Geriatric risk model for older patients with diffuse large B-cell lymphoma (GERIAD): a prospective multicenter cohort study
Article information
Abstract
Background/Aims
Optimal risk stratification based on simplified geriatric assessment to predict treatment-related toxicity and survival needs to be clarified in older patients with diffuse large B-cell lymphoma (DLBCL).
Methods
This multicenter prospective cohort study enrolled newly diagnosed patients with DLBCL (≥ 65 yr) between September 2015 and April 2018. A simplified geriatric assessment was performed at baseline using Activities of Daily Living (ADL), Instrumental ADL (IADL), and Charlson’s Comorbidity Index (CCI). The primary endpoint was event-free survival (EFS).
Results
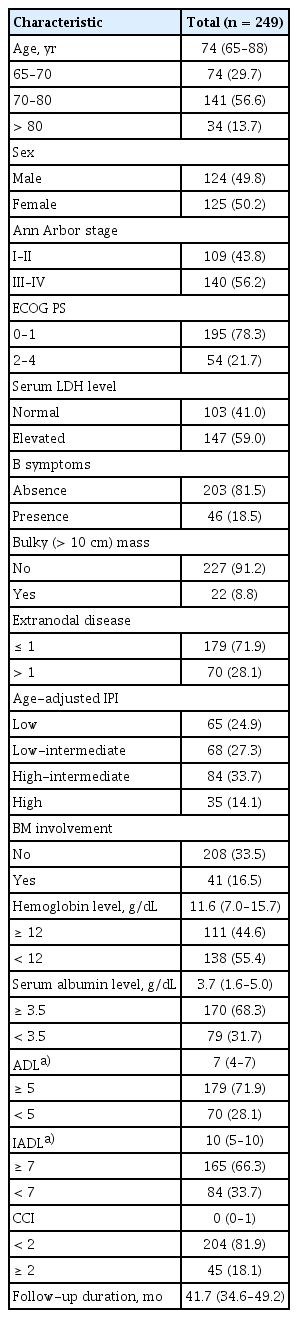
The study included 249 patients, the median age was 74 years (range, 65–88), and 125 (50.2%) were female. In multivariable Cox analysis, ADL, IADL, CCI, and age were independent factors for EFS; an integrated geriatric score was derived and the patients stratified into three geriatric categories: fit (n = 162, 65.1%), intermediate-fit (n = 25, 10.0%), and frail (n = 62, 24.9%). The established geriatric model was significantly associated with EFS (fit vs. intermediate-fit, HR 2.61, p < 0.001; fit vs. frail, HR 4.61, p < 0.001) and outperformed each covariate alone or in combination. In 87 intermediate-fit or frail patients, the relative doxorubicin dose intensity (RDDI) ≥ 62.4% was significantly associated with worse EFS (HR, 2.15, 95% CI 1.30–3.53, p = 0.002). It was related with a higher incidence of grade ≥ 3 symptomatic non-hematologic toxicities (63.2% vs. 27.8%, p < 0.001) and earlier treatment discontinuation (34.5% vs. 8.0%, p < 0.001) in patients with RDDI ≥ 62.4% than in those with RDDI < 62.4%.
Conclusions
This model integrating simplified geriatric assessment can risk-stratify older patients with DLBCL and identify those who are highly vulnerable to standard dose-intensity chemoimmunotherapy.
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) primarily affects older individuals, with approximately 43% of new diagnoses and 62% of deaths occurring in those aged 65 years or older [1]. Although age-related functional decline and comorbidities are highly heterogeneous and associated with treatment outcomes in DLBCL [2–8], they are usually under-recognized in clinical practice. Comprehensive geriatric assessment is used in older patients with cancer to evaluate physical functioning, psychological health, level of comorbidities, and social well-being and can detect frail individuals who are not usually captured in routine assessment [9–11]. However, comprehensive geriatric assessment is not widely accepted for patients with DLBCL because it is time-consuming and has not been validated as a treatment guide. Thus, an appropriate risk stratification model based on optimal geriatric assessment tools need to be established. Recently, the Italian lymphoma group classified older patients (≥ 65 yr) with DLBCL into three categories (fit, unfit, and frail) using three simplified geriatric assessment tools, Activities of Daily Living (ADL), Instrumental Activities of Daily Living (IADL), and the Cumulative Illness Rating Scale for Geriatrics, and age and validated them in an external cohort [12]. However, since that study, no prospective study on patients with DLBCL has reported the prognostic impact of these simplified geriatric assessment tools on treatment outcomes.
