Clinical efficacy of angiotensin receptor-neprilysin inhibitor in de novo heart failure with reduced ejection fraction
Article information
Abstract
Background/Aims
We aimed to analyze the efficacy of angiotensin receptor-neprilysin inhibitor (ARNI) by the disease course of heart failure (HF).
Methods
We evaluated 227 patients with HF in a multi-center retrospective cohort that included those with left ventricular ejection fraction (LVEF) ≤ 40% undergoing ARNI treatment. The patients were divided into patients with newly diagnosed HF with ARNI treatment initiated within 6 months of diagnosis (de novo HF group) and those who were diagnosed or admitted for HF exacerbation for more than 6 months prior to initiation of ARNI treatment (prior HF group). The primary outcome was a composite of cardiovascular death and worsening HF, including hospitalization or an emergency visit for HF aggravation within 12 months.
Results
No significant differences in baseline characteristics were reported between the de novo and prior HF groups. The prior HF group was significantly associated with a higher primary outcome (23.9 vs. 9.4%) than the de novo HF group (adjusted hazard ratio 2.52, 95% confidence interval 1.06–5.96, p = 0.036), although on a higher initial dose. The de novo HF group showed better LVEF improvement after 1 year (12.0% vs 7.4%, p = 0.010). Further, the discontinuation rate of diuretics after 1 year was numerically higher in the de novo group than the prior HF group (34.4 vs 18.5%, p = 0.064).
Conclusions
The de novo HF group had a lower risk of the primary composite outcome than the prior HF group in patients with reduced ejection fraction who were treated with ARNI.
INTRODUCTION
Heart failure (HF) is a clinical syndrome that typically presents with symptoms associated with an inadequate cardiac output. Structural remodeling–along with a compensatory response to HF–is another mechanism of disease progression that leads to exacerbation of HF. This condition has been known to be fatal, with a 5-year mortality rate exceeding 50% from the onset of symptoms [1].
Angiotensin receptor-neprilysin inhibitor (ARNI) has a dual effect of blocking the renin-angiotensin-aldosterone system pathway and modulating the natriuretic peptide system [2], which could be more effective for prevention of HF progression. Thus, in recent years, there has been an increasing interest in the efficacy of ARNI for HF management.
In the Prospective comparison of ARNI with Angiotensin-converting Enzyme Inhibitors (ACEIs) to Determine Impact on Global Mortality and morbidity in Heart Failure (PARADIGM-HF) trial, it was noted that ARNI was superior to ACEI treatment in reducing the risk of cardiovascular (CV) death and hospitalization for HF (HHF) among patients with chronic HF [3]. Similar results were observed in the comparison of sacubitril-valsartan versus enalapril on effect on NT-pro BNP in Patients Stabilized from an Acute Heart Failure Episode (PIONEER) trial for patients with acute decompensated HF (ADHF) [4]. Based on these studies, guideline-directed medical therapy (GDMT), which includes ARNI, is strongly recommended for patients with HF to reduce mortality [5,6]. Moreover, remarkable results were obtained regarding the feasibility and safety of early initiation of ARNI treatment after stabilization of ADHF in the TRANSITION trial and its subsequent study [7,8]. However, studies on how the effects of ARNI treatment vary according to the underlying HF status and disease duration are still lacking. Therefore, we aimed to evaluate the clinical efficacy of ARNI treatment in de novo patients with HF with reduced ejection fraction (HFrEF) compared to those with a previous history of HF.
