Sex differences in chronic obstructive pulmonary disease characteristics: the Korea National Health and Nutrition Examination Survey 2007–2018
Article information
Abstract
Background/Aims
Chronic obstructive pulmonary disease (COPD) is less prevalent in females than males, but it affects mortality in females. There may be sex differences in the clinical characteristics of COPD.
Methods
We analyzed the Korea National Health and Nutrition Examination Survey dataset from 2007 to 2018. We compared the clinical characteristics and comorbidities in subjects with COPD according to sex. We adjusted the multivariate logistic regression of lung cancer prevalence according to COPD and sex by age and smoking amount.
Results
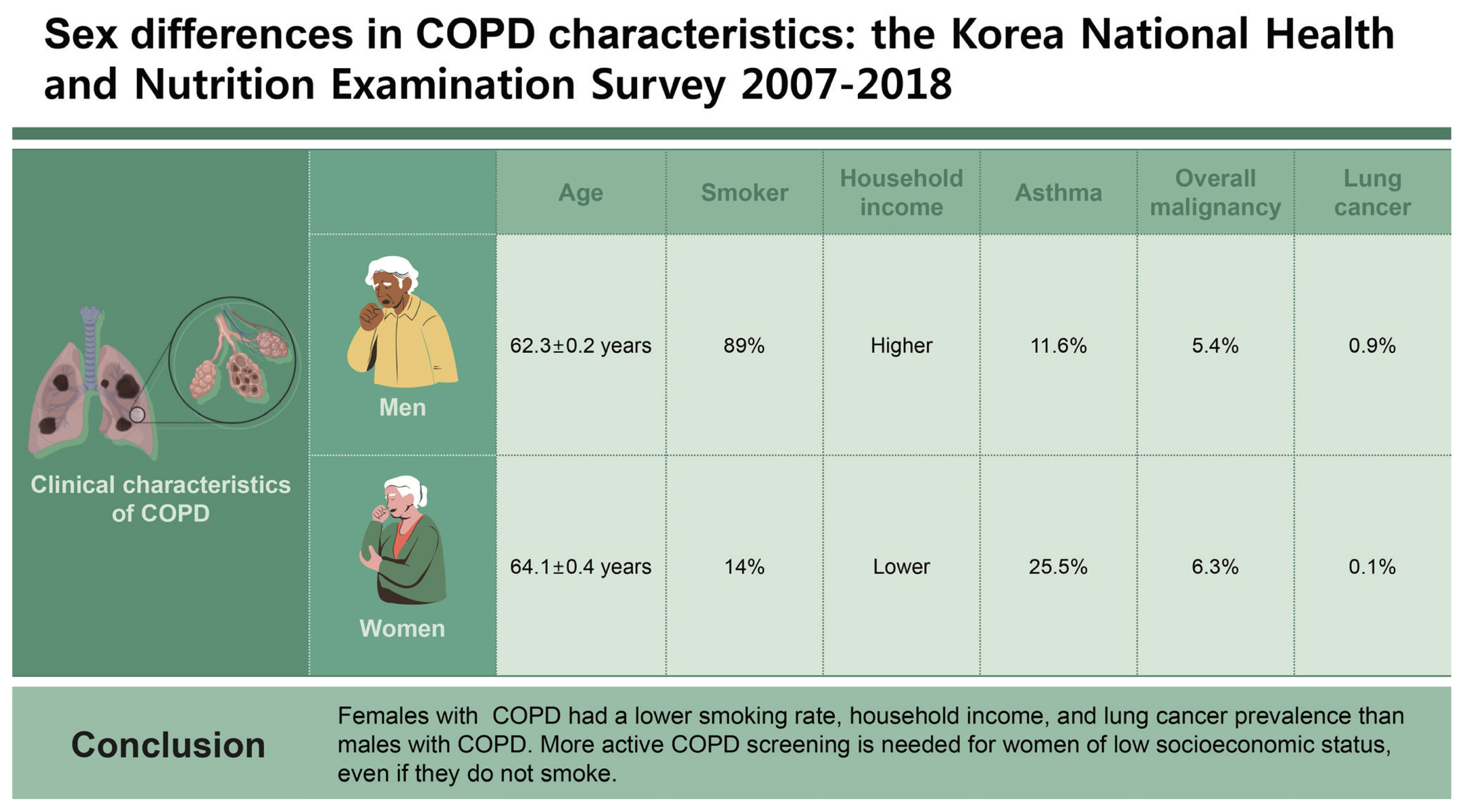
Females with COPD tended to be older than males with COPD (64.1 ± 0.4 yr vs. 62.3 ± 0.2 yr, respectively, p < 0.001). Approximately 89% of males with COPD had a smoking history, while 86% of females with COPD were non-smokers (p < 0.001). Household income was lower (p < 0.001) and asthma and overall malignancy were more prevalent in females with COPD than males with COPD (25.5 vs. 11.6%, respectively, p < 0.001; (6.3 vs. 5.4%, respectively, p < 0.001). However, lung cancer was more common in males with COPD than females with COPD (0.9 vs. 0.1%, respectively, p < 0.001). Lung cancer prevalence increased in males with moderate COPD compared to subjects without COPD (OR, 4.409; 95% CI, 1.741–9.419).