Several retrospective studies have attempted to address the impact of dose intensity of rituximab plus cyclophosphamide, doxorubicin (in particular), vincristine, and prednisone (R-CHOP) in older patients with DLBCL [5,13–17]. Despite the heterogeneity in the study population and in the definition and calculation of dose intensity, there is considerable agreement that maintaining a standard dose intensity of R-CHOP is of importance even in older patients with DLBCL [17]. However, this agreement gradually diminishes with increasing age, particularly in patients aged 80 years or older. Most retrospective studies have shown no significant association between dose intensity and survival in very older patients [5,13–16]. One study [18] even reported that a doxorubicin dose intensity ≥ 85% was associated with worse outcomes compared with dose intensity < 85%. This is explained by increased treatment-related toxicities and early mortality associated with higher dose intensities in patients with advanced age [18]. Given that pretreatment geriatric assessment can predict treatment-related toxicities in older patients with DLBCL [2–5,12,19,20], it would be worthwhile to evaluate the impact of geriatric assessment-defined non-fitness on doxorubicin dose intensity and toxicities in older patients with DLBCL. To date, no study has prospectively evaluated the impact of non-fitness on the relative doxorubicin dose intensity (RDDI), toxicity, and survival outcomes. Therefore, this GERIAD study aimed to develop a risk model based on a simplified geriatric assessment and other geriatric variables to predict event-free survival (EFS) in older patients with DLBCL and to evaluate the impact of geriatric non-fitness defined by the established risk model on RDDI, treatment-related toxicities, and survival outcomes.
METHODS
Study design and patients
This prospective multicenter observational cohort study included older patients with newly diagnosed DLBCL who underwent curative-intent chemoimmunotherapy at 13 institutions of the Korean Society of Hematology Lymphoma Working Party. Eligible patients had a histologic diagnosis of DLBCL, were 65 years or older, and intended to undergo treatment with the R-CHOP regimen. Patients with other histologies, including high-grade lymphoma, follicular lymphoma grade 3b, or primary central nervous system DLBCL were excluded. Additionally, patients with a history of indolent lymphoma or other malignancies requiring active treatment were also excluded. Demographics and baseline clinical, pathological, and laboratory data were collected using study-specific case record forms. Performance status was assessed using the Eastern Cooperative Oncology Group performance status, and the age-adjusted International Prognostic Index (aaIPI) was used to determine prognosis using baseline clinical variables.
Patients received the standard R-CHOP regimen (rituximab 375 mg/m2 intravenously [IV], cyclophosphamide 750 mg/m2 IV, doxorubicin 50 mg/m2 IV, and vincristine 1.4 mg/m2 [maximum 2.0 mg] IV, all administered on day 1; and prednisone 100 mg orally administered on days 1–5) every 21 days for 6–8 cycles. However, the actual dose and interval of R-CHOP therapy were left to the discretion of treating physician and were not based on the results of the geriatric assessment. All patients received primary prophylaxis with pegylated granulocyte-colony stimulating factor and prophylactic trimethoprim-sulfamethoxazole once daily throughout treatment.
The study protocol was approved by the Institutional Review Board of each participating center (Chonnam National University Hwasun Hospital, CNUHH-2015-154; Jeonbuk National University Hospital, CUH-2015-08-007) and conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent before enrollment, and the study was registered at www.clinicaltrials.gov (#NCT02555267 and #NCT03211702).
Assessment and endpoints
Geriatric assessment was performed by trained study staff prior to administering R-CHOP therapy using three tools: the modified Korean version of the Katz ADL [21], Lawton IADL [21], and Charlson’s Comorbidity Index (CCI) [22] (Supplementary Table 1, 2). The ADL and IADL scales assessed seven items of basic self-care activities and 10 items regarding household work and independence status, respectively (Supplementary Table 1), and were determined by the total number of items performed independently. The CCI was calculated by summing the scores based on comorbidities and their severity. Comorbidities, such as leukemia (acute or chronic), metastatic solid tumor, and other lymphoma, were not considered as they were excluded from this study (Supplementary Table 2).
