Korean treatment recommendations for patients with axial spondyloarthritis
Article information
Abstract
We aimed to develop evidence-based recommendations for treating axial spondylarthritis (axSpA) in Korea. The development committee was constructed, key clinical questions were determined, and the evidence was searched through online databases including MEDLINE, Embase, Cochrane, KoreaMed, and Kmbase. Systematic literature reviews were conducted, quality of evidence was determined, and draft recommendations were formulated according to the Grading of Recommendations Assessment, Development, and Evaluations methodology. Recommendations that reached 80% consensus among a voting panel were finalized. Three principles and 21 recommendations were determined. Recommendations 1 and 2 pertain to treatment strategies, regular disease status assessment, and rheumatologist-steered multidisciplinary management. Recommendations 3 and 4 strongly recommend patient education, exercise, and smoking cessation. Recommendations 5–12 address pharmacological treatment of active disease using nonsteroidal anti-inflammatory drugs, glucocorticoids, sulfasalazine, biologics, and Janus kinase inhibitors. Recommendations 13–16 address treatment in stable disease. We suggest against spa and acupuncture as therapies (Recommendation 17). Recommendations 18 and 19 pertain to total hip arthroplasty and spinal surgery. Monitoring of comorbidities and drug toxicities are recommended (Recommendations 20 and 21). Recommendations for axSpA treatment in a Korean context were developed based on comprehensive clinical questions and evidence. These are intended to guide best practice in the treatment of axSpA.
INTRODUCTION
Axial spondyloarthritis (axSpA) is a chronic inflammatory rheumatic disease with axial, peripheral, and non-articular manifestations. It predominantly presents with axial manifestations, such as spondylitis and sacroiliitis; peripheral manifestations, including oligoarthritis, dactylitis, and enthesitis; and non-articular manifestations, including psoriasis, uveitis, and inflammatory bowel disease (IBD). AxSpA is classified as non-radiographic axSpA (nr-axSpA), an early stage of the disease, or ankylosing spondylitis (AS), diagnosed based on radiographic sacroiliitis that fulfills the modified New York criteria for AS [1]. Timely and appropriate treatment is necessary for axSpA, as it is a progressive disease that leads to irreversible structural damage, loss of spinal mobility, functional disability, and ultimately reduced quality of life (QoL).
Evidence-based treatment guidelines are essential for quality care and healthcare policymaking. Academic rheumatology societies, including the European Alliance of Association for Rheumatology (EULAR) and American College of Rheumatology (ACR), periodically publish and update official treatment recommendations and clinical practice guidelines [2–8]. There are variations in population characteristics, cultures, and medical systems across countries. Therefore, societal context is an important consideration when developing and adapting treatment recommendations.
Real-world practice is not consistent with evidence accumulated for the management of patients with axSpA. The use of biologics, such as tumor necrosis factor (TNF) inhibitors and interleukin (IL)-17 inhibitors, in pharmacological therapies has facilitated remarkable advances in axSpA treatment. Novel drugs such as Janus kinase (JAK) inhibitors have been introduced as therapeutic options against active axSpA. Non-Pharmacological management with exercise and surgery are also important in providing optimal care for patients with axSpA. Thus, comprehensive and evidence-based treatment recommendations covering both pharmacological and non-pharmacological therapies are essential to provide the best care for patients with axSpA.
RECOMMENDATION DEVELOPMENT
We referred to the standardized operating procedures of the EULAR and the National Evidence-based Healthcare Collaborating Agency to develop treatment recommendations for axSpA [9,10]. First, the convener (HJB) organized the development committee (DC), which was responsible for developing the treatment recommendations, including the determination of key clinical questions (KCQs), selection of literature, review of evidence, and recommendation formulations. The DC comprised 18 rheumatologists from the Korean Society of Spondyloarthritis Research (KSSR) at the Korean College of Rheumatology (KCR), one methodologist, one nurse, and two patients from patient organizations. Seven rheumatologists and one methodologist comprised the core working group that coordinated and supported the development process, including systematic literature review and evidence synthesis. The DC established the operating terms and conditions, and conflict of interest management standards.
The DC made the following decisions: (1) the topic of recommendations was treatment for adult patients with axSpA, not including juvenile spondyloarthritis and psoriatic arthritis; (2) these recommendations cover overarching principles, treatment strategies, non-pharmacological and non-surgical treatments, pharmacological treatments, surgery, and monitoring; (3) target users of the recommendations are rheumatologists (primary) and physicians treating rheumatic and musculoskeletal disorders (secondary); and (4) healthcare settings covered by the recommendations ranged from primary clinics to tertiary hospitals.
After reviewing clinical questions regarding existing treatment guidelines for axSpA [2,5,7,8,11], the DC identified 88 KCQs after discussion and online surveys. The KCQs were described according to the population, intervention, comparator, and outcome (PICO) systems. Critical outcomes included musculoskeletal symptoms (pain, stiffness, and fatigue), QoL, mental health, disability, physical function, workability, safety, complications, comorbidities, and survival rate. Important outcomes included disease activity, treatment response, inflammatory markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level, structural damage on imaging, inflammation on magnetic resonance imaging (MRI), and spine mobility.
