Relationship between metformin use and mortality in tuberculosis patients with diabetes: a nationwide cohort study
Article information
Abstract
Background/Aims
To determine whether metformin, which is considered a host-directed therapy for tuberculosis (TB), is effective in improving the prognosis of patients with TB and diabetes mellitus (DM), who have higher mortality than those without DM.
Methods
This cohort study included patients who were registered as having TB in the National Tuberculosis Surveillance System. The medical and death records of matched patients were obtained from the National Health Information Database and Statistics Korea, respectively, and data from 2011 to 2017 were collected retrospectively. We classified patients according to metformin use among participants who used diabetes drugs for more than 28 days. The primary outcome was all-cause mortality during TB treatment. Double propensity score adjustment was applied to reduce the effects of confounding and multivariable Cox proportional hazard models were used to estimate adjusted hazard ratio (aHR) with 95% confidence interval (CI).
Results
The all-cause mortality rate during TB treatment was lower (9.5% vs. 12.4%, p < 0.01) in the metformin user group. The hazard of death due to all causes after double propensity score adjustment was also lower in the metformin user group (aHR 0.76, 95% CI 0.67–0.86, p < 0.01). There was no significant difference in mortality between metformin users and non-users for TB-related deaths (p = 0.22); however, there was a significant difference in the non-TB-related deaths (p < 0.01).
Conclusions
Metformin use in patients with TB–DM co-prevalence is associated with reduced all-cause mortality, suggesting the potential for metformin adjuvant therapy in these patients.
INTRODUCTION
Tuberculosis (TB), one of the leading causes of death worldwide, had an estimated incidence of 10.6 million and a mortality rate of 1.4 million in individuals without human immunodeficiency virus (HIV) in 2021 [1]. Diabetes mellitus (DM) is more prevalent in patients with TB than in the general population and has a detrimental effect on the prognosis of patients with TB. The prevalence of DM is increasing as lifestyle and dietary changes occur in low- and middle-income countries with a high TB prevalence. Thus, the prevalence of DM in patients with TB may also continuously increase [2].
Patients with TB and DM (TB–DM) have higher risks of treatment failure, mortality, and recurrence than do those with only TB [3,4]. Regarding mortality, Baker et al. [3] found that patients with TB–DM had a 1.89-times (risk ratio 1.89, 95% confidence interval [CI] 1.52–2.36) higher risk of death than did those with only TB. Other studies reported a 6.5-times higher risk of death (odds ratio 6.5, 95% CI 1.1–38.0) in patients with TB–DM than in those with only TB after covariate adjustment [4].
Host-directed therapy (HDT) has recently been suggested to mitigate the poor treatment outcomes of TB [5,6]. Specifically, metformin, a drug commonly prescribed to patients with DM, is one of the promising candidates as a potential adjunctive agent for TB [7]. It reduces the blood glucose level by inhibiting hepatic gluconeogenesis, decreasing glucose transport from the intestinal lumen into the blood [8], enhancing glucose utilization [9], and reducing the intracellular growth of Mycobacterium tuberculosis by enhancing autophagy [7,10]. Metformin use in people with type 2 DM has shown a protective effect against the development of active TB [11,12], and a favorable treatment outcome could be expected among patients with TB–DM treated with metformin [5,6,13]. Nevertheless, few studies have directly examined the effect of metformin use on mortality in patients with TB–DM [7,13], and clinical evidence for metformin as an HDT is limited.
Therefore, in this study, we aimed to investigate the effect of metformin use on mortality in patients with TB–DM co-prevalence using a nationwide cohort to obtain advanced clinical evidence for the positive effects of metformin.
METHODS
Study design and patients
This study was a nationwide observational cohort study called The Korean Tuberculosis and Post-Tuberculosis (TBPOST) cohort constructed by linking three national databases. Information on TB diagnoses from the Korean National Tuberculosis Surveillance System (KNTSS) between 2011 and 2018, claims data regarding medical history from the National Health Information Database (NHID) between 2006 and 2018, and death records from Statistics Korea from 2011 to 2018 were collected retrospectively and aggregated for matched patients [14]. The study population included patients with TB–DM aged ≥ 18 years and undergoing treatment for drug-susceptible TB between 2011 and 2017. We excluded patients with end-stage renal disease and those who did not use DM medication due to having a relatively mild DM status.
