklist might be supported by previous study conducted by Basoor et al. [13]. It was a small-scaled study enrolling 48 patients with use of the checklist and compared with 48 ‘randomly selected’ patients from 1,597 patients admitted with acute HF in the USA, 2013. The checklist was applied anytime from admission to discharge in non-randomized manner. Therefore, it was rather a one-page pocket-guide of HF patient care, not the predischarge checklist. They showed lower readmission rate in 30-day and 6-month. However, the patients in the checklist group were received one-on-one counselling about the listed HF interventions in the checklist, which might significantly influence the more positive effect of patient education beyond the physician’s awareness by the checklist alone [22]. Another study supported a benefit of the checking system called as the high guideline adherence indicator (GAI), defined as prescribing GDMT two or more medications [23]. In the Survey of Guideline Adherence for Treatment of Systolic HF in Real World (SUGAR) trial, which is a multicenter observational study using treatment adherence indicators with high GAI, there was a significant reduction in 90-day mortality compared to poor GAI (p = 0.001) [23]. Furthermore, a 2018 meta-analysis found that a high GAI score was associated with a significant 71% reduction in relative risk of mortality and 36% rehospitalization (p < 0.005) [24]. Our study showed a higher prescription of GDMT, especially beta-blockers, in the checklist group, counting 68%. When we investigated a smaller scope of the HFrEF subtype, beta-blocker prescriptions reached 79.5% in the discharge checklist group. A discharge checklist may increase the awareness of physicians on GDMT prescriptions. This can be seen from the differences in the type and number of prescriptions in the checklist and non-checklist groups. Legallois et al. [25] conducted a prospective cohort study to evaluate the discharge checklist effect in patients with HF. This study showed a similar result: the discharge checklist group had a better quality of care, particularly on therapeutic optimization of GDMT, such as beta-blocker dose. However, there was no significant reduction in readmission and mortality, which might be related to the small sample size of 103 patients compared to the previous control of 137 patients [25].
Our study had several limitations. First, selection bias might have occurred in an observational study. However, to minimize selection bias, this study included subgroup analysis to exclude possible confounding factors. The impact of attending physicians specializing in HF treatment might have influenced GDMT. However, there was no difference in proportion of patients managed by HF specialists between the two groups at admission (24.2% vs. 25.7%, p = 0.719). In addition, the rate of readmission at 2 months, all-cause death, and primary endpoint were not significantly different according to the HF specialists (chi-square statistical analysis; Supplementary Table 3). And, using univariable and multivariable Cox proportional hazards regression analyses to estimate the HR of the composite outcome, this study also confirmed that there was no association between the HF specialist and the two composite outcomes. However, despite all our efforts to exclude possible confounding factors and relatively comparable clinical characteristics between the checklist and non-checklist groups, the non-randomized design might exert a confounding effect even though we performed several multivariable analyses to overcome this limitation. We hope to conduct clinical trials to confirm the beneficial effects of discharge checklist as observed in this study. However, clinical trials of non-profit interventions are not easy due to limited financial support, which demands support from public organizations. Secondly, there were limited evidences of GDMT in HFpEF and HFmrEF. As a result, most HFpEF and HFmrEF patients have been treated similarly with HFrEF patients. Therefore, we applied the same criteria of GDMT regardless of EF, which might be a cause of lower GDMT score in both groups. However, the effect of discharge check list on primary outcome was consistent in our subgroup analysis. Lastly, current treatment with SGLT2 inhibitors is only covered by insurance in patients with comorbid diabetes; therefore, it was not included in calculating the adequacy score for this study sample.
In conclusion, the use of a discharge checklist was associated with reduced readmission rates for patients admitted with HF and better GDMT prescriptions, especially beta-blockers. Significantly, the completion of the discharge checklist was associated with better clinical outcomes in patients with HF. In particular, we believe that the complete introduction of the discharge checklist can be used conveniently and effectively to improve the clinical skills of resident doctors and the quality of care of patients with HF in the hospitals.
KEY MESSAGE
1. The completion of a discharge checklist was significantly associated with a higher rate of guideline- directed medical therapy prescriptions.
2. 59% decrease in the rate of occurrence of the primary endpoint (rehospitalization or all-cause mortality within two months of discharge) was observed in the group that completed the discharge checklist.
3. Using a discharge checklist is a simple but effective strategy for optimal management of patients with heart failure.
Notes
No potential conflict of interest relevant to this article was reported.