The impact of antimicrobial de-escalation therapy in culture-negative pneumonia: a systematic review and meta-analysis
Article information
Abstract
Background/Aims
Antimicrobial de-escalation (ADE) remains a challenging strategy in the treatment of pneumonia. We investigated the outcomes of ADE as measured by mortality and duration of the use of antibiotics in patients with culture-negative pneumonia.
Methods
We performed a systematic review and meta-analysis in accordance with PRISMA guidelines. The primary outcome was inpatient mortality.
Results
We examined six studies comprising 11,933 subjects, of whom 1,152 received ADE. Overall, the ADE strategy was associated with a statistically lower risk of in-hospital mortality compared with non-ADE (risk ratio [RR] = 0.60, 95% confidence interval [CI] = 0.38 to 0.93). Although substantial heterogeneity was found among the included studies (I2 = 66%), a meta-regression analysis could not reveal plausible sources of heterogeneity. And ADE was associated with a shorter duration of total and initial antibiotic therapies and total length of hospital stay compared with non-ADE.
Conclusions
Our findings suggest that ADE seems to be significantly associated with better clinical outcomes compared with non-ADE. Caution is demanded when interpreting data of this study because of substantial between-study heterogeneity.
INTRODUCTION
In patients with nosocomial pneumonia, empirical antimicrobial therapies targeting multidrug-resistant (MDR) pathogens such as Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus (MRSA) are associated with favorable outcomes [1]. However, excessive use of broad-spectrum antimicrobials has been linked to the development of adverse drug effects and superinfections such as Clostridium difficile, as well as the emergence of MDR pathogens [2]. Interventions that minimize antibiotic burdens are needed.
Antimicrobial de-escalation (ADE) is a strategy to reduce the spectrum of antimicrobial regimens and prevent the development of drug resistance [3]. Current guidelines set by the Infectious Diseases Society of America and American Thoracic Society advocate ADE based on culture results and drug-susceptibilities once patients have shown clinical response [1]. The ADE strategy is recommended in particular for cases of sepsis and nosocomial pneumonia in which the potential risk of MDR pathogens is relatively high. Several studies have reported that ADE is associated with lower mortality compared with non-ADE treatments and can be performed safely in these patients [4–6].
Despite current extensive diagnostic modalities, the positive rate of microbiological cultures is approximately 40–60% for patients with sepsis, and pathogens were absent in more than 60% of patients with community-acquired pneumonia (CAP) [7,8]. Previous studies investigating sepsis and healthcare-associated pneumonia revealed that culture-negative patients experience reduced severity of illness and hospital mortality, and shorter hospital stays compared with culture-positive patients [9,10].
The ADE strategy has been evaluated primarily in cases of nosocomial pneumonia and sepsis in which the risk of MDR pathogens is relatively high [11]. In a previous meta-analysis, although a high risk of bias was evident among the cohort studies, a pooled estimate indicated that ADE resulted in lower mortality rates [11]. The aim of the present study was to investigate the impact of ADE in culture-negative pneumonia through a systematic review and meta-analysis. We also examined factors affecting the clinical outcomes of ADE.
METHODS
Data sources and search strategy
This systematic review and meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [12]. The study protocol was registered with the PROSPERO International Prospective Register of Systematic Reviews (https://www.crd.york.ac.uk/prospero/display_record.php?Recor-dID=376375). The following terms were used to search the available literature: [“antimicrobials” OR “antibiotics” OR “antibacterials”] AND [“de-escalation” OR “discontinuation” OR “narrowing” OR “step-down”]) AND [“hospital-acquired” OR “ventilator-associated” OR “healthcare-associated” OR “nosocomial”] AND [“pneumonia” OR “lower respiratory tract infection”]. Three electronic databases (PubMed, Embase, and the Cochrane Central Register of Controlled Trials) were searched for relevant articles published before January 2023. The reference section lists all research papers investigated, and appropriate reviews were examined manually to identify potentially relevant articles. As the present study is a systematic review of previous published articles, neither informed consent nor ethics approval was required.
Study selection, definitions, and outcomes
This meta-analysis included all studies that met the following criteria: (1) the subjects were patients with culture-negative pneumonia; (2) an examination of the clinical outcomes of ADE was included; (3) in-hospital mortality outcomes were included; (4) relative risk ratios (RRs) and 95% confidence intervals (CIs) were reported or were able to be calculated using information in the article; and (5) the study was published in a peer-reviewed English-language journal. Review journals, case reports or series, commentaries, and extension or post-hoc articles were excluded.