Definitions and outcomes
TB–DM was defined as DM diagnosed before or 1 year after reporting TB to the KNTSS based on World Health Organization (WHO) standards [15]. DM was diagnosed when at least one claim was founded on a diagnosis of DM based on the relevant International Classification of Diseases (ICD) codes and the prescription of DM drugs for > 4 weeks. Alternatively, at least two claims were founded on a diagnosis using ICD codes for DM (E11–E14) were also diagnosed with DM. The follow-up period was defined from the time of TB diagnosis until the endpoint of treatment of TB or the point of death during treatment. Consequently, the death status was only investigated in cases of death during treatment.
Patients were categorized according to the use of metformin, the primary exposure. Metformin users were defined as patients who had used metformin for > 28 days within 6 months after TB diagnosis. Metformin non-users were defined as patients who did not use metformin during the study period but used other DM medication for > 28 days.
The primary outcome was all-cause mortality during the TB-treatment period. The treatment outcomes were defined according to the criteria suggested by the WHO [15]. The sum of cured individuals and those with treatment completion was designated as treatment success.
Data sources
TB information, such as TB history and type, treatment outcome, clinical laboratory test results, notification year, public– private mix (PPM), and TB drug resistance were extracted from the KNTSS. Demographic characteristics, comorbidities, and records of DM medication use were obtained from the NHID. Lastly, death records were confirmed by Statistics Korea.
Statistical analysis
Statistical analysis was performed using STATA/MP version 17 (Stata Corp. LLC, College Station, TX, USA) and SAS Enterprise Guide version 8.3 (SAS Institute Inc., Cary, NC, USA). The chi-square test was used to examine categorical variables. Continuous variables with a normal distribution were assessed using Student’s t-test and are presented as means and standard deviations. Variable with a non-normal distribution were assessed using the Mann–Whitney U test and are presented as medians and interquartile ranges. Baseline characteristics comprising categorical variables are reported as numbers and percentages. For the metformin user and non-user groups, we conducted propensity score (PS) matching using the STATA “psmatch2” syntax by applying the nearest-neighbor algorithm with 1:1 matching for age, gender, the Charlson Comorbidity Index (CCI), and years of TB diagnosis and discarding in both groups. Subsequently, multivariable Cox proportional hazards analysis was conducted to perform double PS adjustment that reduces bias caused by residual confounding [16]. The Kaplan–Meier method was used to evaluate the difference in mortality rate between metformin users and non-users. Statistical significance was set at p < 0.05 and all p values were two-tailed.
Ethics statement
The study protocol was reviewed and approved by the Institutional Review Board of the National Evidence-Based Healthcare Collaborating Agency (NECAIRB19–008–1). Informed consent was waived because of the retrospective nature of the study and use of public de-identified data.
RESULTS
In the overall cohort, 31,641 patients were included. After PS matching, 5,310 metformin users and 5,310 non-users were included in the study (Fig. 1).