METHODS
Study population
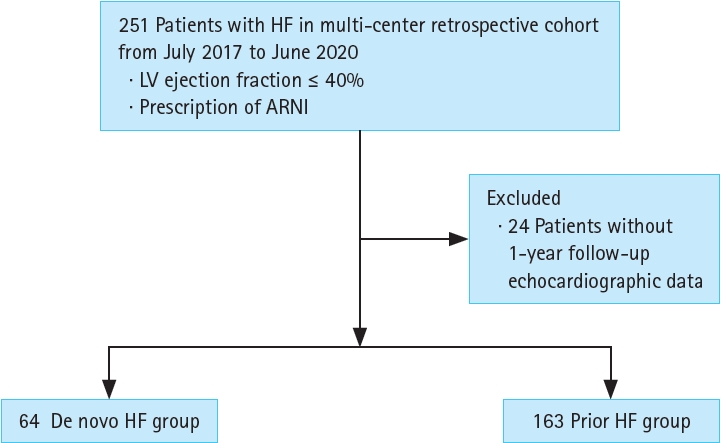
We evaluated patients with HF enrolled in the RECORD SV registry (cris.nih.go.kr; Identifier: KCT0005747). Briefly, the RECORD SV registry is a retrospective multicenter cohort study of 251 patients with HF treated with ARNI between July 2017 and June 2020, who had been followed for > 1 year until June 2021. Patients who met all the following criteria were enrolled in this registry: (1) left ventricular ejection fraction (LVEF) < 40% and (2) patients who had been treated with ARNI. We excluded 24 patients who had no echocardiography records from their 1-year follow-up (Fig. 1). We defined patients with ‘de novo HF’ as those who were newly diagnosed with HF and ARNI treatment was started within 6 months of HF diagnosis. The patients with ‘prior HF’ were defined as those who were diagnosed with or hospitalized for aggravation of HF > 6 months before the initiation of ARNI treatment. The study protocol was approved by the Institutional Review Board of Soonchunhyang University Bucheon Hospital (approval number: 2020-11-010). The need for informed consent by the participants was waived by IRB approval.
Data collection
The following data were collected from the RECORD-SV registry: key patient demographic data including age, sex, etiology, comorbidities, relevant information about their HF status, HF medication history, relevant laboratory tests, and echocardiography reports. Ischemic etiology was defined as development of HF in patients who have a history of myocardial infarction (MI), percutaneous coronary intervention, coronary artery bypass graft surgery, moderate or severe coronary artery disease (> 50% stenosis) or vasculitis, and did not have other that could cause HF.
Echocardiographic data were collected from the medical records. Transthoracic echocardiography (TTE) was performed at the time of study enrollment and after 12 ± 6 months from enrollment. LVEF was measured by the biplane Simpson technique, and other variable parameters were measured as per the standard protocol.
Outcomes
The primary outcome was a composite of CV death and worsening of HF within 12 months. Worsening of HF included HHF and an urgent visit to the emergency room (ER) for HF symptoms. We defined urgent ER visits as visits required for patients who needed HF management, including intravenous therapy without hospitalization. HHF was defined as admission for symptom control and further HF management. The secondary outcomes were as follows: (1) clinical events of worsening HF for 1 year; (2) CV death within 1 year; (3) changes in echocardiographic parameters, including LVEF, left atrial volume index (LAVI), early diastolic mitral inflow velocity/early diastolic mitral annular tissue velocity (E/e’), LV mass index (LVMI), LV end-systolic volume (LVESV), and LV end-diastolic volume (LVEDV) after 1 year; (4) discontinuation rate of loop diuretics use after 1 year in patients who had taken diuretics before. All occurrences of clinical events were identified from the medical records.
Statistical methods
All continuous variables were reported as mean with standard deviation or median with interquartile range (IQR). Categorical variables were reported as numbers and frequencies (%). A normality test was performed by using the Shapiro–Wilk test. Continuous variables were tested by the two-sample t-test and categorical variables were tested by the chi-squared test to identify differences. Incidence rates for outcome were calculated per 100 person-years of follow-up days. Cumulative incidences of clinical outcomes were calculated using the Kaplan–Meier (KM) method with the log-rank test. Cox regression analysis was performed to evaluate the association between variables and clinical outcomes and presented with 95% confidence intervals (CIs). We adjusted the variables with clinical and statistical significance for multi-varied Cox regression analysis. All p values were two-sided and a value of p < 0.05 was considered statistically significant. All the statistical analyses were performed by Rex software (Version 3.6.0; RexSoft Inc., Seoul, Korea).
RESULTS
Baseline characteristics
Our study included a total of 227 patients, 163 of whom had a prior HF diagnosis while 64 had de novo HF. The baseline characteristics of the two groups are shown in Table 1. There were no significant differences between the two groups for age, sex, and comorbidities, such as hypertension (HTN), diabetes mellitus, atrial fibrillation, history of previous MI, and chronic kidney disease.