The ADE strategy involves discontinuing broad spectrum antibiotics or narrowing the regimen based upon negative culture results following reassessment of a patient’s status after initiation of therapy [3]. Appropriate MDR-directed antimicrobial therapy was defined as a combination of at least one anti-pseudomonal beta-lactam antibiotic and at least one active anti-MRSA agent.
The primary outcome for this study was in-hospital mortality at the study index date. We also analyzed the duration of the initial antimicrobial therapy, total duration of antimicrobial therapy, and length of hospitalization.
Data extraction, assessment of literature quality, and publication bias assessment
Two reviewers independently screened the titles and abstracts of the studies retrieved from the search to identify relevant papers. We obtained full-text articles and extracted data according to the predefined inclusion criteria. The following variables were extracted: the first author, year of publication, study sites, number of patients, age, sex, initial intensive care unit (ICU) admission rate, type of pneumonia, ADE definition, ADE rate, patient mortality, duration of initial and total antimicrobial therapy, and primary outcomes.
As recommended by the Cochrane Collaboration, the methodological quality of included studies was evaluated using the in the Risk of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool [13]. The ROBINS-I uses seven criteria to assess the potential risk of bias in each study including confounding, selection bias, classification of interventions, deviation from interventions, missing data, measurement of outcome, and selection of reported result. The risk of bias was graded as low, moderate, serious, critical, or no information [13]. The overall risk of bias was considered