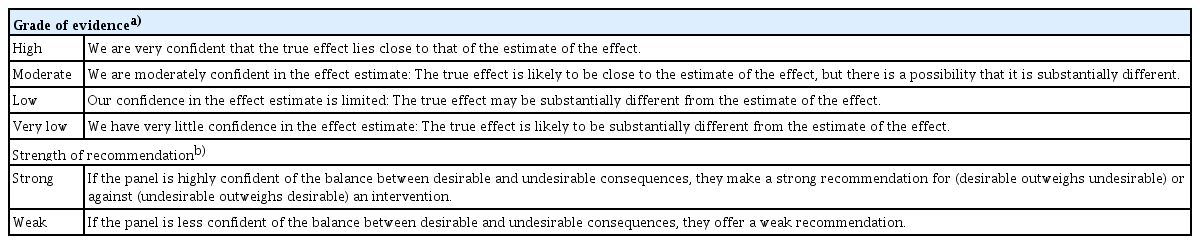
DC members identified Korean and English search terms for each KCQ. A literature search for Korean or English articles published between 1990 and 2021 was performed using the following databases: MEDLINE, Embase, Cochrane, KoreaMed, and KMbase (Korean Medical Database). Evidence from randomized controlled trials (RCTs) and/or high-quality comparative studies involving patients with axSpA aged 18 years or older was considered. Observational studies were included as evidence in the absence of RCTs or high-quality comparative studies. If required, manual searches were performed to obtain additional evidence. Finally, 160 reports were selected for supporting evidence. The risk of bias was assessed using the Cochrane risk of bias tool for randomized trials (RoB 2) [12]. The working group conducted systematic reviews and meta-analyses using RevMan software version 5.4 (Cochrane Collaboration, Oxford, UK). The grade of evidence (GoE) was rated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method (Table 1) [13].
The DC decided not to address 39 KCQs for which no quality evidence was found. Evidence for the remaining KCQs was summarized using the GRADE table and/or a summary of supporting studies [14]. Evidence and preliminary recommendations were presented to the DC members who discussed these at an off-line meeting and through online group chats. Some relevant items were combined into one recommendation. The strength of a recommendation (SoR) was described as “strong” or “weak” (Table 1) [15]. The verb “recommend” or “should” was used for strong recommendations; “suggest” or “can” was for weak recommendations. The formulated recommendations were prepared for voting on the consensus panel through further electronic surveys of the DC members.
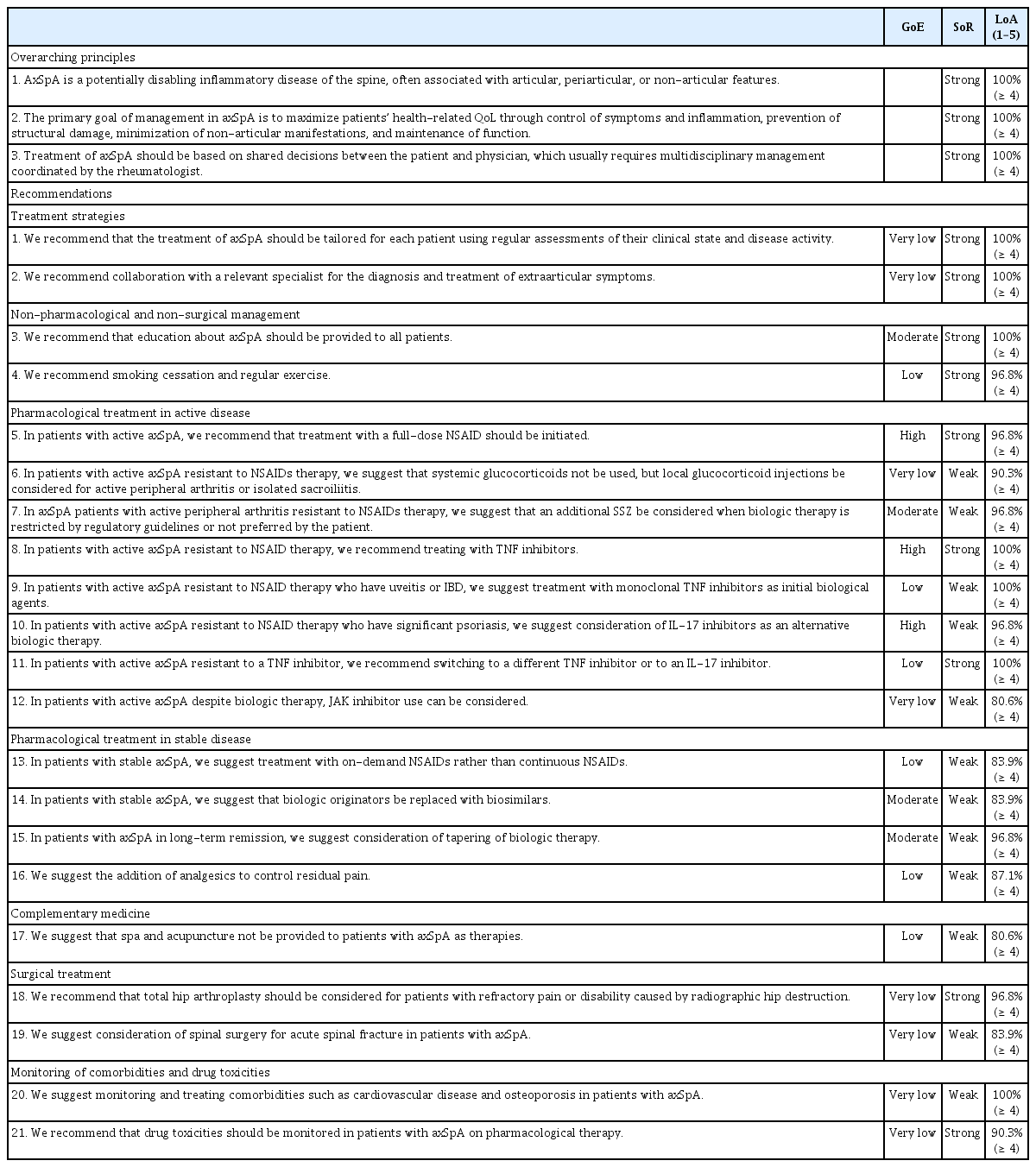
The consensus-voting panel comprised the directors of the KCR, steering committee members of the KSSR, and members of the DC. The formulated recommendations, summaries of the evidence, and voting guidelines were presented to the panel. Voting was based on a level of agreement (LoA) scale from 1 to 5 (1, strongly disagree; 2, disagree; 3, neither agree nor disagree; 4, agree; and 5, strongly agree). Consensus was achieved if more than 80% of the panel voted 4 or 5 for a recommendation. Consensus was reached by the first vote on all recommendations, except for recommendation 12, for which it was reached by the second vote. Treatment recommendations for axSpA, comprising three overarching principles and twenty-one recommendations, were finalized (Table 2, Supplementary Table 1). A schematic of the final treatment recommendations was presented at the next DC meeting (Fig. 1). The steering committee of the KSSR endorsed these recommendations on June 14, 2022.
RECOMMENDATIONS FOR THE TREATMENT OF PATIENTS WITH AXIAL SPONDYLOARTHRITIS
Overarching principles
AxSpA is a potentially disabling inflammatory disease of the spine, often associated with articular, periarticular, or non-articular features (SoR, strong; LoA, 100%)