Primary outcome
The median follow-up duration was 365 days (IQR, 333–388 d). In total, 45 cases of primary composite outcomes occurred during the 12 months follow-up period―6 cases (9.4%) occurred in the de novo HF group and 39 cases (23.9%) in the prior HF group (Table 2). Moreover, the prior HF group had a higher risk of primary outcome than the de novo HF group as per the KM curves (Log-rank p = 0.014) (Fig. 2). In total, four cases of CV death occurred during the follow-up period, and all of them were identified as patients from the prior HF group. Three patients experienced prior HHF aggravation. Of all the patients, 9.4% in the de novo and 23.3% in the prior HF group experienced worsening of HF symptoms.
The prior HF group had a higher risk of primary composite outcomes than the de novo HF group. In multi-variate Cox regression analysis, previous history of HF was an independent prognostic factor of a primary outcome in comparison with the de novo group (adjusted hazard ratio [HR] 2.52, 95% CI 1.06–5.96, p = 0.036). In addition, HTN (adjusted HR 2.69, 95% CI 1.25–5.79, p = 0.011) was associated with an increased risk of a primary outcome. Increase in LVEF after 1 year by 1% corresponded to a decrease in the primary outcome by 0.04 times (adjusted HR 0.96, 95% CI 0.93–0.98, p = 0.004) (Table 3).