Conclusions
Females with COPD had a lower smoking rate, household income, and lung cancer prevalence than males with COPD. More active COPD screening is needed for women of low socioeconomic status, even if they do not smoke.
INTRODUCTION
Globally, chronic obstructive pulmonary disease (COPD) is the third leading cause of death [1]. Approximately 3.2 million people died from COPD in 2015 [2], and its prevalence and mortality rate is increasing [1,2]. COPD is characterized by persistent respiratory symptoms and airflow limitation [3], which manifest the airway and/or alveolar abnormalities typically caused by exposure to noxious particles or gases. Cigarette smoking is the most important risk factor for COPD, but factors such as indoor air pollution, work environment exposure, and passive smoking also contribute to its development [4,5]. Average smoking exposure is associated with an increased risk of COPD [6]. There is a dose-response relationship between smoking and a decline in lung function [5,7]; however, this relationship varies by individual. A substantial proportion of patients with COPD have never smoked [8], while others have minimal to no symptoms despite a history of heavy smoking [9].
A recent systematic review and meta-analysis reported that a COPD prevalence of 9.2% in men and 6.2% in women [10]. However, COPD is gradually becoming an important factor affecting women’s mortality and quality of life [11]. In general, the smoking rate is lower in women than men [12,13], but women generally experience a greater decline in lung function than men at the same smoking exposure. There may be sex differences in lung damage susceptibility associated with cigarette smoking [14], and women who have never smoked may have prolonged exposure to organic dust [8]. Therefore, the phenotypes of women with COPD may differ from those of men with COPD, which has not been evaluated on a nationwide scale. In the present study, we aimed to identify the difference in baseline characteristics and comorbidities, including lung cancer, in patients with COPD according to sex using a Korean nationwide database.
METHODS
Korea National Health and Nutrition Examination Survey
The Korea National Health and Nutrition Examination Survey (KNHANES) is an ongoing, population-based, nationwide, cross-sectional survey conducted annually by the Korea Disease Control and Prevention Agency (KDCA), which has been assessing the health and nutritional status of Koreans since 1998. The KNHANES represents the general Korean population because it involves a stratified, multistage, clustered sampling based on national census data [15]. Health trends can be deduced by merging yearly data. The KDCA institutional review board approved all KNHANES survey protocols (2015-01-02-6C). The questionnaire and dataset had guidelines for calculating a health-related index indicated by the KDCA (available at https://knhanes.kdca.go.kr/knhanes/main.do). We evaluated data from 2007 to 2018 as KNHANES has used spirometry to measure pulmonary function tests (PFTs) since 2007.
The study protocol was reviewed and approved by the Institutional Review Board of Chung-Ang University Hospital (approval no. 2312-004-19500). The requirement for informed consent was waived as all subjects were de-identified.
COPD diagnosis
PFT was performed on subjects older than 40 years of age during the KNHANES health examination by well-trained technicians according to the guidelines [16,17]. Because post-bronchodilator PFT was not available in the KNHANES dataset, we used pre-bronchodilator PFT findings. A diagnosis of COPD was established based on PFT parameters using the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines [3]. Subjects with a ratio of forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) < 0.7 were determined to have COPD. Airflow limitation severity was classified according to the GOLD criteria, as follows: GOLD I (mild): FEV1 ≥ 80%; GOLD II (moderate): 50% ≤ FEV1 < 80%; GOLD III (severe): 30% ≤ FEV1 < 50%; GOLD IV (very severe): FEV1 < 30% predicted [3].