Overarching principle (OAP) 1 pertains to the definition of axSpA and reflects a comprehensive view of the disease. AxSpA is an inflammatory disease that can cause disability in patients’ daily lives. It involves not only the spine, but also peripheral joints and periarticular tissues. Many patients experience extra-musculoskeletal symptoms such as uveitis, IBD, and psoriasis [16–18].
The primary goal of management in axSpA is to maximize patients’ health-related QoL through control of symptoms and inflammation, prevention of structural damage, minimization of non-articular manifestations, and maintenance of function (SoR, strong; LoA, 100%)
The goal of caring for axSpA patients is to help them achieve the best health-related QoL (HrQoL). The main factors that determine the HrQoL in patients with axSpA include inflammatory activity, structural damage, and physical function [19,20]. As axSpA is fundamentally an inflammatory disease, controlling disease activity is important to relieve symptoms, prevent structural damage, and maintain and improve function and QoL [21–23]. Extra-musculoskeletal involvement is associated with decreased QoL and may be with increased cardiovascular risk and mortality [24–26]. Thus, controlling these symptoms in patients with axSpA is another concern. Similar to the treatment of other rheumatic and musculoskeletal disease, both pharmacological and non-pharmacological treatment such as education, physical therapy, and surgery should be used for optimal management of axSpA.
Treatment of axSpA should be based on shared decisions between the patient and physician, which usually requires multidisciplinary management coordinated by the rheumatologist (SoR, strong; LoA, 100%)
Quality care for individual patient is based on shared decision-making (SDM) between the patient and health professionals. In SDM, patient and caregivers work together to build a treatment plan that incorporates evidence-based information, clinical experts’ experiences, and patients’ preferences, values, and goals [27]. This includes determining the treatment objective, selecting the treatment method, and considering how to taper therapies if the treatment objective is achieved. SDM success requires provision of sufficient information to patients and appropriate trust and communication between patients and health professionals. Patient and physician commitment to SDM maximizes treatment concordance and success. SDM is strongly supported as a general principle and is foundational in treatment recommendations by international organizations such as the Assessment of SpondyloArthritis International Society (ASAS), EULAR, and ACR [28,29].
Care for patients with axSpA who show various clinical symptoms, including extra-musculoskeletal symptoms, and need both pharmacological and non-pharmacological treatment requires a multidisciplinary approach involving ophthalmologists, dermatologists, gastroenterologists, orthopedic surgeons, physiatrist, and other health professionals, along with rheumatologists. Multidisciplinary care is most effectively coordinated by rheumatologist, who have a broad understanding of the spectrum of axSpA diagnoses, disease course, and treatments.