as serious or critical if the study achieved serious or critical ratings at least in one domain. Studies that obtained low in all domains were regarded as having a low risk of bias, and articles without any serious and critical ratings were regarded as having a moderate risk of bias [13].
The funnel plot was drawn to evaluate inherent publication bias and the Egger’s test was used to identify the evidence of bias. Any disagreements regarding study search, data extraction, and quality assessment were discussed and resolved by the authors.
Statistical analysis
We extracted the RRs and associated 95% CIs for the ADE strategy to evaluate clinical outcomes for dichotomous data compared with non-ADE treatments. For continuous data, the weighted mean difference (MD) with 95% CIs was extracted. Between-study statistical heterogeneity was evaluated using I2 statistics on a scale of 0–100% (low heterogeneity for I2 < 49%, moderate I2 = 50–74% and high for I2 > 75%, respectively) [14]. A random-effects model was used for moderate or high heterogeneity. A p value < 0.05 was considered statistically significant. Statistical analyses were performed with Stata statistical software (Version 14.2; Stata Corp LP, College Station, TX, USA) and Review Manager (Version 5.3; Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
RESULTS
Study search, characteristics of included studies, and study quality
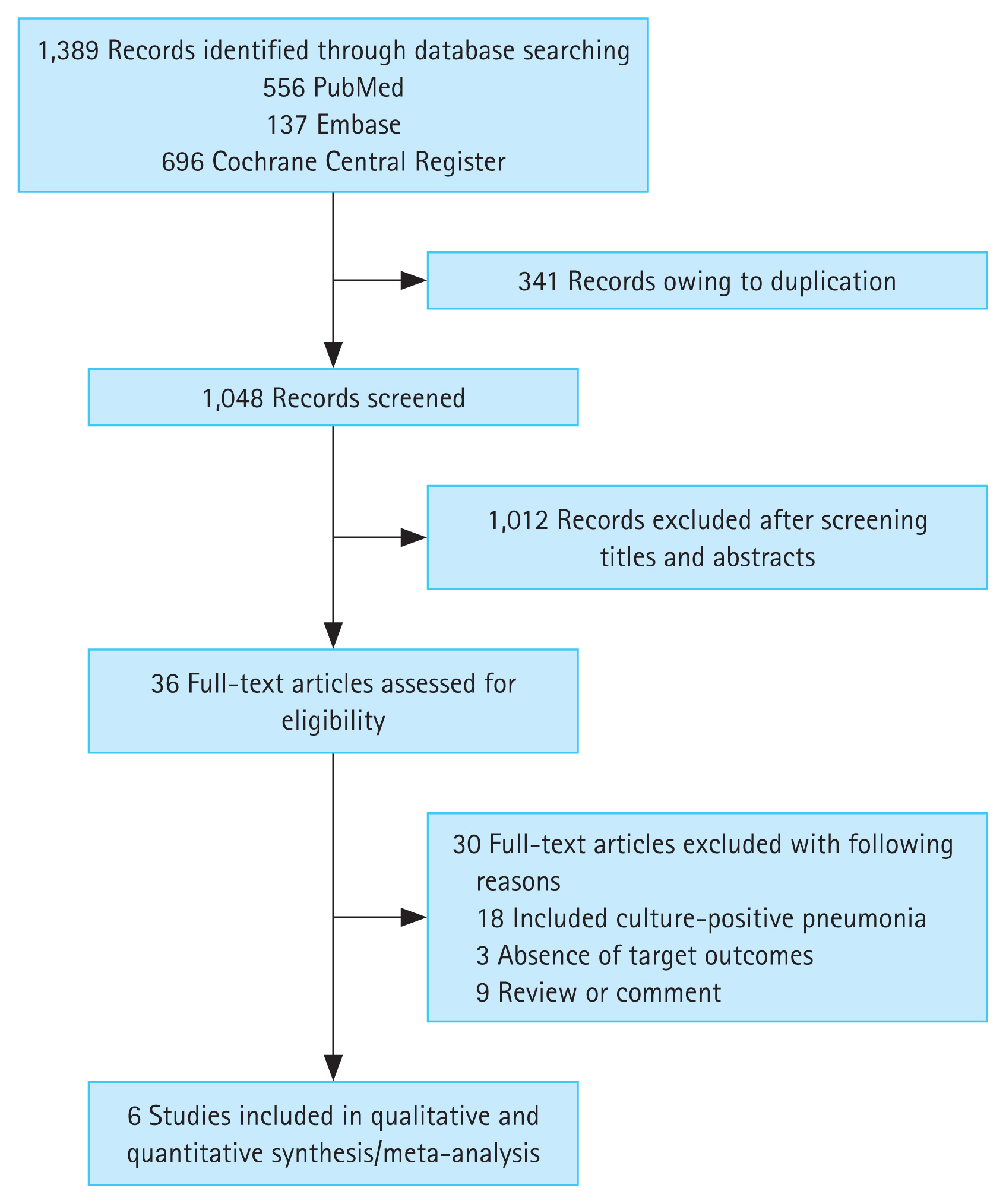
Figure 1 presents the results of the literature search. A total of 1,389 published articles were initially identified from three databases; 556 articles from PubMed, 137 articles from Embase, and 696 articles from the Cochrane Central Register of Controlled Trials. After removing duplicate articles, 1,048 potentially eligible articles were screened. A review of titles and abstracts resulted in the removal of 1,012 search records, and the remaining 36 articles were assessed by reading the full text. Thirty articles were excluded for the reasons presented in Figure 1, leaving six studies for our analysis [15–20].