The primary endpoint was EFS, which was calculated from the date of inclusion to the date of disease progression, relapse from complete responders, unplanned changes in therapy during treatment, death from any cause, or last follow-up, as appropriate. The secondary endpoints were progression-free survival (PFS), calculated from the date of inclusion to the date of progression, relapse, death from any cause, or last follow-up, and overall survival (OS), which was measured from the date of inclusion to the date of death from any cause or last follow-up. The dose intensity of doxorubicin was calculated by dividing the total dose of doxorubicin administered by the number of weeks of treatment. The RDDI was then determined by dividing the received dose intensity by the projected dose intensity of doxorubicin.
All patients underwent baseline, mid-treatment (after three cycles of R-CHOP), and post-treatment response assessments, using the Lugano criteria [23,24]. Patients were followed up every 3 months for the first 2 years after treatment, then every 4 months for the third year, and every 6 months thereafter. Adverse events (AEs) were evaluated using the NCI-CTCAE version 4.03, from study enrollment to 4 weeks after the last R-CHOP therapy.
Statistical analysis
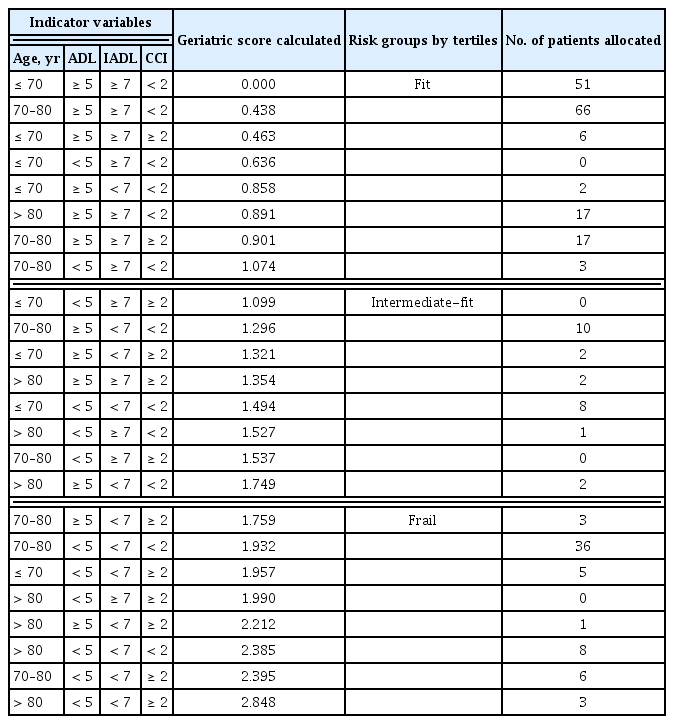
The Kaplan–Meier method was used to estimate EFS, PFS, and OS, and comparisons between categorical variables were performed using the log-rank test. To develop a geriatric risk model based on geriatric variables, we performed univariable analysis to evaluate the association between EFS and different baseline variables. To define the categorization of continuous clinical and geriatric assessment variables, two different methods, X-tile analysis [25] and receiver operating characteristic (ROC) analysis, were used to determine the best cutoff values of these variables for event prediction. Clinical variables with p < 0.05 in the univariable analysis were included in the multivariable analysis using the Cox proportional hazard regression model. Covariates that remained significant after multivariable adjustment were selected to build a geriatric model by summing the products of indicator variables (encoded as 0 for reference and 1 if a risk factor was present) multiplied by their respective beta coefficients (natural logarithm of the HR) from the multivariable Cox regression model. To define categorical risk groups (i.e., fit, intermediate-fit, and frail), tertiles were defined within the distribution of geriatric scores. The predictive ability of the geriatric model was compared to models relying on individual geriatric variables or combinations using the concordance index (C-index) [26]. Descriptive statistics were reported as percentages for categorical variables and as medians and interquartile ranges (IQRs) for continuous variables. All statistical analyses were performed using R version 4.0 (the R Foundation for Statistical Computing, Vienna, Austria at https://www.r-project.org) and X-tile 3.6.1 software (Yale University, New Haven, CT, USA) [25].