Recommendations
Treatment strategies
Recommendation 1. We recommend that the treatment of axSpA should be tailored for each patient using regular assessments of their clinical state and disease activity (GoE, very low; SoR, strong; LoA, 100%)
This recommendation was derived from the KCQs related to the treat-to-target (T2T) strategy and disease monitoring. There is considerable indirect evidence for effective disease monitoring in the management of axSpA [21,30–48]. Although treatment strategies for remission or low disease activity have attracted widespread attention to achieve the goal of care for patients with axSpA referred to in OAP2, the T2T strategy for ax SpA remains controversial. One RCT reported no significant difference between the T2T strategy and the traditional method in terms of the primary endpoint [49]. As it is difficult to judge the definite benefits of the T2T strategy, it was not directly included in this recommendation. However, the DC believes that individualized treatment adjustment using periodic evaluation of the patient’s clinical state centered on disease activity is essential; therefore, they strongly recommend it. Disease activity should be assessed using validated indicators such as the Ankylosing Spondylitis Disease Activity Score (ASDAS) and the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) [31–33].
Recommendation 2. We recommend collaboration with a relevant specialist for the diagnosis and treatment of extra-articular symptoms (GoE, very low; SoR, strong; LoA, 100%)
This recommendation is related with OAP 3. Despite the limited direct evidence, recommendation 2 was strongly agreed upon by all the experts. IBD, uveitis, and psoriasis are common extra-musculoskeletal symptoms in patients with axSpA. The relevant specialists should participate in the diagnosis and management of these symptoms.
Non-pharmacological and non-surgical management
Recommendation 3. We recommend that education about axSpA should be provided to all patients (GoE, moderate; SoR, strong; LoA, 100%)
Education is crucial for patients with axSpA, who must cope with the disease and may not know it well. Most patients with axSpA wish to receive education on the disease, treatment, required exercises, and self-management. Patients who received education about axSpA showed better results of the BASDAI, Bath Ankylosing Spondylitis Functional Index (BASFI), and Ankylosing Spondylitis QoL (ASQoL) compared to those who did not [50]. Patient education may also improve SDM and patient participation in treatment, as mentioned in OAP 3.
Recommendation 4. We recommend smoking cessation and regular exercise (GoE, low; SoR, strong; LoA, 96.8%)
Smoking may be detrimental in terms of disease activity, bony progression, and QoL in patients with axSpA [37]. Considering this and the effects of smoking on general health, smoking cessation is strongly recommended. Exercise significantly improved fatigue and the BASFI and EuroQoL scores in patients with axSpA [50–54]. Supervised or institutional exercise better improved the BASDAI, BASFI and Bath Ankylosing Spondylitis Metrology Index (BASMI) scores, but did not differ from unsupervised or homebased exercise in terms of pain, chest expansion, and Bath AS patient global score [55–59]. Aquatic exercise was more beneficial for short-term pain and the modified Schober test results than was land-based exercise; however, the difference was modest [60]. Unfortunately, standardized axSpA-appropriate programs for supervised, institutional, or aquatic exercise are not easily accessible for patients. Passive physical therapy has been shown to have short-term effects; however, no studies have reported on its long-term effects [61,62]. Further, while manual therapy is popular, it remains unverified in terms of harmful effect in patients with axSpA [63]. Thus, we strongly recommend regular exercise without specifying the type and location of exercise, in consideration of accessibility and availability.
Pharmacological treatment in active disease
Recommendation 5. In patients with active axSpA, we recommend that treatment with a full-dose nonsteroidal anti-inflammatory drug (NSAID) should be initiated (GoE, high; SoR, strong; LoA, 96.8%)
Active axSpA refers to the presence of axial and/or peripheral symptoms attributed to inflammation, usually defined as a BASDAI score or ASDAS of >4.0 or ≥2.1, respectively [2,64]. NSAIDs have demonstrated significant beneficial effects on active axSpA in terms of outcome parameters such as pain and BASFI [65–67]. There are not certain NSAIDs being more advantageous in their efficacy than others [65–73]. However, a full dose of NSAIDs is more effective than a minimal dose in terms of the patient global assessment, ASAS20, and BASDAI scores [65,68–71]. Although worsening of occult bowel inflammation is a concern when using NSAIDs in patients with axSpA, there is no definite relationship between NSAID use and IBD exacerbation [74,75]. We strongly recommend a full-dose NSAID as the first-line therapy in patients with active axSpA. However, safety issues associated with long-term NSAID use remain a concern. In addition, NSAID use is restricted in patients with renal insufficiency, cardiovascular disease, peptic ulcer disease, aspirin-exacerbated respiratory disease, or advanced chronic liver disease. Therefore, as directed in Recommendation 1, in axSpA, NSAID use should be tailored for each patient according to the associated benefits and risks.
Recommendation 6. In patients with active axSpA resistant to NSAIDs therapy, we suggest that systemic glucocorticoids not be used, but local glucocorticoid injections be considered for active peripheral arthritis or isolated sacroiliitis (GoE, very low; SoR, weak; LoA, 90.3%)
Only one RCT reported that the short-term use of systemic glucocorticoid was effective in active axSpA refractory to NSAIDs therapy [76]. The efficacy of long-term systemic glucocorticoid treatment in patients with active axSpA has not been clarified, although it is associated with a high risk of adverse effects. Biological agents are good treatment options for patients with active axSpA despite NSAID use. Therefore, we suggest that systemic glucocorticoids not be used in these patients. Intraarticular glucocorticoid injections for peripheral arthritis are popular in rheumatology [77]. Although evidence of their efficacy in axSpA is scarce, experts have suggested that these injections might help control active peripheral arthritis in patients with axSpA. A small RCT reported that local glucocorticoid injections are effective in controlling isolated sacroiliitis in axSpA [78]. Appropriate evidence on the efficacy of local glucocorticoid injections for enthesitis in patients with axSpA, which could have a risk of causing tendon rupture, was unavailable. Therefore, we suggest consideration of local glucocorticoid injections only for active peripheral arthritis or isolated sacroiliitis resistant to NSAIDs in patients with axSpA.
