Mechanical ventilation in patients with idiopathic pulmonary fibrosis in Korea: a nationwide cohort study
Article information
Abstract
Background/Aims
The prognosis of patients with idiopathic pulmonary fibrosis (IPF) and respiratory failure requiring mechanical ventilation is poor. Therefore, mechanical ventilation is not recommended. Recently, outcomes of mechanical ventilation, including those for patients with IPF, have improved. The aim of this study was to investigate changes in the use of mechanical ventilation in patients with IPF and their outcomes over time.
Methods
This retrospective, observational cohort study used data from the National Health Insurance Service database. Patients diagnosed with IPF between January 2011 and December 2019 who were placed on mechanical ventilation were included. We analyzed changes in the use of mechanical ventilation in patients with IPF and their mortality using the Cochran-Armitage trend test.
Results
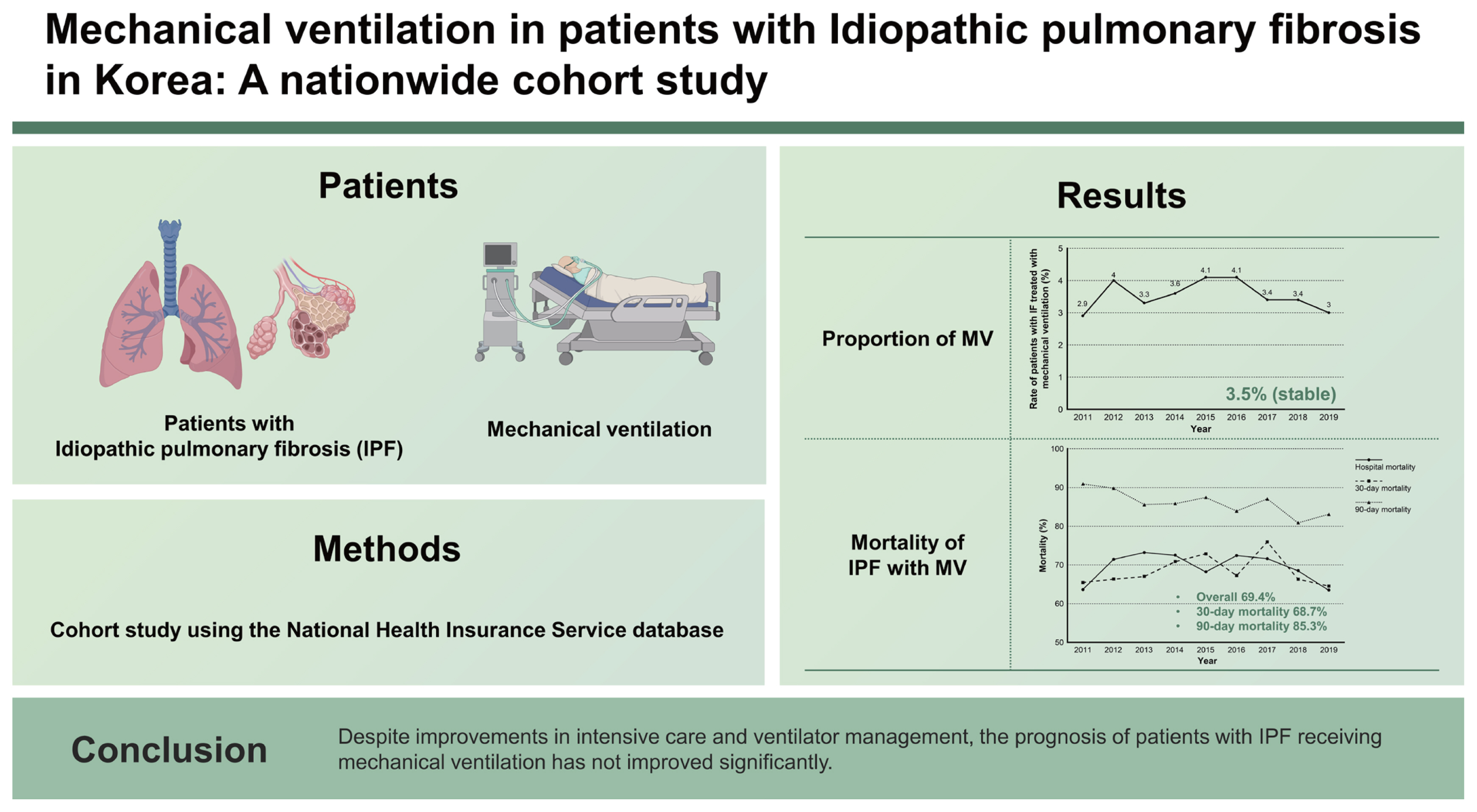
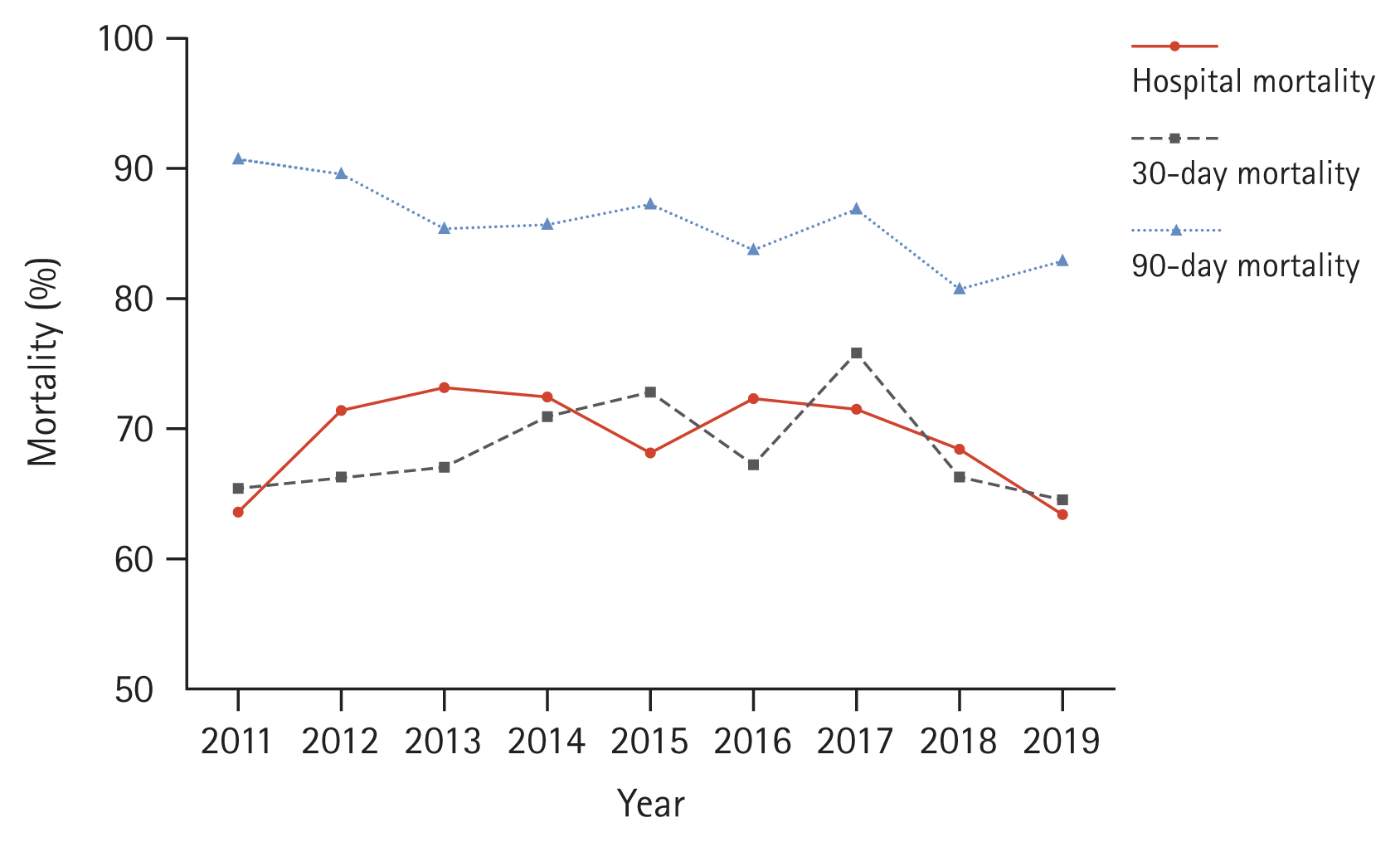
Between 2011 and 2019, 1,227 patients with IPF were placed on mechanical ventilation. The annual number of patients with IPF with and without mechanical ventilation increased over time. However, the ratio was relatively stable at approximately 3.5%. The overall hospital mortality rate was 69.4%. There was no improvement in annual hospital mortality rate. The overall 30-day mortality rate was 68.7%, which did not change significantly. The overall 90-day mortality rate was 85.3%. The annual 90-day mortality rate was decreased from 90.9% in 2011 to 83.1% in 2019 (p = 0.028).