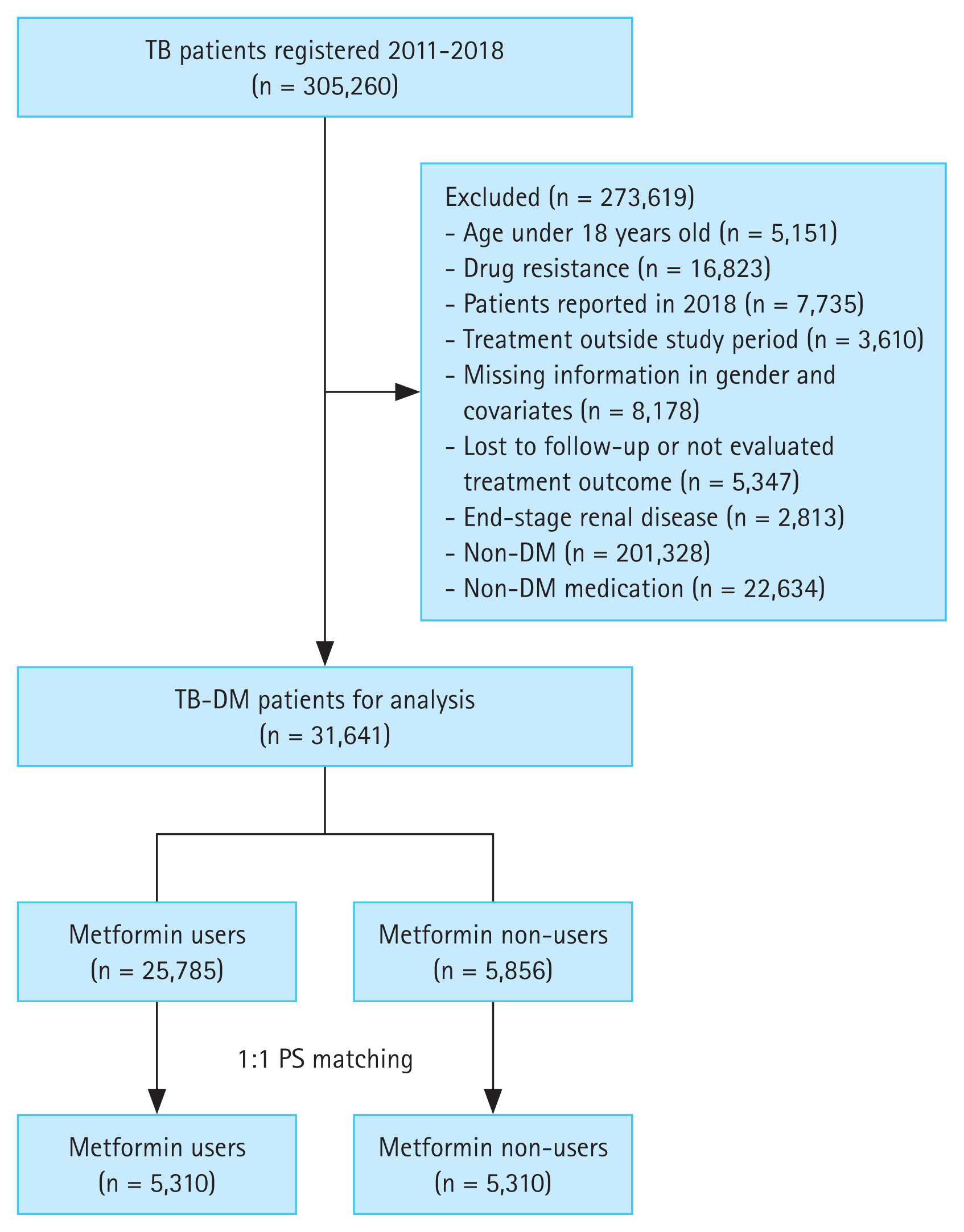
Flow chart classifying metformin use in patients with TB–DM with PS matching. DM, diabetes mellitus; PS, propensity score; TB, tuberculosis.