Echocardiography data
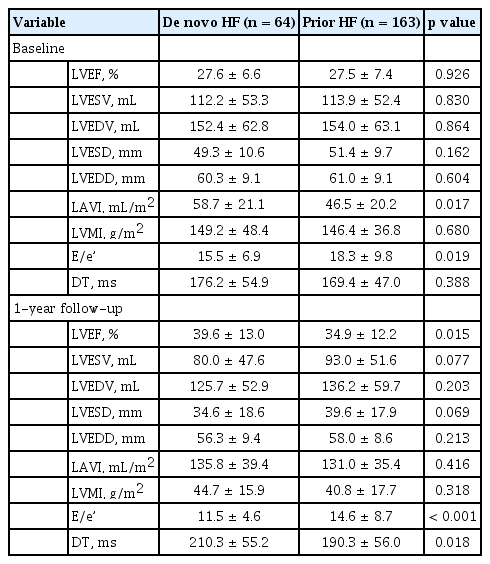
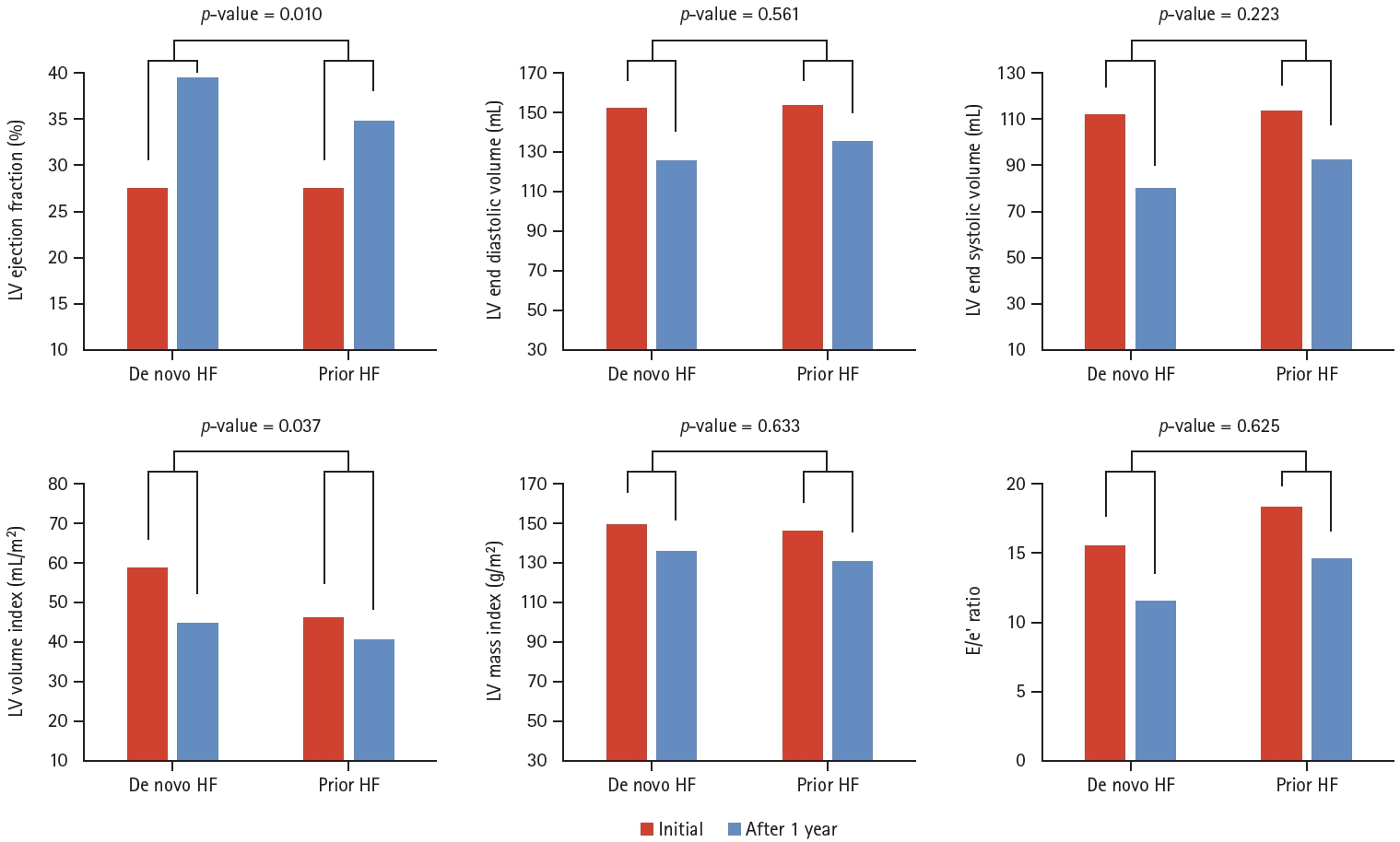
Table 4 shows the echocardiographic data at hospitalization and at the time of 1-year follow up. In the initial TTE data, there were no significant differences in LVEF (27.6 vs. 27.5%, respectively) and other parameters including LAVI, LVMI, E/e’, LVESV, and LVEDV. However, after 1 year, LVEF showed greater improvement in the de novo HF group as compared to the prior HF group (LVEF 39.6 vs. 34.9%, p = 0.015). In Figure 3, the values of average LVEF increase in each group were 12% for the de novo HF group and 7.4% for the prior HF groups, respectively (p = 0.010). LVESV and LVEDV decreased in both the groups (-26.6 vs. -22.4 mL in LVEDV, p = 0.561, -32.1 vs. -23.6 mL in LVESV, p = 0.223). Additionally, LAVI greater decrease in the de novo HF group was observed as compared to the prior HF group (-14.0 vs. -5.7 mL/m 2 , p = 0.037). Further, E/e’ was higher in the prior HF patients compared to the de novo HF patients, both at initial hospitalization (15.5 vs. 18.3, p = 0.019) and after 1 year (11.5 vs. 14.6, p < 0.001).