RESULTS
Patient characteristics and geriatric assessment
Between September 2015 and April 2018, a total of 264 patients were screened, 15 of whom were ineligible for inclusion in the cohort (Supplementary Fig. 1). Therefore, a total of 249 patients were enrolled in this study. The median age was 74 years (range, 65–88 yr), 34 patients (13.7%) were > 80 years old, and 125 (50.2%) were female. The baseline demographic information and disease characteristics are shown in Table 1.
Geriatric assessment and survival outcomes
Of the 249 patients, 191 (76.7%) completed the planned R-CHOP treatment. Treatment was discontinued because of toxicities in most patients who did not complete the planned treatment (74.1%) (Supplementary Table 3). With a median follow-up of 41.7 months (IQR, 34.6–49.2 mo), 116 patients died during treatment or follow-up, 72 of whom died because of lymphoma progression, 18 related to therapy toxicities, 9 deemed unrelated to toxicities or to disease progression, and 17 because of unknown causes. Eleven patients experienced disease progression but were still alive at the time of analysis. Additionally, nine patients had unplanned therapy changes during R-CHOP treatment (six to radiotherapy, and three to systemic therapy with palliative intent); five of these patients had lymphoma progression and died. A total of 127 PFS and 131 EFS events were documented. The 2-year EFS, PFS, and OS were 53.5% (95% CI, 47.2–59.8), 55.9% (95% CI, 49.6–62.1), and 63.9% (95% CI, 57.8–70.0), respectively.
The median ADL and IADL scores were 7 (IQR, 4–7) and 10 (IQR, 5–10), respectively. Frequently impaired activities in ADL were related to independence in bathing (42.9%), transferring (31.7%), and dressing (22.0%). Similarly, the most frequently impaired activities in IADL were transportation (45.7%), laundry (39.7%), and housework (38.5%). The median CCI score was 0 (IQR, 0–1), and the frequent comorbidities were diabetes without complications (18.1%), mild liver disease (7.6%), and cerebrovascular disease (5.6%).
To identify subgroups of patients with different prognosis according to geriatric assessment results, we evaluated the prognostic value of each geriatric assessment tool. From the identical results of the X-tile software and ROC analysis, an ADL score of 5 (sensitivity 43.5%, specificity 89.8%), an IADL of 7 (sensitivity 54.2%, specificity 87.3%), and a CCI of 2 (sensitivity 36.0%, specificity 90.7%) were determined to be the best cutoffs for predicting EFS. Seventy patients (28.1%) had an ADL score < 5, 84 (33.7%) an IADL score < 7, and 45 (18.1%) a CCI ≥ 2. Patients with ADL score < 5, IADL score < 7, and CCI ≥ 2 had worse outcomes with 2-year EFS of 24.3% (HR 3.38, 95% CI 2.37–4.82; p < 0.001), 26.7% (HR 3.84, 2.70–5.47; p < 0.001), and 39.6% (HR 1.95, 1.32–2.89; p = 0.001) respectively, compared to 65.2%, 68.0%, and 56.6% for those with ADL score 5–7, IADL score 7–10, and CCI 0–1, respectively (Fig. 1A–C). Hence, each geriatric assessment tool identified two distinct survival outcome subgroups.

Survival analysis according to each simplified geriatric assessment tool and the established geriatric risk model. Event-free survival and (A) ADL, (B) IADL, and (C) CCI. (D) Event-free survival, (E) progression-free survival, and (F) overall survival based on the established geriatric risk model. ADL, Activities of Daily Living; IADL, Instrumental Activities of Daily Living; CCI, Charlson’s Comorbidity Index; HR, hazard ratio; CI, confidence interval.