Demographic data
We collected sociodemographic data, such as age, sex, body mass index (BMI), waist circumference, household income level, educational level, and marital status. Household income was divided into four quartiles: the lowest quartile, lower-middle quartile, upper-middle quartile, and highest quartile. Educational levels were divided according to graduation level (elementary school, middle school, high school, and college or more). Marital status was classified as not married, married, married but spouse deceased, and divorced). Comorbidities included a self-reported physician diagnosis of pulmonary tuberculosis, asthma, hypertension, diabetes, chronic renal disease, liver cirrhosis, and malignancies, including lung cancer. We obtained urinary cotinine and laboratory results, including white blood cells, hemoglobin, alanine aminotransferase, creatinine, fasting glucose, glycated hemoglobin levels, and lipid profiles.
Smoking status
All subjects were asked if they had smoked at least 100 cigarettes (five packs) in their lifetime. Those who answered ‘no’ were categorized as ‘non-smokers.’ Subjects who indicated that they do not currently smoke but have smoked more than 100 cigarettes were categorized as ‘ex-smokers,’ and those who answered ‘yes’ were defined as ‘current smokers.’
Statistical analysis
Continuous variables are presented as means ± standard deviations and categorical data as frequencies and percentiles. We performed an intergroup comparison of demographic data and airflow obstruction severity between men and women with COPD by t-test and an intergroup comparison of categorical data between men and women with COPD with the chi-square test. Major comorbidities included pulmonary tuberculosis, asthma, hypertension, diabetes, chronic renal disease, liver cirrhosis, and malignancies, including