The usage of HF medications
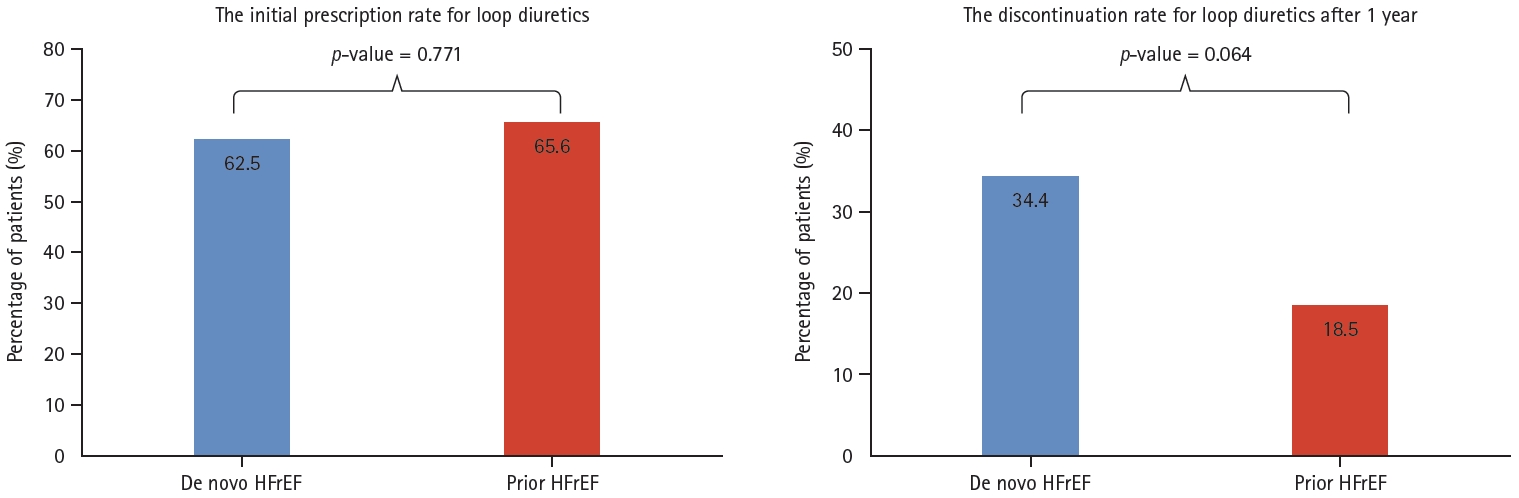
There were no significant differences in the concomitant use of HF medications between the two groups, except for mineralocorticoid receptor antagonist (MRA) (Table 5). Upon comparison of the initial dosage of ARNI, it was observed that the prior HF group had a higher proportion of patients were administered ARNI at daily doses of 200 mg or more as compared to the de novo HF group (52.8 vs. 31.2%, p = 0.001). However, after 1 year, there was no difference in ARNI dosage distribution (p = 0.520) (Fig. 4). In addition, the initial use of loop diuretics did not differ between the two groups (62.5% in de novo HF vs. 65.6% in prior HF group, p = 0.771). However, the rate of discontinuation of diuretics after 1 year was higher in the de novo HF group as compared to the prior HF group (34.4 vs. 18.5%, p = 0.064) (Fig. 5).
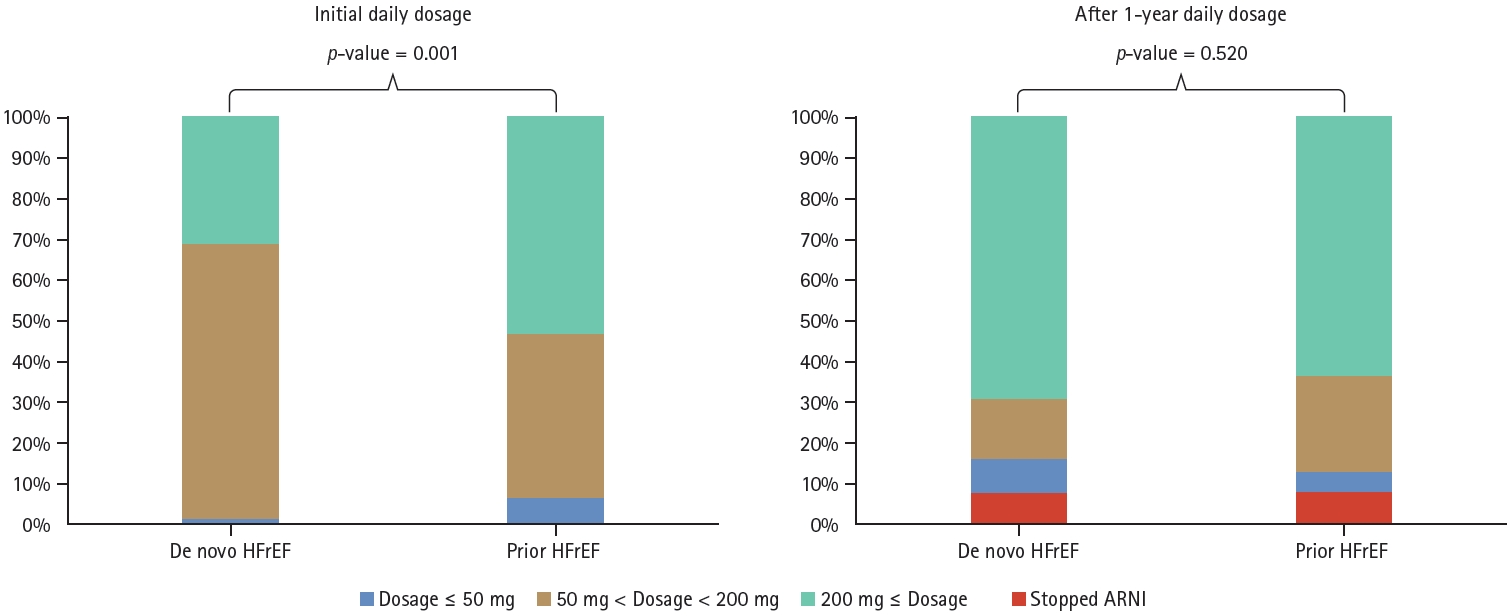
ARNI daily dosage prescription pattern. ARNI, angiotensin receptor-neprilysin inhibitor; HFrEF, heart failure with reduced ejection fraction.
DISCUSSION
Our study revealed a reversal of remodeling effects as well as clinical benefits associated with ARNI treatment in patients with de novo HF. The main findings of our study were as follows: (1) The risk of a primary composite outcome including CV death and worsening HF was lower in the de novo HF group than the prior HF group. (2) There was a significant improvement in patients with LVEF in the de novo HF group compared to the prior HF group. (3) Also, previous history of HF as well LVEF after 1 year were both independent prognostic factors of a primary composite outcome.
Clinical outcomes and cardiac reverse remodeling