Development of geriatric risk model
Based on the identification of subgroups with worse survival outcomes using each geriatric tool, we next investigated whether a geriatric risk model integrating geriatric and clinical variables could more accurately predict outcomes compared to each geriatric tool alone or in combination. In univariable analysis, all variables, except sex and bulky disease status, were significantly associated with EFS, PFS, and OS (Supplementary Table 4). In multivariable Cox proportional hazard analysis, which included significant variables from the univariable analysis, geriatric variables (age, ADL and IADL scales, and CCI) and aaIPI were associated with EFS (Supplementary Table 5). To build a stricter geriatric risk model, the Cox model was re-fitted with only geriatric variables (i.e., age, ADL and IADL scales, and CCI) and showed that all these variables were independent factors for EFS (Table 2). Thus, the final geriatric risk model included age, ADL and IADL scales, and CCI. The beta coefficients for each indicator variable were determined based on the final Cox model (Table 2), and the geriatric score was calculated for each group (Table 3). Finally, patients were stratified into three groups according to their geriatric scores: fit (≤ 33rd percentile; n = 162, 65.1%), intermediate-fit (> 33rd percentile and ≤ 66th percentile; n = 25, 10.0%), and frail (> 66th percentile; n = 62, 24.9%), with 2-year EFS of 67.7% (HR 1), 44.0% (HR 2.61, 95% CI, 1.52–4.49; p < 0.001), and 21.0% (HR 4.61, 95% CI 3.15–6.75; p < 0.001), respectively (Fig. 1D). The established geriatric model demonstrated the highest predictive ability (C-index 0.7060) among the geriatric variables alone or in various combinations (Supplementary Table 6) and was also strongly associated with PFS and OS (Fig. 1E, F).
RDDI and treatment-related toxicities according to established geriatric risk model
To determine whether geriatric intermediate-fit or frail patients may benefit from standard dose-intensity therapy, we compared the RDDI, treatment-related toxicities, and survival outcomes of fit patients with those of intermediate-fit or frail patients. The median RDDI administered to all patients was 61.6% (IQR, 55.5–75.4), which was significantly higher in fit patients (66.2%; IQR, 56.8–79.7) compared to that in intermediate-fit or frail patients (60.0%; IQR, 51.1–70.0; p = 0.001). To define the optimal RDDI cutoff for predicting EFS, two different approaches, X-tile and ROC analyses, were performed. In the X-tile analysis, the best cutoff of RDDI for EFS was 60.2%, and ROC analysis identified a 64.6% RDDI as an optimal cutoff. Therefore, we chose a RDDI threshold of 62.4% (sensitivity 39.4%, specificity 87.5%), corresponding to the mean of the cut-offs identified from the X-tile (60.2%) and ROC analysis (64.6%). A RDDI of 62.4% separated the study cohort into two groups of 133 patients with a RDDI < 62.4% (fit, n = 75; intermediate-fit or frail, n = 58) and 116 patients with a RDDI ≥ 62.4% (fit, n = 87; intermediate-fit or frail, n = 29). In 87 patients of the intermediate-fit or frail group, patients with RDDI ≥ 62.4% had significantly worse EFS (2-year, 10.3% vs. 36.2%; HR 2.15, 95% CI 1.30–3.53; p = 0.002), PFS (2-year, 13.8% vs. 41.1%; HR 1.95, 95% CI 1.19–3.20; p = 0.007), and OS (2-year, 20.7% vs. 48.0%; HR 2.16, 95% CI 1.30–3.59; p = 0.002) than those with RRDI < 62.4% (Fig. 2A, Supplementary Fig. 2). However, in 162 fit patients, EFS (2-year, 66.4% vs. 69.5%; HR 0.94, 95% CI 0.56–1.56; p = 0.810), PFS (2-year, 67.5% vs. 70.8%; HR 0.96, 95% CI 0.57–1.60; p = 0.884), and OS (2-year, 78.0% vs. 77.1%; HR 0.88, 95% CI 0.51–1.53; p = 0.661) did not differ significantly between those with RDDI ≥ 62.4% and < 62.4% (Fig. 2B, Supplementary Fig. 2).

Event-free survival according to RDDI administered. (A) Intermediate-fit or frail patients (n = 87) and (B) fit patients (n = 162). RDDI, relative doxorubicin dose intensity; HR, hazard ratio; CI, confidence interval.