Baseline characteristics before and after PS matching
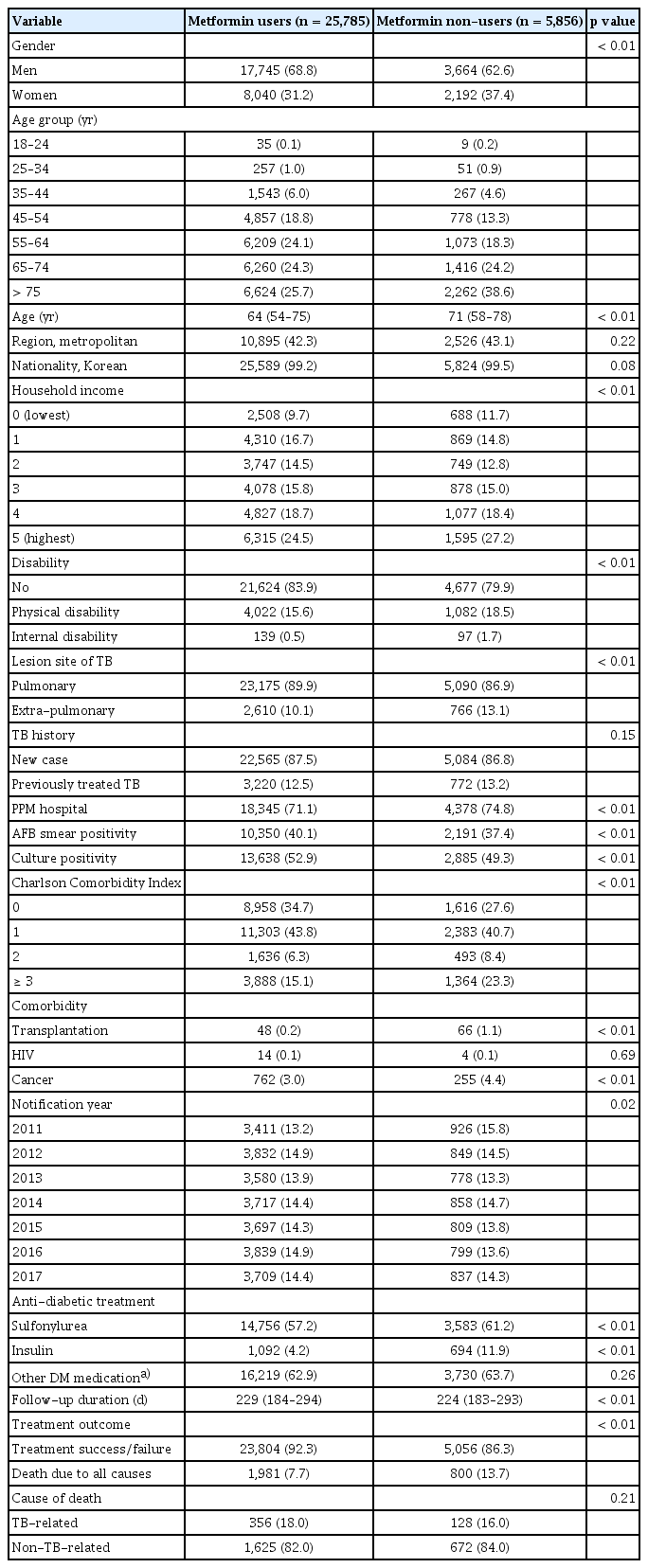
Tables 1 and 2 show the baseline characteristics of metformin users and non-users before and after PS matching, respectively. Before PS matching, the metformin user group was younger and had a higher ratio of men. The CCI score of the metformin user group was relatively lower than that of the metformin non-user group. However, after PS matching, differences between the metformin user and non-user groups in terms of age, gender ratio, and CCI scores were not observed. In addition, the nationality, household income, previous TB history, notification year, and follow-up duration of the two groups were similar after PS matching. The metformin non-user group comprised more individuals with disability, extra-pulmonary TB, transplantation, and living in the metropolitan area. In the metformin user group, more patients were culture-positive (53.1% vs. 49.7%, p < 0.01). The difference in acid-fast bacilli (AFB)-positive smears between the two groups was not significant (39.4% vs. 38.0%, p = 0.07). The results of examining the baseline characteristics of gender-specific subgroups before and after PS matching are provided in the supplementary files (Supplementary Table 1–4). Similar results in terms of baseline characteristics were observed in the metformin user and non-user groups after subgroup analysis by gender.