Recommendation 7. In axSpA patients with active peripheral arthritis resistant to NSAIDs therapy, we suggest that an additional sulfasalazine (SSZ) be considered when biologic therapy is restricted by regulatory guidelines or not preferred by the patient (GoE, moderate; SoR, weak; LoA, 96.8%)
There is little evidence that conventional disease-modifying antirheumatic drugs such as methotrexate and leflunomide are effective in patients with axSpA who do not respond to initial NSAID therapy [79–81]. Although biologic therapy may be a more effective treatment option in these patients, SSZ demonstrated efficacy and is commonly used for peripheral arthritis in patients with axSpA [82–84]. A few studies that compared SSZ with biological agents showed that SSZ was effective in relieving peripheral symptoms in the patients with active axSpA despite NSAID use [85,86]. Therefore, the DC conditionally recommends SSZ for active peripheral arthritis resistant to NSAID therapy, in cases where biologic therapy is not affordable or preferable, for patients with axSpA.
Recommendation 8. In patients with active axSpA resistant to NSAID therapy, we recommend treating with TNF inhibitors (GoE, high; SoR, strong; LoA, 100%)
In Korea, biological agents, including TNF inhibitors such as etarnercept, infliximab, adalimumab, and golimumab, and IL-17 inhibitors such as secukinumab and ixekizumab, have been approved and used to treat patients with axSpA. Compared with placebos, TNF inhibitors have pronounced effects on various parameters, including ASAS response criteria, disease activity, BASFI, BASMI, 36-item Short Form Survey (SF-36) scores, and peripheral symptoms, in patients with active axSpA despite NSAID treatment [87–105]. TNF inhibitors were more effective than SSZ for most parameters in these patients [85,87,89]. Therefore, we recommend the use of TNF inhibitor as initial biologic therapy for active axSpA despite NASID use. There is no evidence regarding certain TNF inhibitors being more effective than others [106].
Although IL-17 inhibitors are also recommended as initial biologic therapy in the recently published EULAR recommendations [107], we did not include IL-17 inhibitors as first-line biological therapy. While there is no evidence that TNF inhibitors are more effective than IL-17 inhibitors, TNF inhibitors are preferred as they have been studied more extensively and have been used in clinical practice for a longer time than have IL-17 inhibitors. Moreover, while switching to IL-17 inhibitors in case of insufficient response to TNF inhibitors has been reported, switching from IL-17 inhibitors to TNF inhibitors has not [108–110]. In other words, evidence regarding the pharmacological therapeutic pathway in cases of IL-17 inhibitor failure in patients with active axSpA is unavailable.
The DC did not address the criteria of initiation of biologic therapy in case of insufficient response to initial NSAID treatment. The reimbursement regulation of the Korean National Health Insurance regarding biological agents for AS patients defines that as BASDAI score of > 4.0 despite of treatment with two or more NSAIDs for more than 3 months. This differs from the global standard, in which early initiation of biological agents is recommended, based on expert judgement, in patients with active axSpA (BASDAI >4.0 or ASDAS ≥2.1) despite the use of two or more NSAIDs consecutively for 1 month [2,3,5,8].
Safety in the use of biological agents has not been addressed in this recommendation and should be referred to in other recommendations [111].
Recommendation 9. In patients with active axSpA resistant to NSAID therapy who have uveitis or IBD, we suggest treatment with monoclonal TNF inhibitors as initial biological agents (GoE, low; SoR, weak; LoA 100%)
There are no direct RCTs related to the KCQs corresponding to this recommendation. Three observational studies and three meta-analyses showed that compared to fusion proteins (etanercept), monoclonal TNF inhibitors (infliximab and adalimumab) generally showed better outcomes in terms of the incidence or flare rates of uveitis or IBD [112–117]. Further, IL-17 inhibitors may exacerbate IBD in patients with axSpA [118].
Recommendation 10. In patients with active axSpA resistant to NSAID therapy who have significant psoriasis, we suggest consideration of IL-17 inhibitors as an alternative biologic therapy (GoE, high; SoR, weak; LoA, 96.8%)
IL-17 inhibitors were more effective than a placebo in patients who responded insufficiently to NSAID therapy [108,119–125]. In particular, IL-17 inhibitors were more effective than TNF inhibitors in treating psoriasis [126]. Therefore, IL-17 inhibitors can be considered the first-line biological agents for patients with axSpA with significant psoriasis, which corresponds to severe or extensive psoriasis and significantly affects QoL [127].