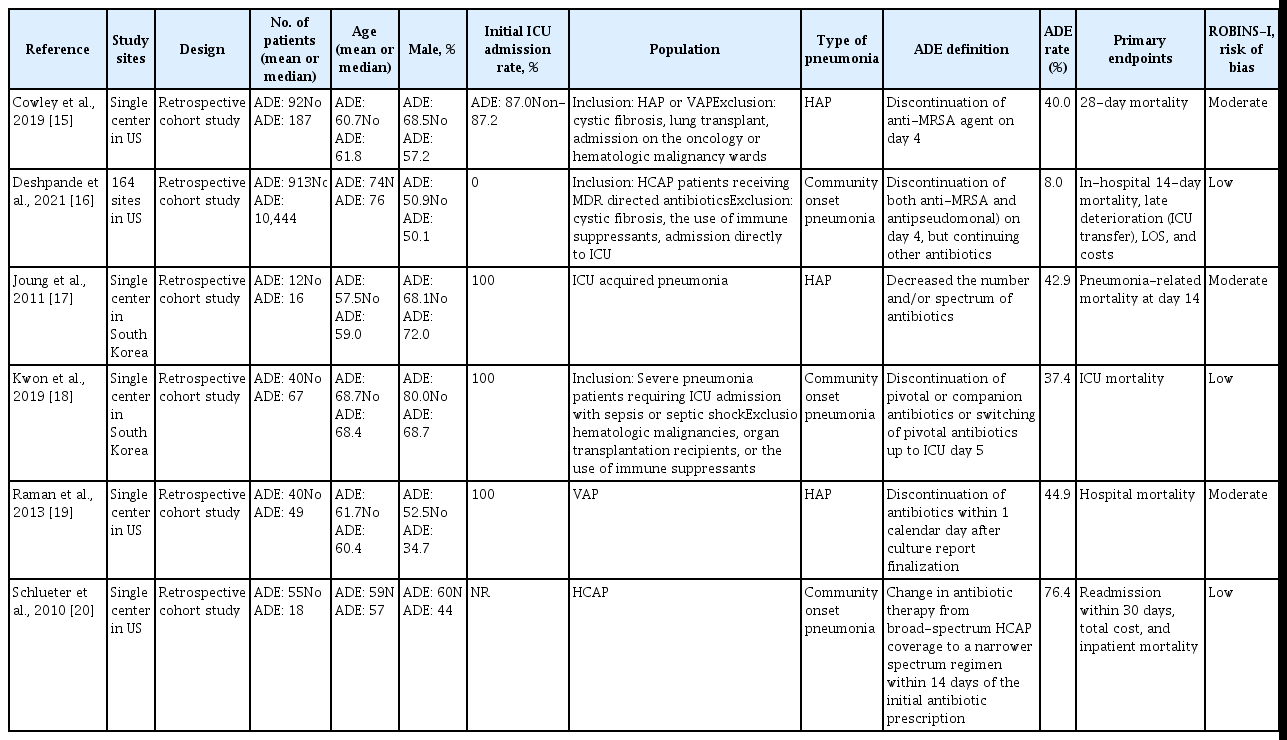
Table 1 summarizes the features of the selected studies. Our systematic review and meta-analysis included 11,933 patients, of whom 1,152 received the ADE strategy and 10,781 did not receive ADE. The type of pneumonia was community onset pneumonia in 3 studies [16,18,20] and hospital-acquired pneumonia (HAP) in 3 studies [15,17,19], respectively. All studies were published between 2010 and 2021. A quality assessment of the included studies is reported in the Supplementary Table. Overall, the methodological quality evaluated by the ROBINS-I appeared to be acceptable.
Primary outcome
Pooled estimates of strategy efficacy with respect to reduced in-hospital mortality rates by using ADE were weighed and combined with a generic inverse variance and random-effects model. Overall, the RR for in-hospital mortality indicated that ADE had a favorable effect compared with non-ADE (RR = 0.60, 95% CI = 0.38 to 0.93; Fig. 2), with a 40% absolute risk reduction. The heterogeneity was high (I2 = 66%). The results of Egger’s test in the included studies indicated no significant publication bias (p = 0.335), although a visual inspection of the plot suggested asymmetry (Supplementary Fig.).
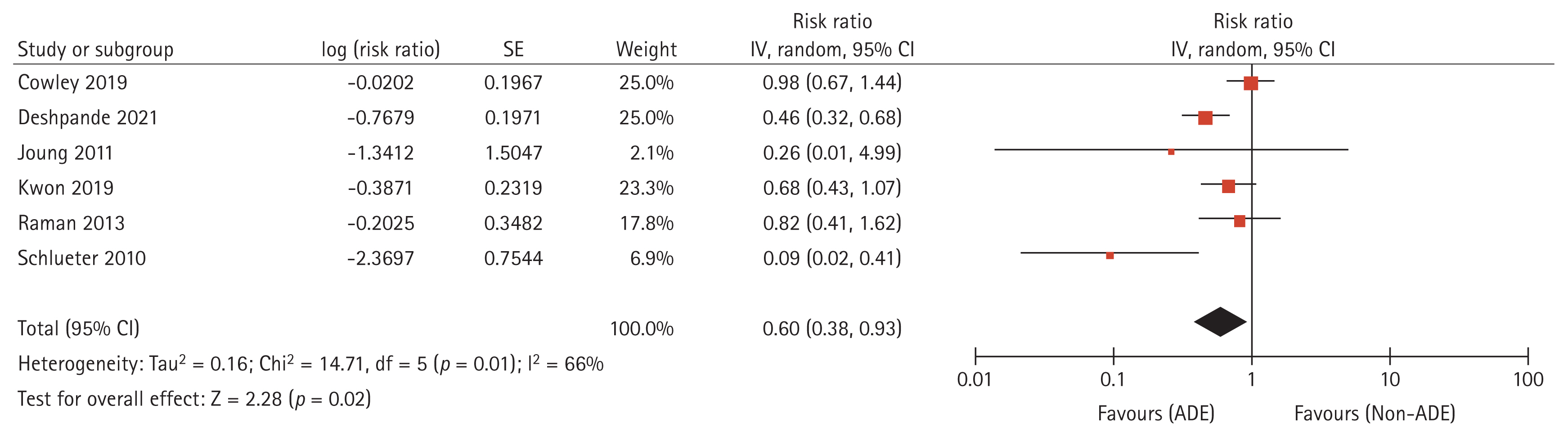
Forest plots for the risk ratio of in-hospital mortality in patients with culture-negative pneumonia receiving the ADE strategy, compared with non-ADE. ADE, antimicrobial de-escalation; CI, confidence interval; df, degrees of freedom; IV, inverse variance; SE, standard error.