lung cancer. We adjusted the multivariate logistic regression of the lung cancer prevalence according to COPD severity by age and smoking amount, and then calculated the odds ratio (OR) and 95% confidence intervals (CIs). The results were analyzed using SPSS statistics complex samples procedures (version 26.0; IBM Corp., Armonk, NY, USA). All proportion estimates were weighted to the general Korean population using longitudinal weights supplied by the KDCA. We conducted two-tailed analyses, and p values lower than 0.05 were considered significant.
RESULTS
Study population
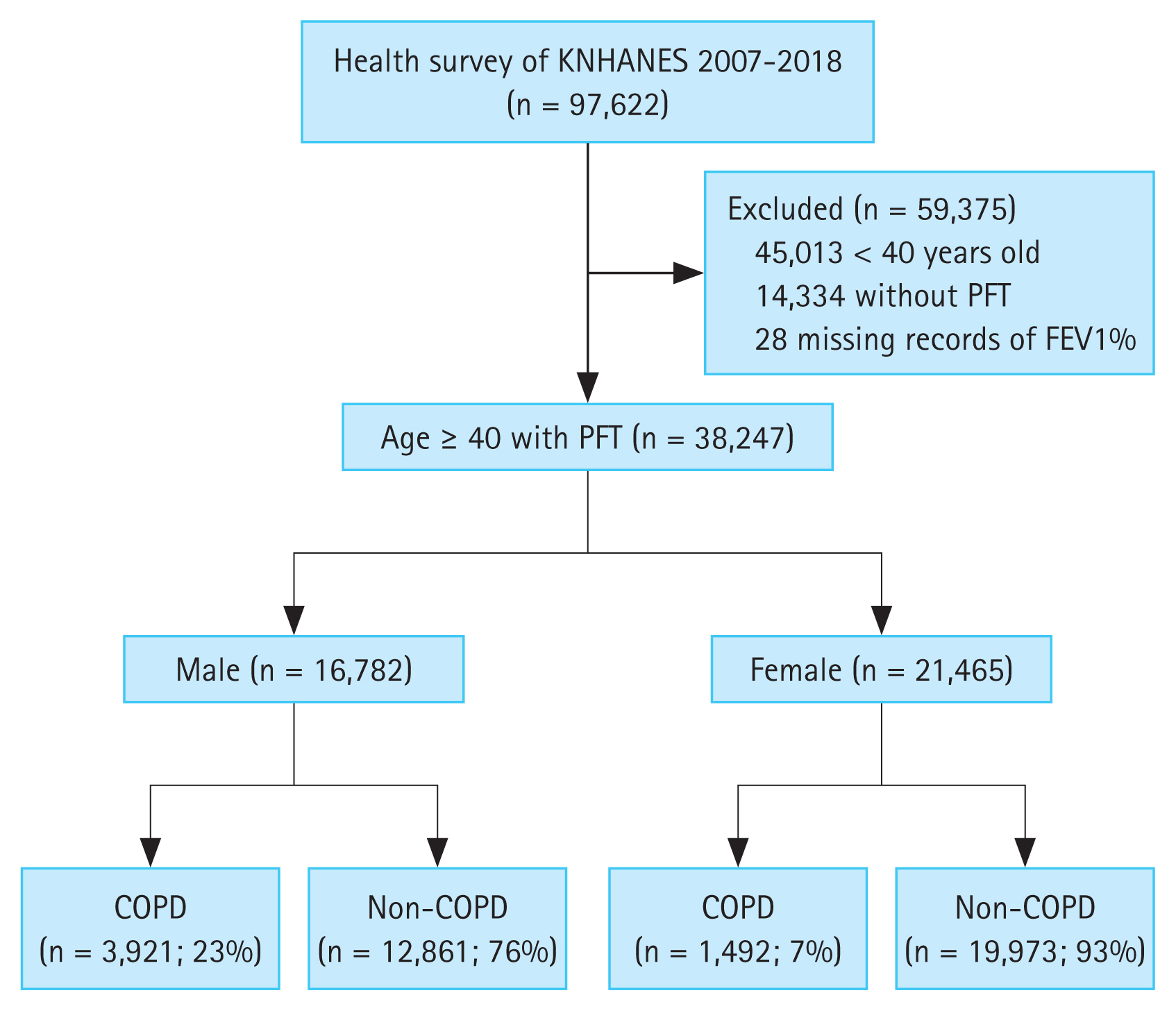
The KNHANES data included 97,622 people during the study period (Fig. 1). We excluded 45,013 who were < 40 years old. Of the remaining 52,609 subjects, we excluded 14,362 who did not have spirometry results or who were missing spirometry values. We analyzed 38,247 subjects aged ≥ 40 years who had spirometry results. Among them, 5,413 (14.2%) met the COPD criteria of FEV1/FVC < 0.7 on PFT. In total, 23.4% of males had COPD (3,921 out of 16,782) and 7.0% of females had COPD (1,492 out of 21,465) (Fig. 1).
Comparison of clinical characteristics in males with and without COPD
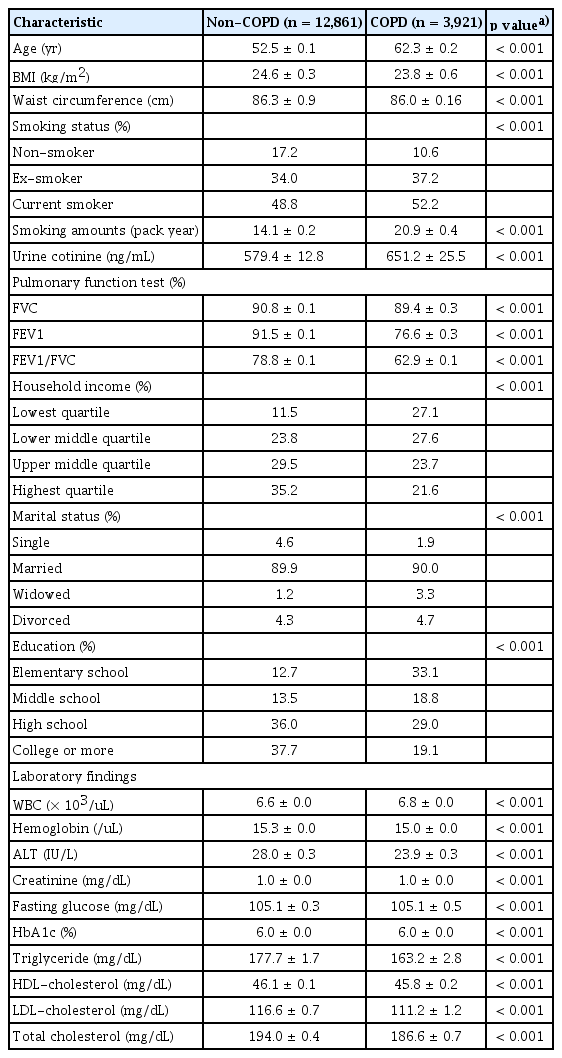
Males with COPD were older than those without COPD (62.3 ± 0.2 yr vs. 52.5 ± 0.1 yr, respectively, p < 0.001) and had a lower BMI (23.8 ± 0.6 kg/m2 vs. 24.6 ± 0.3 kg/m2, respectively, p < 0.001) (Table 1). Males with COPD were more likely to have a smoking history (89.4 vs. 82.8%, respectively, p < 0.001) and higher urinary cotinine levels (651.2 ± 25.5 ng/mL vs. 579.4 ± 12.8 ng/mL, respectively, p < 0.001) than those without COPD. Males with COPD had lower PFT values, including FVC (%), FEV1 (%), and FEV1/ FVC, and lower household income and education levels than in those without COPD.