Recommendation 11. In patients with active axSpA resistant to a TNF inhibitor, we recommend switching to a different TNF inhibitor or to an IL-17 inhibitor (GoE, low; SoR, strong; LoA, 100%)
Switching to another TNF inhibitor is effective in a significant number of patients with axSpA, in cases of intolerance to or persistence of active disease with the first TNF inhibitor [128–133]. However, this appears less effective in patients with an initial lack of response than in those with relapse after first TNF inhibitor use [128]. IL-17 inhibitors have also demonstrated efficacy in patients with AS being refractory to or intolerant to the TNF inhibitors [108–110]. Therefore, in patients with axSpA with active disease resistant to a TNF inhibitor, we strongly recommend switching to a different TNF inhibitor or to an IL-17 inhibitor, irrespective of the presumed reason behind failure of the first TNF inhibitor.
Recommendation 12. In patients with active axSpA despite biologic therapy, JAK inhibitor use can be considered (GoE, very low; SoR, weak; LoA 80.6%)
Recently, JAK inhibitors, such as tofacitinib and upadacitinib, have shown significant effects on several outcomes, including the ASAS20, ASAS40, BASFI, BASMI, and ASDAS scores in patients with active axSpA with an insufficient response to NSAID therapy [134–136]. However, data regarding JAK inhibitor use in clinical practice remains scarce. Although there are no RCTs on the effectiveness of JAK inhibitors in patients with axSpA who have an insufficient response to biologic therapy, we conditionally suggest JAK inhibitor use in such patients.
Pharmacological treatment in stable disease
Recommendation 13. In patients with stable axSpA, we suggest treatment with on-demand NSAIDs rather than continuous NSAIDs (GoE, low; SoR, weak; LoA, 83.9%)
In axSpA, stable disease corresponds to an inactive disease state that persists for more than six months [2,3]. Long-term studies showed that continuous NSAID treatment was not better than on-demand NSAID treatment for inhibiting structural damage [137,138], and there was no statistical difference in the mean BASDAI and BASFI scores between patients with continuous and on-demand NSAID use over 24 months [138]. Long-term use of NSAIDs is associated with concerns regarding safety rather than their efficacy. Therefore, we suggest the use of on-demand NSAIDs over continuous NSAIDs for patients with stable axSpA.
Recommendation 14. In patients with stable axSpA, we suggest that biologic originators be replaced with biosimilars (GoE, moderate; SoR, weak; LoA, 83.9%)
A biosimilar is a biological agent with highly similar physicochemical characteristic and biological activities as the biological originator. Further preclinical and clinical studies are required to confirm their equivalent efficacy, safety, and immunogenicity [139–141]. Several biosimilars based on infliximab, etanercept, and adalimumab originators have been developed and approved for use in patients with axSpA. Biosimilars are intended to be used in the same manner as the originator biological agents, but physicians may prefer treating with originators because they usually have more experience with these. Although switching from an originators to a biosimilar can save costs, it may result in a nocebo response such as a subjective increase in disease activity or adverse events [141]. However, several studies have confirmed that there is no significant difference in the ASAS response criteria and adverse events between biosimilars and biological originators [142–144]. Biosimilars are used more and more in rheumatic diseases; this is true even among physicians and patients in Korea. The voting panel agreed that a biological originator can be replaced with a biosimilar in patients with stable axSpA.
Recommendation 15. In patients with axSpA in long-term remission, we suggest consideration of tapering of biologic therapy (GoE, moderate; SoR, weak; LoA, 96.8%)
The appropriateness of discontinuation or dose reduction for biological agents in well-controlled axSpA is a common and important question for both patients and physicians. Among patients with axSpA in long-term remission, discontinuation of biologic agents resulted in a higher flare rate, but biologic agent dose reduction by half or increasing dosing intervals resulted in well-maintained remission without flares when compared to that with continuation of biological agents [145–148]. Therefore, tapering of biologic therapy can be considered in these patients.
In axSpA, remission is a state in which both disease activity and progression are absent over a long period of time. However, there are currently no universally accepted criteria for remission in axSpA. Some authors have proposed the following remission criteria: ASDAS <1.3, absence of peripheral symptoms, absence of extra-articular symptoms, normal CRP levels, and absence of radiographic progression [149]. Herein, remission for over 6 (or 12) months could be considered long-term.
Recommendation 16. We suggest the addition of analgesics to control residual pain (GoE, low; SoR, weak; LoA, 87.1%)
Although the incidence of side effects increased slightly, the addition of analgesics, such as tramadol and acetaminophen, helped relieve pain in patients with axSpA [150]. Use of analgesics must not hinder or delay the appropriate anti-inflammatory therapies. When residual pain persists despite standard treatments, analgesics can be administered.
Complementary medicine
Recommendation 17. We suggest that spa and acupuncture not be provided to patients with axSpA as therapies (GoE, low; SoR, weak; LoA, 80.6%)
Spa and acupuncture are traditional complimentary remedies for controlling musculoskeletal pain that are familiar to Koreans. A few small studies showed that spas helped relieve symptoms and improve the QoL in patients with axSpA; however, these effects lasted for a short period [51,151,152]. Currently, there is no standardized spa therapy for patients with axSpA. Further, in a small RCT, acupuncture was not more effective than sham therapy [153]. Therefore, we suggest that spa and acupuncture not be used in patients with axSpA as therapies.