Treatment outcomes in the metformin user and non-user groups
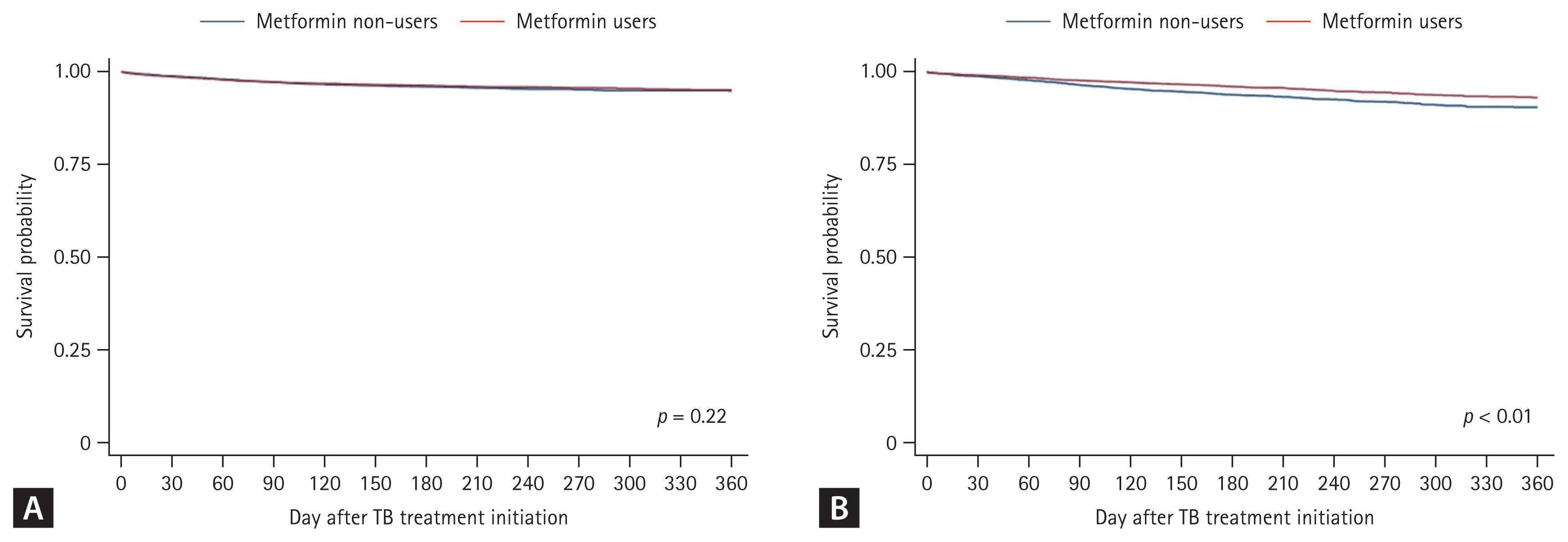
The treatment success rate was higher in the metformin user group than in the non-user group (90.3% vs. 87.6%, p < 0.01). Furthermore, the all-cause mortality was lower in the metformin user group (9.5% vs. 12.4%, p < 0.01, Table 2). However, there was no difference between the groups in the proportion of TB-related and non-TB-related deaths among the deceased patients. The Kaplan–Meier curve in Figure 2 shows that the all-cause mortality rate was significantly lower in the metformin group after (Fig. 2A) and before (Fig. 2B) PS matching (p < 0.01). A comparison of the survival probability according to the cause of death (TB-related vs. non-TB-related death) revealed a significant difference in survival probability between the groups only for non–TB-related death (p < 0.01, Fig. 3).

Kaplan–Meier curve showing the effect of metformin use on mortality during TB treatment after and before propensity score matching. After propensity score matching (A), a survival analysis showed a higher rate of overall survival in metformin users. Even before propensity score matching (B), lower overall survival rates were maintained in metformin non-users. TB, tuberculosis.