Comparison of clinical characteristics in females with and without COPD
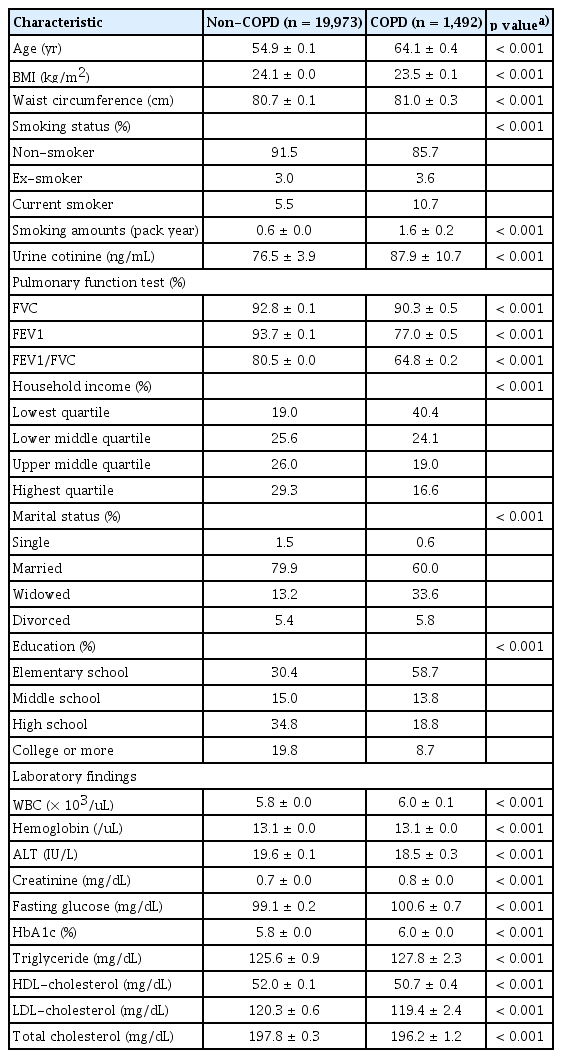
The females COPD group was older (64.1 ± 0.1 yr vs. 54.9 ± 0.1 yr, respectively, p < 0.001) and had a lower BMI (23.5 ± 0.1 kg/m2 vs. 24.1 ± 0.0 kg/m2, respectively, p < 0.001) than the non-COPD group (Table 2). The female COPD group had a higher smoking history (14.3 vs. 8.5%, respectively, p < 0.001) and higher urinary cotinine levels (87.9 ± 10.7 ng/ mL vs. 76.5 ± 3.9 ng/mL, respectively, p < 0.001) than the non-COPD group. Females with COPD had lower spirometry values, such as FVC (%), FEV1 (%), and FEV1/FVC, and lower household income and education levels than those without COPD. There were more widowed or divorced subjects in the COPD group than the non-COPD group (39.4 vs. 18.6%, respectively, p < 0.001).