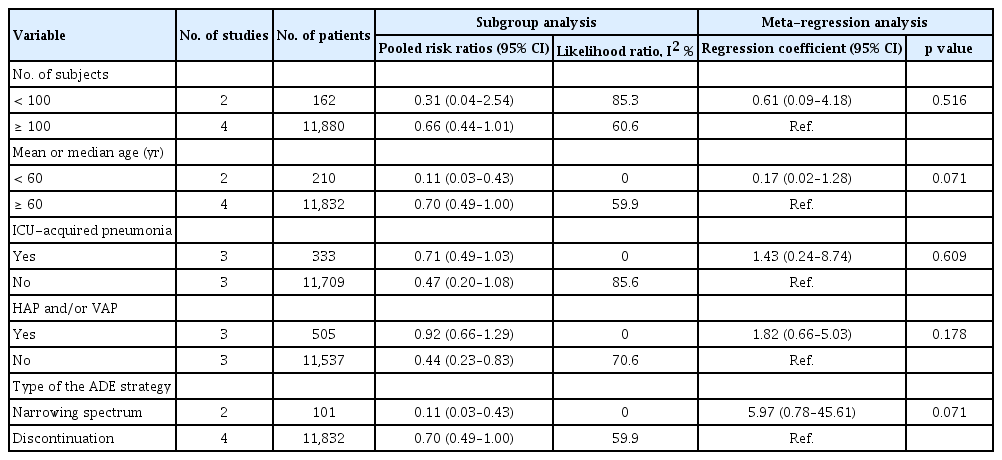
Because a substantial degree of heterogeneity existed among the included studies, we conducted subgroup and meta-regression analyses to explore heterogeneity according to strategy used in response to culture-negative pneumonia. Table 2 provides details of the subgroup and meta-regression analysis regarding the number of subjects (< 100 and ≥ 100), age (< 60 and ≥ 60 yr), ICU-acquired pneumonia, HAP or ventilator-associated pneumonia (VAP), and the type of ADE (discontinuation and narrowing spectrums). In the meta-regression analysis, probable sources of between-study heterogeneity for included studies were not found.
Secondary outcomes
Four studies reported data on the duration of antibiotic therapy [15,16,18,19]. The ADE strategy resulted in shorter durations of total and initial antibiotic administration compared with non-ADE (MD = −3.88 days, 95% CI = −6.21 to −1.54, p < 0.01, I2 = 98% and MD = −3.01 days, 95% CI = −6.93 to −0.91, p < 0.01, I2 = 99%, respectively) (Fig. 3A, B). Data regarding the total lengths of hospital stay, which were available for three trials, were analyzed using the generic inverse variation method [15,16,20]. The duration in total length of hospital stays was significantly shorter with ADE compared with non-ADE (MD = −3.85 days, 95% CI = −7.52 to −0.18, p = 0.05, I2 = 66%) (Fig. 3C). The mean ICU length of stay was similar between the ADE and non-ADE groups (MD = −0.93 days, 95% CI = −5.33 to 3.47, p = 0.09, I2 = 64%) (Fig. 3D) [15,18].