Effect of metformin use on all-cause mortality during TB treatment
Survival analysis was performed using a Cox proportional hazard model (Table 3) to identify the effect of metformin use on all-cause mortality during the treatment of TB in patients with DM. In the analysis of the PS-matched cohort, metformin users had a lower risk of death compared with metformin non-users in the univariable analysis (hazard ratio [HR] 0.77, 95% CI, 0.68–0.86, p < 0.01). Multivariate analysis in Models 1–4 showed similar statistical results. Model 1 was adjusted for demographic and socioeconomic factors, and Model 2 was additionally adjusted for clinical test results and clinical status. The risk of death of metformin users was lower than that of non-users in both these models. In Models 3 and 4, which considered comorbidities and anti-diabetic medications including sulfonylurea, insulin, and other anti-diabetic drugs, the risk of death in the metformin user group was also consistently lower than that in the metformin non-user group (Model 4: adjusted HR [aHR] 0.76, 95% CI 0.67–0.86, p < 0.01). In addition, the favorable effect of metformin on mortality was consistent with the results of the analysis before PS matching and stratification by gender. The aHR in Model 4 was 0.76 (95% CI 0.65–0.89, p < 0.01) in metformin users in men (Supplementary Table 5) and 0.75 (95% CI 0.62–0.91, p < 0.01) in women (Supplementary Table 6).
DISCUSSION
By analyzing a nationwide integrated TB cohort, we found that metformin had a favorable effect on all-cause mortality during the TB treatment of patients with TB–DM.
DM is known to be a poor prognostic factor for the treatment outcome of TB. As previously reported, in patients with combined TB–DM, the all-cause mortality rate increased by 2.18–6.5 times [4,17]. TB recurrence after the completion of TB treatment increases by 3.89 times [3] in the presence of DM. Therefore, the Collaborative Framework for Care and Control of Tuberculosis and Diabetes recommends appropriate glucose control as one of the treatments for TB [18].
Metformin is used as a first-line treatment for patients with DM [19] and it has been investigated for its possible positive effects in patients with TB–DM. Previous studies have shown its ability to lower TB risk in people with DM. Among patients with DM, the risk of TB development in metformin users was reduced by 32–61%, as revealed in a meta-analysis [12]. In terms of the dose-dependent effect of metformin, Pan et al. reported a dose-response relationship between metformin use and TB risk reduction [11]. However, data on the effect of metformin on mortality during TB treatment in patients with DM are lacking. Degner et al. [13] reported a reduced mortality rate in patients with TB–DM who use metformin (aHR 0.56, 95% CI 0.39–0.82), and similar mortality rates between patients without DM and patients with DM when metformin was used during TB treatment. This suggests that the use of metformin could offset an increase in mortality due to DM in patients with TB. Similarly, in our study, among patients with DM, metformin use reduced the risk of all-cause mortality during TB treatment, even after adjusting for potential confounders, including age, gender, household income, AFB smear positivity, the presence of various comorbidities, and use of other anti-diabetic drugs. This result was consistent with that before PS matching and after subgroup analysis by gender.
We cannot fully explain how metformin showed favorable effects on all-cause mortality in patients with TB–DM in this study. Some researchers have suggested the following possible explanations. First, metformin may have anti-mycobacterial capacity in patients with TB–DM. The primary target of metformin, mitochondrial respiratory chain complex I, is structurally similar to bacterial respiratory chain complex-I (NDH-I). As metformin inhibits mitochondrial respiratory chain complex I, it can have a similar effect on NDH-I [20]. Therefore, a bactericidal effect on M. tuberculosis may be possible if bacterial NDH-I is suppressed by metformin. In addition, metformin activates the adenosine monophosphate-activated protein kinase pathway, which eventually promotes phagosome–lysosome fusion and plays a crucial role in controlling the intracellular growth of M. tuberculosis [7]. Metformin can also downregulate the expression of matrix metalloproteinases (MMPs), which play a role in lung parenchymal destruction in patients with TB [21,22]. TB-induced inflammatory tissue damage is responsible for morbidity and mortality [23]. Thus, inhibition of the effect of critical MMPs on a TB-infected lung could be a possible mechanism underlying the positive impact of metformin on mortality in patients with TB–DM.