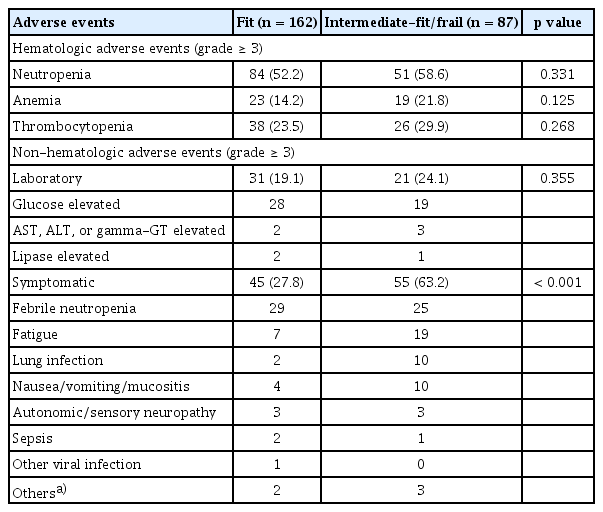
Grade ≥ 3 hematologic and non-hematologic AEs are listed in Table 4. Grade ≥ 3 neutropenia, anemia, and thrombocytopenia were reported in 135 (54.2%), 42 (16.9%), and 64 (25.7%) patients, respectively. The risk of grade ≥ 3 hematologic AEs was not significantly higher in intermediate-fit or frail patients than in fit ones (Table 4). Similarly, laboratory non-hematologic AEs (grade ≥ 3) were documented in 52 (20.9%) patients, where the most common abnormality was transient hyperglycemia during steroid therapy, but the risk of these AEs did not differ significantly according to geriatric risk groups (Table 4). However, the risk of grade ≥ 3 symptomatic non-hematologic AEs, such as febrile neutropenia, fatigue, and lung infection significantly increased in intermediate-fit or frail patients compared to that in fit patients (63.2% vs. 27.8%, p < 0.001; Table 4). Consequently, the rates of early treatment discontinuation due to therapy-related AEs were significantly higher in intermediate-fit or frail patients compared to those in fit patients (30 [34.5%] vs. 13 [8.0%]; p < 0.001). Notably, the risk of treatment discontinuation due to toxicities was 1.9% in fit and 14.9% in intermediate-fit or frail patients in the first cycle, and 6.2% in fit and 29.9% in intermediate-fit or frail patients by the third cycle, which was significantly higher in intermediate-fit or frail patients (p < 0.001). Taken together, we established a geriatric risk model integrating age, ADL and IADL scales, and CCI score which showed better predictive power than each geriatric variable alone or in combination. Our geriatric risk model identified a vulnerable older patient subset at high risk of non-hematologic toxicities, early treatment discontinuation, and mortality following R-CHOP therapy.
DISCUSSION
This prospective study demonstrated that a geriatric risk model integrating age, functional status, and comorbidities can predict survival outcomes, therapy-related toxic effects, and is useful in determining the feasibility of standard dose-intensity R-CHOP therapy. Older non-fit patients had an increased risk of disease progression, death, symptomatic non-hematologic AEs, and early treatment discontinuation, regardless of aaIPI, which were consistent with previous reports [2–5,12,19,20]. Notably, a higher RDDI was associated with adverse outcomes in older, non-fit patients. These findings indicated that geriatric fitness itself is a relevant surrogate marker for long-term outcomes in DLBCL, and thus novel treatment strategies for non-fit patients need to be explored in this highly vulnerable patient subgroup.
Although evidence-based treatment guidance could not be offered from this study because the choice of regimen and dose intensity was not based on geriatric assessment and was left to the discretion of the treating physician, our data provides relevant information on geriatric assessment-based treatment approaches in older patients with DLBCL. Given the acceptable rates of non-hematologic toxicities and early treatment discontinuation among fit patients in the present study, these patients may be candidates for a standard dose-intensity chemoimmunotherapy approach, which is consistent with previous data [27,28]. By contrast, the main cause of early treatment discontinuation in intermediate-fit or frail patients in our study cohort was treatment-related toxicities (34.5%). Most toxicities associated with treatment discontinuation were grade ≥ 3 non-hematologic toxicities such as febrile neutropenia, fatigue, and lung infection, which was in line with previous studies [17,20,29]. These data indicated that reducing treatment-related toxic effects may be a reasonable approach for improving short-term outcomes in older, non-fit patients. In this study, intermediate-fit or frail patients with an attenuated RDDI (< 62.4%) had better survival outcomes than those with higher RDDI (≥ 62.4%), suggesting the feasibility of dose-attenuated chemoimmunotherapy strategy in older non-fit patients. This idea was further supported by observations from two previous phase 2 trials evaluating the efficacy and safety of R-miniCHOP [30] and ofatumumab-miniCHOP [31] in patients older than 80 years that reported 2-year OS rates of 59% and 65%, respectively, with acceptable toxicity profiles. However, although these results suggest the feasibility of a dose-attenuated chemoimmunotherapy strategy in older non-fit patients, reducing treatment-related toxicities without compromising therapy efficacy remains a challenging issue. Other data suggest that reducing treatment-related toxicities using anthracycline-free chemoimmunotherapy may compromise therapeutic efficacy and result in inferior outcomes in older non-fit patients [12–14,16,19]. Furthermore, although non-fit patients with an attenuated RDDI showed superior survival outcomes to those with higher RDDI in our study, it was slightly disappointing that the 2-year EFS of patients with attenuated RDDI was only 36.2%, indicating that there is a still considerable room for improvement in this highly selected population. Currently, new treatment strategies incorporating novel agents, such as bispecific antibodies, are being actively investigated in older/unfit patients with DLBCL, and early clinical data have shown promising results [32]. Thus, future randomized trials incorporating these novel strategies in older non-fit patients will offer optimal treatment options for this highly vulnerable patient subgroup. Our geriatric risk model might be beneficial in determining the patients most likely to benefit from such a novel approach.