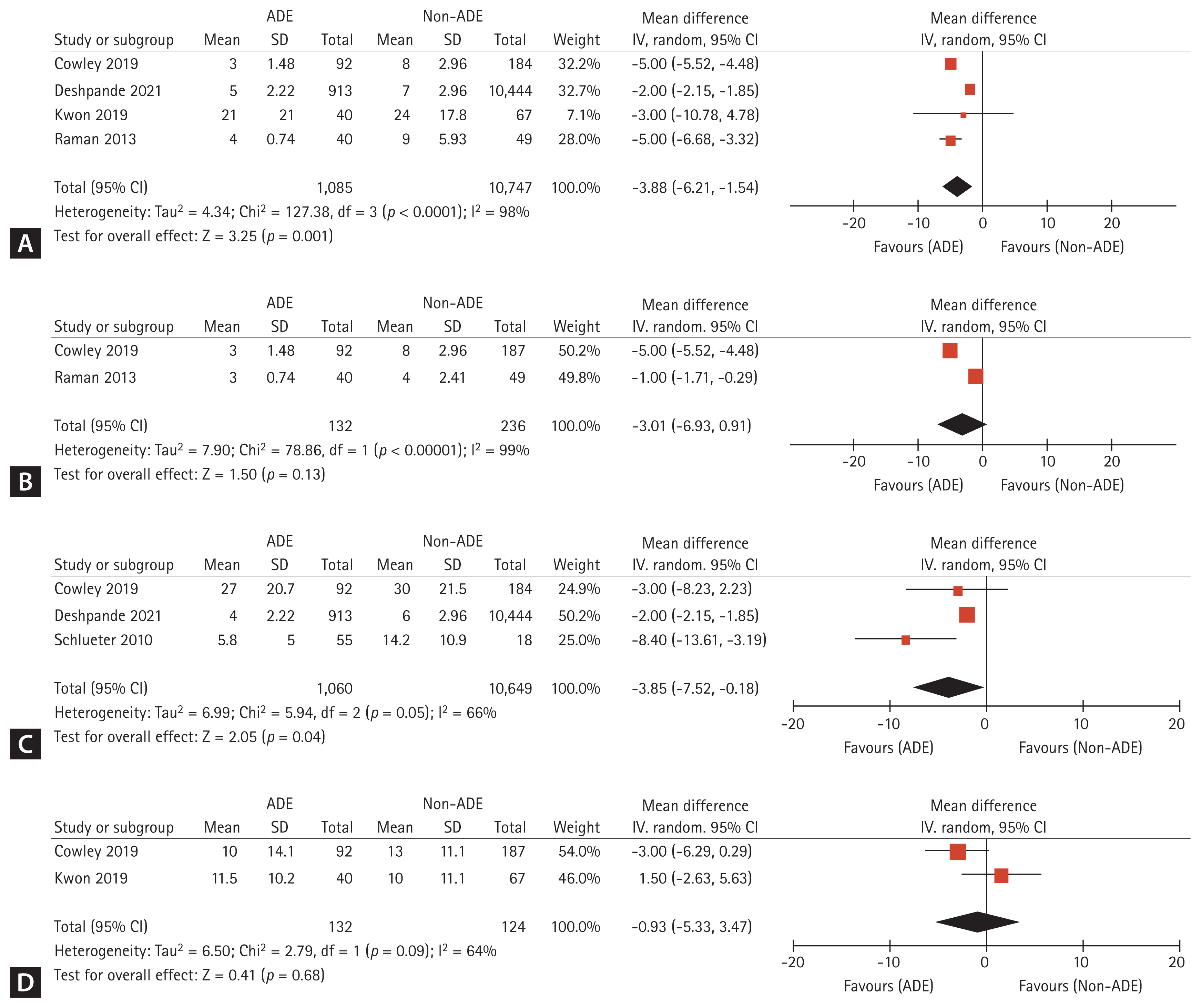
Forest plots for the mean difference of the duration of (A) total antibiotic therapy, (B) initial antibiotic therapy, (C) total length of hospital stay, (D) ICU length of stay in patients with culture-negative pneumonia receiving ADE, compared with non-ADE. ADE, antimicrobial de-escalation; CI, confidence interval; df, degrees of freedom; IV, inverse variance; SE, standard error.
DISCUSSION
Current guidelines for the management of adults with HAP or VAP recommend antibiotic therapy with de-escalation rather than fixed broad-spectrum antibiotic regimens, considering culture results and drug-susceptibilities [1,21]. A lack of de-escalation may increase the possibility of acquiring antibiotic resistance [1,21]. However, current guidelines do not provide guidance regarding ADE in culture-negative pneumonia [1,21]. Although physicians commonly consider ADE when microorganisms are detected, these cases represent less than 40% of all cases of pneumonia [8]. Major studies of treatment of pneumonia have focused on microbiologically confirmed disease [10]. Culture-negative pneumonia is a relatively common but understudied disease.