Comparison of clinical characteristics in subjects with COPD according to sex
The female COPD group was older than the male COPD group (64.1 ± 0.4 yr vs. 62.3 ± 0.2 yr, respectively, p < 0.001) (Table 3). In total, 89.4% of the male COPD group had a smoking history, however, 85.7% of females with COPD were non-smokers (p < 0.001). Urinary cotinine levels were higher in males than females (651.2 ± 25.5 ng/mL vs. 87.9 ± 10.7 ng/mL, respectively, p < 0.001). Overall rates of secondhand smoke and exposure at the workplace were higher in the male COPD group compared to the female COPD group (44.7 vs. 37.3%, respectively, p < 0.001; and 33.4 vs. 26.1%, respectively, p < 0.001); however, exposure at home was higher in females with COPD compared to males with COPD (6.6 vs. 4.5%, respectively, p < 0.001).
Approximately 5.7% of males with COPD and 5.6% of females with COPD were classified as GOLD levels III and IV (p = 0.046). Household income was lower in females with COPD than in males with COPD: 64.5% of females and 54.7% of males were in the lowest and lower middle household income quartiles (p < 0.001). In terms of comorbidities, asthma was more prevalent in the female COPD group than in the male COPD group (25.5 vs. 11.6%, respectively, p < 0.001). A higher prevalence of overall malignancy was observed in females than males (6.3 vs. 5.4%, respectively, p < 0.001); however, lung cancer was more common in the male COPD group than the female COPD group (0.9 vs. 0.1%, respectively, p < 0.001).
The association between lung cancer and COPD according to sex
Lung cancer was more common in those with COPD than in those without COPD (OR, 2.033; 95% CI, 1.112–3.715) (Table 4). There was a difference in lung cancer prevalence according to sex. In males, lung cancer was more common in the COPD group than the non-COPD group (OR, 2.574; 95% CI, 1.214–3.715). However, in females, lung cancer was more common in the non-COPD group than the COPD group (OR, 0.519; 95% CI, 0.295–0.910).
Compared to the non-COPD group, the prevalence of lung cancer increased in those with moderate COPD (GOLD II), all subjects (OR, 3.300; 95% CI, 1.642–6.629), and males with COPD (OR, 4.409; 95% CI, 1.741–9.419). However, the ORs for lung cancer were not high in the mild COPD (GOLD I) and severe COPD (GOLD III and IV) groups (Table 5). The relationship between lung cancer and COPD severity is unclear in females because there were no lung cancer patients with COPD GOLD levels of I, III, and IV.
DISCUSSION
This study compared subjects with and without COPD according to sex. In both males and females, those with COPD were older and had lower household income and educational levels than those without COPD. Smokers and those with a high urinary cotinine level were also more prevalent in the COPD group. Furthermore, several differences in patients with COPD according to sex were noted: males with COPD were associated with a smoking history and lung cancer, while females with COPD had a lower household income and a higher prevalence of asthma.
There are some sex differences in patients with COPD. Females with COPD were less likely to smoke and had lower education and income levels than males with COPD [18,19]. According to Kanervisto et al. [20], subjects with a lower educational level had a 1.8 (95% CI, 1.2–2.6) times higher risk of COPD than those with a higher educational level. Socioeconomic status is also an important risk factor for COPD. According to nationwide data from China, subjects with a low income had a 1.64 (p < 0.001) times higher COPD risk than those with a high income, particularly in rural areas [21]. Furthermore, socioeconomic and educational levels are inversely associated with smoking prevalence.
A meta-analysis from Latin America revealed that low income was associated with a 1.62 (95% CI, 1.34–1.96) times higher smoking prevalence than a high income [22]. Kim et al. [23] reported that the risk of smoking was 1.65 (95% CI, 1.44–1.88) for men and 1.90 (95% CI, 1.14–3.16) for women who graduated from middle school or lower than those who graduated from high school or higher. COPD is more frequently underdiagnosed in women than in men [24]. Therefore, more active COPD screening may be needed in women with a low socioeconomic status.
Considering women with COPD have a smoking prevalence as low as 15%, other factors such as secondhand smoke [25] or household air pollution [5,26,27] exposure may contribute to an increased COPD risk. In our study, although the prevalence of secondhand smoke exposure was higher in men, women were more likely to be exposed to smoke in the home. Furthermore, the overall prevalence of secondhand smoke in females was approximately 37%. Secondhand smoke is a significant risk factor for COPD (OR, 3.80; 95% CI, 1.29–11.2) [25]. Household air pollution from biomass fuels, coal, and kerosene burned in open fires, primitive stoves, and lamps are major risk factors for COPD, particularly for women [5,26,27]. Although household air pollution is a common risk factor for COPD in developing countries, there are still many people with COPD who have a history of exposure to firewood or briquettes in Korea [28]. Korean women are more often in charge of household chores, such as cooking, than men. Household air pollution is a major risk factor for Korean non-smoking females with COPD.