Currently, several studies have reported on the efficacy and safety of ARNI treatment [3,4,9]. However, there is limited data on the prognostic implications of ARNI administration according to the disease course of HF. Thus, we analyzed the clinical outcomes of ARNI by classifying HF patients into two categories (de novo vs. prior HF). First, we discovered that the de novo HF group had a lower risk of a composite outcome, including HF-related hospitalization and CV death as compared to the prior HF group. These findings were consistent with the TRANSITION subgroup analysis data that had reported a greater benefit for biomarker and clinical events with initial ARNI therapy as compared to that with other GDMTs in the de novo group compared to the prior HF group [8].
Additionally, in our study, the HR for a primary outcome was associated with LVEF at 1 year, which had a negative correlation with the HR of a primary composite outcome. LVEF improvement could be described as a kind of cardiac reverse remodeling (CRR) effect for ARNI therapy. Currently, there have been several studies demonstrating the efficacy of ARNI for CRR [10-12]. In the above studies, CRR was regarded as an improvement of LVEF, LV volume, or dimensional index. These studies demonstrated a favorable CRR effect with ARNI treatment. However, it remains controversial whether patients with HF with long-standing clinical symptoms and signs would respond to therapy. One prospective study on patients with HFrEF demonstrated the effect of treatment with ARNI on both LV and LA reverse remodeling, despite the inclusion of patients with long-lasting disease [12]. In addition, Maizels et al. [13] reported that the effect of ARNI therapy on CRR was greater in selective patients, especially in those receiving ARNI treatment within 3 years from HF diagnosis, thereby demonstrating that they had greater LVEF improvement than other patients. Similarly, our study showed that increase of LVEF in the de novo group was approximately 1.5 times higher than that in the prior HF group (12.0 vs. 7.4%, p = 0.010).