Previous studies did not differentiate between TB-related deaths and non-TB-related deaths when evaluating the impact of metformin [7,13]. However, we conducted separate analyses for these groups in this study. As shown in Figure 3, metformin use did not have a significant impact on the mortality rate in terms of TB-related deaths, whereas it did have a significant effect in terms of non-TB-related deaths, similar to the findings in the overall population. The retrospective nature of this study presents challenges in comparing the DM control status between metformin users and non-users. Consequently, it remains uncertain whether the diverse effects of metformin, contingent on the cause of death, are primarily linked to DM regulation or result from metformin’s broader physiological impacts. Hence, the necessity for future prospective investigations on this matter is evident.
Our study has several strengths. First, this was a large, national data-based cohort study investigating the impact of metformin use on all-cause mortality during TB treatment in patients with TB–DM. This cohort included all patients with TB who were registered and followed up for an appropriate amount of time. Second, we analyzed and adjusted for relevant covariates, including socioeconomic status and the presence of multiple co-morbidities, which are crucial contributing factors to the mortality of patients with TB–DM, by integrating three different national datasets. In addition, we performed a sensitivity analysis including PS matching, and it showed the positive effect of metformin on mortality in patients with TB–DM, which was consistent in various sets of analyses.
Despite these strengths, this study has some limitations. We could not access some information because the data used were from a retrospective cohort. First, we included patients with stage III chronic kidney disease (CKD) because we could not distinguish stage IIIa and stage IIIb CKD from our integrated dataset, which lacked information on CKD stage. Second, we could not fully account for potential confounding factors such as DM-related factors in PS matching and different characteristics between metformin users and non-users even after matching. To overcome this problem, we performed additional regression analyses after matching, including DM medications, CCI score, and multiple other prognostic variables [16,24]. Third, the data used in the analysis were based on the KNTSS, which is secondary data. There might be errors in the reporting of the treatment outcomes and missing data from each institution. However, the KNTSS is the most reliable national TB registry managed by the Korea Disease Control and Prevention Agency. Finally, we were unable to account for potential confounding factors, including glucose control status (e.g., HbA1c), smoking, drinking, and body mass index, which have a significant influence on DM- and TB-related deaths [25,26]. Considering HbA1c might have also impacted our findings because poorly controlled DM can influence poor outcomes of TB and mortality [27,28]. Therefore, further research is warranted.
In summary, our study demonstrated that the mortality rate during TB treatment was 8.8%, and this rate was lower in the metformin user group than in the non-user group. After adjusting for multiple covariates in the PS-matched cohort, metformin use was associated with a lower risk of all-cause mortality during TB treatment, suggesting its potentially protective role as an HDT in patients with TB–DM.
KEY MESSAGE
1. DM is known to increase mortality in patients with TB.
2. Metformin use is associated with reduced all-cause mortality in patients with TB-DM.
3. Metformin is considered a potential adjunctive agent for patients with TB-DM.
Supplementary Materials
Baseline characteristics of metformin users and non-users before propensity score matching in men
Baseline characteristics of metformin users and non-users before propensity score matching in women
Baseline characteristics of metformin users and non-users after propensity score matching in men
Baseline characteristics of metformin users and non-users after propensity score matching in women
Notes
CRedit authorship contributions
Eunki Chung: writing - original draft, writing - review & editing, visualization; Dawoon Jeong: formal analysis, writing - review & editing; Jeongha Mok: methodology, writing - review & editing; Doosoo Jeon: methodology, writing - review & editing; Hee-Yeon Kang: data curation, writing - review & editing; Heejin Kim: data curation, writing - review & editing; Heesun Kim: data curation, writing - review & editing; Hongjo Choi: conceptualization, formal analysis, writing - review & editing; Young Ae Kang: conceptualization, writing - original draft, writing - review & editing, visualization
Conflicts of interest
The authors disclose no conflicts.
Funding
This work was supported by the National Evidence-Based Healthcare Collaborating Agency, funded by the Ministry of Health and Welfare (NC19-002, NC20-003, and NC21-001) and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (HI19C1235).