One notable finding in our study is that the prevalence of lung cancer was higher in men with COPD than in those without COPD, but not in females. Smoking is a well-known independent risk factor for lung cancer [29]. Therefore, the smoking exposure of female patients with COPD may have influenced the difference in lung cancer prevalence between men and women with COPD. Another possible explanation for the differences in lung cancer prevalence between men and women is that COPD prevalence differs by sex. COPD increases oxidative stress and damages DNA [30,31]. However, according to a recent large-scale study in Korea, there may be an increased prevalence of lung cancer in those with COPD, even in non-smokers [32]. Furthermore, Nagasaka et al. [33] reported that females with COPD were 1.64 times more likely to develop lung cancer than those without COPD. Lung cancer in women has increased worldwide [30], and secondhand smoke [34] or wood smoke exposure [35] may contribute to the development of lung cancer in women. Therefore, caution is warranted in interpreting our results to indicate a relationship between lung cancer and COPD in women.
Another notable finding was that the prevalence of lung cancer increased in men with moderate COPD. However, there was insufficient evidence to determine this relationship in women. There are conflicting findings on COPD severity and lung cancer prevalence [36–38]. Mannino et al. [36] reported that patients with moderate-to-severe COPD had a 2.8 (95% CI, 1.8–4.4) times higher prevalence of lung cancer. A Cox regression analysis by de Torres et al. [37] showed that GOLD stages I and II are risk factors for lung cancer, but GOLD stages III and IV are not. However, this study included a small number of patients with lung cancer in stage GOLD IV. There may be an increased risk of lung cancer due to impaired clearance of carcinogens and inflammation-related factors with worsening lung function [39]. This relationship may be statistically insignificant because there were few women with lung cancer in our study population. Therefore, further research is warranted.
This study has several limitations. First, airway reversibility results were not available for the COPD diagnosis, as a bronchodilator was not used in the lung function test. Therefore, patients with diseases involving airway reversibility, such as asthma, may have been included in the COPD patient group. Moreover, in our study, the female COPD group included a small number of individuals with lung cancer. Therefore, COPD’s contribution to lung cancer development may have been underestimated in females. Second, we did not determine inhalant or steroid use history, which may have impacted disease staging or caused us to incorrectly classify patients with COPD in the normal group during the diagnostic process. Third, it is difficult to evaluate COPD risk factors other than smoking history because we did not evaluate lung damage due to occupational hazards, environment, and biomass smoke. Fourth, we assessed comorbid diseases, educational background, and income through a self-questionnaire, so reliability may vary by the respondent. Despite these limitations, this study is meaningful in that it investigated the differences between men and women with COPD in a large population.
In conclusion, males with COPD were associated with a smoking history and lung cancer, while females with COPD had a lower smoking rate and household income levels. This study showed that people with COPD demonstrated varying phenotypes according to sex. Due to these differences, COPD may be more underdiagnosed in women than in men. More active COPD screening and management are needed in women of low socioeconomic status, even in non-smokers.
KEY MESSAGE
1. Females with COPD had a lower smoking rate, household income, and a lower prevalence of lung cancer than males with COPD.
2. More active COPD screening and management are needed for women of low socioeconomic status, even if they do not smoke.
Notes
CRedit authorship contributions
Moon Seong Baek: conceptualization, data curation, methodology, writing - original draft, writing - review & editing; Haegwang Shin: data curation, formal analysis, writing - original draft, writing - review & editing; Kang-Mo Gu: data curation; Hae In Jung: data curation; Won Young Kim: data curation; Jae-Woo Jung: data curation, formal analysis, methodology; Jong-Wook Shin: data curation; Sun-Young Jung: formal analysis, methodology; Jae Yeol Kim: conceptualization, project administration, writing - original draft, writing - review & editing
Conflicts of interest
The authors disclose no conflicts.
Funding
None
Availability of data and materials
The dataset used and analyzed in this study is available from the corresponding author upon reasonable request.