Pharmacologically, ARNI treatment could attenuate cardiac structural remodeling by diminishing myocardial cell death, impaired myocyte contraction, and hypertrophy [14]. However, this reversible effect may be difficult to attain if the underlying structural abnormality has already progressed considerably. A Korean study reported that patients with de novo HF had more remarkable prognostic effects of GDMT than patients with prior HF [15]. They showed that early initiation of ACEI or ARB therapy during the course of HF progression reduced the rate of rehospitalization, and one of the mechanisms for this is early neurohormonal modulation affecting cardiac remodeling.
The authors of the Korean study also suggested differences in comorbidities as another mechanisms that may lead to different clinical outcomes [15]. In concordance with this study, we identified differences in prognoses between different types of HF―the prior HF group had a poorer prognosis than that observed in the de novo HF group. However, there was no significant difference in comorbidities between the prior and de novo HF groups, unlike that in previous studies which demonstrated higher comorbidities in patients with prior HF [15,16]. Instead, our data showed significantly higher LVEF improvement in patients with de novo HF, and LVEF was an independent prognostic factor of primary composite outcome including HHF or CV death. This finding is consistent with data from the European Society of Cardiology-Heart Failure (ESC-HF) registry on the association between LVEF and HF outcome [17]. Considering these findings, although we could not make clear the pathophysiologic mechanisms for the difference in prognoses according to the course of HF, we suspect that ARNI therapy in the early stage of HF may have a more positive effect on CRR and that it may be associated with a reduced risk of clinical outcomes.
The dosage of ARNI and its effect
Interestingly, in our data, the distribution of initial ARNI dosage was different between the two groups, showing that there were more patients with a lower ARNI dosage in the de novo than the prior HF group. However, after 1 year, the proportion of patients with a daily ARNI dose ≥ 200 mg increased in both the groups, and there were no significant differences in the distribution pattern between them (Fig. 4). Our data showed that the proportion of patients with a daily dose of 200 mg or more increased from 46.7% to 64.9% in all the patients, of which 38% reached the target dose. This was consistent with the findings of a retrospective cohort study conducted on the Korean population, which showed that the proportion of patients with a daily dose ≥ 200 mg increased from 33% at baseline to 53% at the 12-month follow-up, and the proportion of patients on the target dose increased from 4.8% to 25% [18]. Our study confirmed the findings of the previous Korean cohort study.
Furthermore, in our study, ARNI treatment was stopped in 16 patients (8.4%) because of severe adverse effects or intolerability such as hyperkalemia, hypotension, or acute renal failure. There were no statistically significant differences in the discontinuation rates of each group (8.2 vs. 8.5%, p > 0.99). This result aligns with the findings of the previous TRANSITION study, demonstrating that ARNI therapy is tolerated and safe in the de novo group compared to the prior HF group [8]. In the TRANSITION study, the prior HF group had more comorbidities such as old age, ischemic etiology, and lower eGFR as compared to the de novo HF group; hence, patients with previous HF could be more vulnerable to adverse events. However, in our study, there were no significant differences in the aforementioned factors between prior and de novo HF groups. These findings could explain why the differences in ARNI discontinuation rates between the two groups were smaller than those of the TRANSITION study.
Additionally, we observed that the incidence of clinical events was lower in the de novo HF group, even though these patients were exposed to a lower initial dose than the prior HF group. According to a Korean prospective observational study [19], dosage titration with a very low initial dose (25 mg twice daily) had a similar clinical event rate (CV death and HHF) compared to initiation with a standard dose (50 mg twice daily). They suggested that initiation with a very low ARNI dose could be available for those patients who cannot use the standard dose for some reason. Thus, results from our study supplement previously reported evidence that early ARNI therapy during the course of the disease may be a useful clinical strategy, although with administration of a very low dose.
The rate of discontinuation of loop diuretics and use of angiotensin receptor-neprilysin inhibitor
When we analyzed the use of diuretics during the follow up period, there was no significant difference in the initial use between the two groups (62.5% in de novo HF vs. 65.6% in prior HF, p = 0.771). However, the discontinuation rate of loop diuretics after 1 year was numerically higher in the de novo HF group (34.4% in de novo HF vs. 18.5% in prior HF), even though this difference narrowly missed the statistical significance (p = 0.064) (Fig. 5).