Surgical treatment
Recommendation 18. We recommend that total hip arthroplasty should be considered for patients with refractory pain or disability caused by radiographic hip destruction (GoE, very low; SoR, strong; LoA, 96.8%)
According to epidemiological data from Western countries, up to one-third of patients with AS have hip involvement [154]. Hip involvement is associated with significant functional decline in patients with axSpA, who may require hip arthroplasty. While hip involvement seems to be less frequent in Korean patients with AS, the rate of hip arthroplasty among patients with hip involvement is similar to that in foreign countries [155]. There are no RCTs on the effectiveness of total hip arthroplasty in patients with axSpA; however, many observational studies have suggested that total hip arthroplasty can reduce pain and improve joint range of motion and function [156–160]. This recommendation emphasizes that total hip arthroplasty is indicated in patients with axSpA who have severe pain or disability caused by hip destruction.
Recommendation 19. We suggest consideration of spinal surgery for acute spinal fracture in patients with axSpA (GoE, very low; SoR, weak; LoA, 83.9%)
Spinal fractures occurs more frequently and at younger ages in patients with axSpA than in controls [161–163]. In addition, axSpA is often accompanied by spinal cord injury, and the clinical outcome is worse in patients with axSpA than in those with general trauma [164–166]. Pain from spinal fractures may be overlooked due to axSpA disease activity, and patients’ abnormal vertebral structure makes radiographic evaluation difficult, often leading to a diagnostic delay [160,161]. Spinal fractures in patients with axSpA usually require surgery; however, conservative treatments are sometimes used when the surgical risk is extremely high. Observational studies have shown that surgery tends to further improve neurological outcomes and reduce complications when compared with conservative treatment [160]. In particular, patients with neurologic deficits or unstable fractures may require surgery, so immediate consultation with a surgeon is essential [167–169]. Therefore, we suggest acute spinal fractures as probable surgical indications in patients with axSpA.
Guidelines for vertebral osteotomy in patients with axSpA are conflicting. The EULAR/ASAS recommendations suggests that patients with severe kyphosis be considered for vertebral corrective osteotomy in a specialized center [107]; however, the ACR/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network guidelines conditionally recommend against elective spinal osteotomy, except in extreme cases, because of the postoperative mortality and neurological complications [3,11]. The DC has set aside recommendation on vertebral osteotomy for the future, considering the lack of specialized surgical institutions in Korea, the risk of surgery, and lower postoperative patient satisfaction. Arthroscopic synovectomy for active peripheral arthritis in patients with axSpA was excluded from the discussion because of a lack of evidence.
Monitoring of comorbidities and drug toxicities
Recommendation 20. We suggest monitoring and treating comorbidities such as cardiovascular disease and osteoporosis in patients with axSpA (GoE, very low; SoR, weak; LoA, 100%)
Comorbidities that can affect the patient mortality or QoL are important concerns for both patients and physicians during long-term care in chronic rheumatic diseases. Osteoporosis, posing a risk of spinal fractures, and cardiovascular diseases are frequently observed in patients with axSpA. In a large observational cohort, the incidence and prevalence of major adverse cardiovascular events in patients with axSpA were similar to those in patients with rheumatoid arthritis after adjusting for traditional cardiovascular risk factors, disease onset age, sex, and disease duration [170]. Patients with axSpA also have a higher prevalence of cardiovascular comorbidities, such as hypertension, dyslipidemia, and obesity, than dose the general population [171]. The bone mineral density of patients with AS is significantly lower than that of healthy controls [172,173]. Osteoporosis is found in approximately one quarter of patients with AS aged >50 years or with a disease duration of ≥10 years [174,175]. The voting panel agreed that monitoring and management of comorbidities in patients with axSpA, especially cardiovascular diseases and osteoporosis, is necessary.
Recommendation 21. We recommend that drug toxicities should be monitored in patients with axSpA on pharmacological therapy (GoE, very low; SoR, strong; LoA, 90.3%)
As there is a substantial possibility that all drugs cause toxicities, monitoring drug toxicity is essential for patient safety. Drug safety monitoring should be conducted for each drug taken by the patient [176]. This should be initiated by the physician with a clinical interview of the patient, considering their comorbidities and past medical history. Periodic blood tests, including complete blood count, liver function tests, and creatinine levels, are often required. Before using biological agents in patients with axSpA, surveillance of tuberculosis and hepatitis is required. Previously published consensus recommendations could be referred to on this [111].
CONCLUSION
Herein, recommendations, covering the comprehensive scope of management of adult patients with axSpA in a Korean context, were first developed based on clinical evidence. These consist of three overarching principles and 21 individual recommendation items, pertaining to treatment strategies, non-pharmacological and non-surgical management, pharmacological treatment in active and stable disease, complementary medicine, surgical treatment, and monitoring of comorbidities and drug toxicities.
However, these recommendations may be limited as some KCQs were not addressed owing to a lack of evidence. Additionally, we did not provide clear and specific consensus definitions of concepts essential for caring for patients, such as activity, remission, and treatment response. Further investigation and discussion are required to address these limitations. These recommendations will be updated when significant or substantial new evidence is identified by the KSSR at the KCR. We hope that these recommendations will guide best practice in the treatment of axSpA until then.