This study has several limitations. First, an independent validation cohort was absent because a simplified geriatric assessment using the three tools was not yet routinely evaluated in Korea. In addition, the number of patients was based on the potential number of patients we were able to feasibly enroll rather than on statistical calculations, owing to the absence of data regarding simplified geriatric assessment in older patients with DLBCL at the time of the study design. Thus, unfortunately, sample size was inadequate for internal validation. Second, as the number of patients was quite small in the intermediate-fit group (n = 25), these patients were not exclusively analyzed but were combined with the frail group, which might be another limitation. Finally, we only considered baseline functional status and did not evaluate dynamic changes in functional fitness over time after the initiation of R-CHOP therapy, which may have underestimated the actual proportion of non-fit patients.
In conclusion, this multicenter prospective cohort study establishes a geriatric risk model integrating age, ADL and IADL scales, and the CCI score that appropriately predicts the risk for treatment-related toxicity, early treatment discontinuation, and death in older patients with newly diagnosed DLBCL. Our geriatric model can assist in identifying potentially vulnerable older patients who may be candidates for new treatment strategies that incorporate novel drugs. Our geriatric risk model and the observed findings warrant further validation in a prospective study with a larger population.
KEY MESSAGE
1. Age-related functional decline and comorbidities affect survival in older patients with DLBCL.
2. A geriatric risk model based on age, ADL and IADL scales, and CCI identified vulnerable older patients at high risk of chemotherapy-induced toxicities and death.
Notes
CRedit authorship contributions
Ho-Young Yhim: conceptualization, methodology, resources, investigation, data curation, formal analysis, writing - original draft, writing - review & editing, project administration, funding acquisition; Yong Park: resources, investigation, data curation, writing - review & editing; Jeong-A Kim: resources, investigation, data curation, writing - review & editing; Ho-Jin Shin: resources, investigation, data curation, writing - review & editing; Young Rok Do: resources, investigation, data curation, writing - review & editing; Joon Ho Moon: resources, investigation, data curation, writing - review & editing; Min Kyoung Kim: resources, investigation, data curation, writing - review & editing; Won Sik Lee: resources, investigation, data curation, writing - review & editing; Dae Sik Kim: resources, investigation, data curation, writing - review & editing; Myung-Won Lee: resources, investigation, data curation, writing - review & editing; Yoon Seok Choi: resources, investigation, data curation, writing - review & editing; Seong Hyun Jeong: resources, investigation, data curation, writing - review & editing; Kyoung Ha Kim: resources, investigation, data curation, writing - review & editing; Jinhang Kim: resources, investigation, data curation, writing - review & editing; Chang-Hoon Lee: resources, investigation, data curation, formal analysis, writing - review & editing; Ga-Young Song: resources, investigation, data curation, formal analysis, writing - review & editing; Deok-Hwan Yang: conceptualization, methodology, resources, investigation, data curation, formal analysis, writing - original draft, writing - review & editing, Supervision, Funding acquisition; Jae-Yong Kwak: resources, investigation, data curation, writing - review & editing, supervision
Conflicts of interest
The authors disclose no conflicts.
Funding
This study was supported by Fund of Biomedical Research Institute, Jeonbuk National University Hospital, the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea Under Grand HR20C0021, and the Chonnam National University Hwasun Hospital Research Institute of Clinical Medicine under Grant HCRI22009.