Previous studies of pneumonia patients with MDR risk factors found that patients with culture-negative pneumonia experienced less-severe illness and hospital mortality and shorter hospital stays compared to patients with culture-positive pneumonia [10,22]. We therefore hypothesized that fixed broad-spectrum antibiotics targeting MDR pathogens may be excessive in patients with culture-negative pneumonia.
To the best of our knowledge, few data are available to assess ADE in cases of culture-negative pneumonia. We investigated the clinical impact of ADE in culture-negative pneumonia through a systematic review and meta-analysis. The RR for in-hospital mortality indicated that ADE in these patients had a favorable effect compared with non-ADE, with an absolute risk reduction of 40%. All studies included in our analysis administered ADE based on clinical signs. Once clinical stability is achieved, ADE may be appropriate for patients with culture-negative pneumonia.
We found ADE was associated with additional favorable outcomes in culture-negative pneumonia, such as a shorter duration of total and initial antibiotic administration and duration of hospital stays. Because excessive antibiotic agents can cause the development of adverse drug effects and antibiotic resistance [1], our findings may be related to a reduction in these risks. A systematic review of 38 studies also found that antimicrobial stewardship in the ICU was associated with clinical improvement in antibiotic resistance and adverse events, exclusive of short-term outcomes [23]. In line with these findings, another observational cohort study of 1,995 adults with community onset pneumonia reported that broad-spectrum antibiotic use targeted MDR pathogens was associated with increased mortality, longer lengths of hospital stay, greater costs, and increased C. difficile infection [24]. Baseline characteristics and comorbidities were similar between patients receiving MDR directed antibiotics and those who did not [24]. These results tend to support our hypothesis.
Our research provided detailed information for a subgroup and meta-regression analysis. The efficacy of ADE for in-hospital mortality was examined for various clinical factors such as age, ICU setting, and HAP/VAP. Of these variables, patients less than 60 years of age, those with non-ICU–acquired pneumonia and CAP, and the narrowing spectrum of the strategy may be better positioned to benefit from ADE. However, we could not a significant factor for substantial between-study heterogeneity in the meta-regression analysis.
The cause of lower mortality with ADE was likely associated with the low severity of pneumonia, rather than the timing of the ADE. We attempted to investigate the severity of disease in the study populations. Among studies included in our analysis, only one addressed the severity of pneumonia, and the pneumonia severity index did not differ between the two groups studied [20]. Two trials used acute physiology and chronic health evaluation (APACHE) or sepsis-related organ failure assessments as ICU scoring systems [18,19]. These scores were similar between the two strategies. The remaining three studies did not describe the severity of illness. Because all studies included in our analysis had retrospective observational cohort designs, differences in severity of illness may exist between ADE and non-ADE, which may have affected the mortality rate.
The present study has some limitations. First, we identified no randomized controlled trials (RCTs) that directly compared the two strategies. All studies in the present analysis had an observational cohort study design and most included studies that collected data from a relatively few subjects. The visual funnel plot asymmetry in our analysis may be an indicator of the effects of the small study sizes. Second, enrolled studies in our analysis were conducted in only two countries, the US and South Korea, making it difficult to generalize the results to all patients with culture-negative pneumonia. Third, no apparent consensus on the definition of the ADE has emerged, and the included studies used different definitions of ADE. The selected studies included heterogenous populations with healthcare associated pneumonia, HAP/VAP, and ICU-acquired pneumonia, which might have introduced bias, and our findings should be interpreted with caution.
CONCLUSIONS
In a systematic review and meta-analysis, we found that ADE would be statistically associated with favorable outcomes in patients with culture-negative pneumonia. Although the present study suffers from between-study heterogeneity, a subgroup and meta-regression analysis could not reveal considerable sources of heterogeneity. Further large-scale RCTs are warranted to assess the efficacy of ADE in culture-negative pneumonia.
KEY MESSAGE
1. The ADE strategy seems to be associated with better clinical outcomes compared with non-ADE in culture-negative pneumonia.
Notes
CRedit authorship contributions
Jae-Uk Song: conceptualization, data curation, formal analysis, methodology, writing - original draft; Jonghoo Lee: conceptualization, data curation, formal analysis, methodology, writing - original draft
Conflicts of interest
The authors disclose no conflicts.
Funding
This work was supported by a research grant from Jeju National University Hospital in 2022.
Availability of data and materials
All data generated or analyzed during this study are included in the published article.