Acknowledgments
We thank Min Kyung Hyun, Dongguk University Graduate School of Korean Medicine; Yoon Jae Lee, Jaseng Medical Foundation for guidance on the methodology of the literature review and clinical guideline development; and all members of the voting panel: Hyun Sik Gong, Seoul National University Bundang Hospital; Jung Soo Song, Chung-Ang University School of Medicine; Wansik Uhm, Uhm’s Hanyang Rheumatism Clinic; Chong-Hyeon Yoon, Eunpyeong St. Mary’s Hospital, the Catholic University of Korea; Sangil Lee, Gyeongsang National University Hospital; Shin-Seok Lee, Chonnam National University Medical School and Hospital; Young Ho Lee, Korea University; Chang Keun Lee, Asan Medical Center, University of Ulsan College of Medicine; Ji Hyeon Ju, The Catholic University of Korea; Seung-Jae Hong, Kyung Hee University Hospital.
Notes
Conflicts of interest
M.R.S. received unrestricted grants from Hanlim Pharm, and speaker fees from Lilly. Y.A.L. received consulting and/or speaker fees from Astellas, Janssen, Lilly, AbbVie, and unrestricted grants from Celltrion. E.H.K. received unrestricted grants from Celltrion. S.R.K. received consulting and/or speaker fees from Janssen, Novartis, AbbVie, and unrestricted grants from Celltrion. S.K.K. has been an editorial board member since May 2018, but has no role in the decision to publish this article. T.J.K. received consulting and/or speaker fees from Janssen, AbbVie, Pfizer, and Novartis. T.H.K. received consulting and speaker fees from AbbVie, Novartis, Lilly, Janssen, and unrestricted grants from Yuhan Corporation. M.C.P. received consulting and/or speaker fees from Novartis, Lilly, AbbVie, Chong Kun Dang Pharm, JW Pharm, and research grants from Novartis, and has been an editorial board member since June 2014, but has no role in the decision to publish this article. S.H.L. received consulting and/or speaker fees from Novartis, Janssen, Lilly, AbbVie, Celltrion, and unrestricted grants from Celltrion. E.Y.L. received consulting or speaker fees from Lilly, JW Pharm, Novartis, Janssen, Samsung Bioepis and unrestricted grants from Yuhan Corporation. H.S.C. received consulting and/or speaker fees from LG Chem, Yuhan Corporation, AbbVie, Janssen, Novartis, and unrestricted grants from Celltrion. S.C.S. received consulting and/or speaker fees from AbbVie, Pfizer, Celltrion, Novartis, Astellas, JW Pharm, Chong Kun Dang Pharm, Amgen, and Otsuka Pharm. H.J.B. received consulting or speaker fees from Janssen, Astellas, Novartis, AbbVie, JW Pharm, Meranini, and unrestricted grants from Celltrion.
This article is co-published by the Korean Journal of Internal Medicine and Journal of Rheumatic Diseases.
CRedit authorship contributions
Mi Ryoung Seo: methodology, resources, investigation, data curation, formal analysis, validation, software, writing - original draft, writing - review & editing, visualization; Jina Yeo: methodology, resources, investigation, data curation, formal analysis, validation, software, visualization; Jun Won Park: methodology, resources, investigation, data curation, formal analysis, validation, software; Yeon-Ah Lee: methodology, resources, investigation, data curation, formal analysis, validation, software; Ju Ho Lee: methodology, resources, investigation, data curation, formal analysis, validation, software; Eun Ha Kang: methodology, resources, investigation, data curation, formal analysis, validation, software; Seon Mi Ji: conceptualization, methodology, investigation, formal analysis, validation, software, supervision; Seong-Ryul Kwon: conceptualization, methodology, resources, investigation, data curation, writing - review & editing, supervision; Seong-Kyu Kim: conceptualization, methodology, resources, investigation, data curation, writing - review & editing, supervision; Tae-Jong Kim: conceptualization, methodology, resources, investigation, data curation, writing - review & editing, supervision; Tae-Hwan Kim: conceptualization, methodology, resources, investigation, data curation, writing - review & editing, supervision; Hye Won Kim: conceptualization, methodology, resources, investigation, data curation, writing - review & editing, supervision; Min-Chan Park: conceptualization, methodology, resources, investigation, data curation, writing - review & editing, supervision; Kichul Shin: conceptualization, methodology, resources, investigation, data curation, writing - review & editing, supervision; Sang-Hoon Lee: conceptualization, methodology, resources, investigation, data curation, writing - review & editing, supervision; Eun Young Lee: conceptualization, methodology, resources, investigation, data curation, writing - review & editing, supervision; Hoon Suk Cha: conceptualization, methodology, resources, investigation, data curation, writing - review & editing, supervision; Seung Cheol Shim: conceptualization, methodology, resources, investigation, data curation, writing - review & editing, supervision; Youngim Yoon: conceptualization, investigation, writing - review & editing, supervision; Seung Ho Lee: conceptualization, investigation, writing - review & editing, supervision; Jun Hong Lim: conceptualization, investigation, writing - review & editing, supervision; Han Joo Baek: conceptualization, methodology, resources, investigation, data curation, formal analysis, validation, software, writing - original draft, writing - review & editing, visualization, supervision, project administration, funding acquisition
Funding
This study was supported by the HANLIM Pharmaceutical and the KCR. The funders played no role in the study design, data collection, data analysis, data interpretation or manuscript writing.