In the post-hoc analysis of the PARADIGM-HF study, the effect of ARNI therapy on dose reduction of diuretics was verified [20]. Patients randomized with respect to ARNI treatment had lower use of diuretics at 24 months (net reduction 6.1%, p < 0.001) relative to ACEI [20]. Additionally, in a retrospective single-center study, decrease in the dosage of loop diuretics was achieved in 37.2% of patients and mean reduction of furosemide was 10 ± 38 mg across the entire population treated with ARNI [21]. The use of loop diuretics is an indicator that reflects clinical volume status. Thus, the effect on diuretics dose reduction of ARNI may be due to potential natriuretic effects by inhibiting degradation of natriuretic peptides [2,20]. From this point of view, the use of ARNI may be effective for discontinuation of diuretics, which might be stronger at the de novo stage of HF.
Hypertension and poor clinical outcome
Several studies have reported a J-shaped curve pattern between CV outcomes and blood pressure rather than a linear correlation [22,23]. Similarly, a study of Korean HF registry data demonstrated a reverse J-shaped curve pattern, whereby mortality and readmission rates for HF increased with treatment both in cases low and high blood pressure [24]. However, in our study, HTN was defined as a disease state, rather than being solely characterized by blood pressure levels. Moreover, the relationship between HTN and HF is complex. HTN is generally known to be one of the most powerful risk factors for the development of HF. Although diastolic dysfunction, which follows left ventricular hypertrophy, is the most common form, HTN also increases the risk for HFrEF [25]. In addition, Lloyd-Jones et al. [26] showed that the lifetime risk of congestive HF is largely attributable to HTN. HTN could be advantageous, since an acceptable high systolic blood pressure can allow the use of a higher dose of RAS blocker or beta-blockers, leading to better outcome in HF patients. However, considering the physiology of the disease itself and the mechanism that causes cardiac remodeling by RAS activation, we speculate that HTN is a factor that influences poor clinical outcomes observed in our data.
Limitations
There were several limitations of this study. First, our study was limited by its retrospective nature, and there is a possibility that several confounding biases may have been introduced. Furthermore, we have analyzed data with limited information, such as the exact prevalence period of prior HF and the number of previous hospitalizations for HF. Thus, a prospective large-scale study with randomization is needed to support the results of our study. Next, upon comparing concomitant use of GDMTs, no significant differences in the prescribed pattern except for MRA were observed. In terms of MRA use, we found that the de novo HF group used more spironolactone than the prior HF group (70% vs. 50%, respectively). These results may be due to the recent emphasis on the use of MRA as a guideline recommendation [5,6]. However, we performed Cox regression analysis to evaluate the independent predictors of primary outcome and observed that the initial use of spironolactone did not influence the outcome. Thus, the impact of differences in the MRA prescription on prognosis may be small in this study.
Conclusion
Among patients with HFrEF who were initiated on treatment with ARNI, those with de novo HF had a better clinical outcome than those with prior HF. These results may be due to an earlier exposure to ARNI in patients with de novo HF as compared to patients with prior HF.
KEY MESSAGE
1. In patients with HFrEF treated with ARNI, patients with de novo HF had a better clinical outcome than those with a previous history of HF, possibly due to an earlier exposure to ARNI.
2. Earlier exposure to ARNI may lead to a better clinical outcome in patients with HFrEF.
Notes
CRedit authorship contributions
Su Yeong Park: writing - original draft; Min Gyu Kong: conceptualization, writing - review & editing; Inki Moon: data curation; Hyun Woo Park: data curation; Hyung-Oh Choi: data curation; Hye Sun Seo: data curation; Yoon Haeng Cho: data curation; Nae-Hee Lee: data curation; Kwan Yong Lee: data curation; Ho-Jun Jang: data curation; Je Sang Kim: data curation; Ik Jun Choi: data curation; Jon Suh: project administration
Conflicts of interest
The authors disclose no conflicts.
Funding
This work was supported by the Soonchunhyang University Research Fund.