Conclusions
Despite improvements in intensive care and ventilator management, the prognosis of patients with IPF receiving mechanical ventilation has not improved significantly.
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is an interstitial lung disease (ILD) characterized by chronic and progressive fibrosis of the lung parenchyma without known causes. It is a common form of ILD with a poor prognosis [1]. The overall mean survival is 4 years [2], and the median survival is only 3–4 months when acute exacerbation occurs [3]. In a pooled analysis, the outcome of patients with IPF who have respiratory failure requiring mechanical ventilation is poor, with a 3-month mortality of 94% [4]. The 2011 international guideline suggests not using mechanical ventilation in patients with IPF and respiratory failure who are not candidates for lung transplantation [5], consistent with subsequent reports on mechanical ventilation [3,6]. However, most previous studies reporting high mortality rates of patients with IPF on mechanical ventilation were conducted at a single center with a small sample size between the 1980s and the early 2000s [4,5]. Recently, short-term mortality of patients on mechanical ventilation has decreased over time, which implies an improved quality of intensive care unit (ICU) care [7,8]. The prognosis of patients with IPF admitted to the ICU has also improved [9]. In addition, the mortality of patients with IPF receiving mechanical ventilation has decreased, becoming lower than previously reported [10]. This discrepancy between the existing guideline and recent literature makes it difficult for clinicians to decide on the application of mechanical ventilation [11,12] or provide proper information to patients or their surrogates, particularly in situations where lung transplantation is not an option. Thus, the aim of this study was to investigate changes in the use of mechanical ventilation in patients with IPF and their outcomes over time.
METHODS
Data source and study population
The National Health Insurance Service (NHIS) in Korea provides health insurance to all citizens residing in Korea, except Medical Aid Beneficiaries and Health Care Beneficiaries for veterans [13]. Thus, abundant health-related data have been accumulated in the NHIS database. Through the National Health Insurance Sharing Service, researchers can access an established database or build a customized one. We constructed a customized database of patients with IPF placed on mechanical ventilation. This study was approved by Institutional Review Boards (IRBs) of Korea University Guro Hospital (2021GR0276) and the National Health Insurance Sharing Service (NHIS-2021-1-767). The requirement for written informed consent was waived by IRBs because all data were de-identified.
We enrolled eligible patients using the International Classification of Disease-Tenth Revision (ICD-10) codes. Patients with IPF were identified using the ICD-10 code for IPF (J84.1). We excluded patients with pulmonary fibrosis secondary to external agents using the following criteria: pneumoconiosis (J60–J64); respiratory conditions due to inhalation of chemicals, gases, fumes, and vapors (J68); and respiratory conditions due to other external agents, including drugs and radiation (J70). The aforementioned diagnostic codes were registered before assigning the IPF code. Patients with hypersensitivity pneumonitis (J67), sarcoidosis (D86), or connective tissue disease (CTD) were also excluded. ICD-10 codes for CTDs were as follows: rheumatoid arthritis (M05, M06), polyarteritis (M30), other necrotizing vasculopathies (M31), systemic lupus erythematosus (M32), dermatopolymyositis (M33), systemic sclerosis (M34), and other systemic involvement of the connective tissue (M35). As diseases mentioned above might be identified after the diagnosis of IPF, patients with both codes were excluded regardless of the order of code registration. Finally, patients who underwent lung transplantation (Z94.2, Z94.3) were excluded. This is because clinicians are not concerned about applying mechanical ventilation to candidates for lung transplantation. In addition, lung transplantation is the only curative treatment for IPF.
We used the NHIS claim code to identify patients treated with invasive mechanical ventilation (M5859, M5860) only when they were issued after registration of the IPF diagnostic code. Patients who underwent general anesthesia during index hospitalization were excluded. Patients who were placed on extracorporeal life support were also excluded. This is because extracorporeal life support is mostly used in lung transplantation candidates for rehabilitation and lung protection.
The claim code for each treatment was as follows: general anesthesia (L0101), partial extracorporeal circulation (O1901, O1902), partial extracorporeal circulation-extracorporeal membrane oxygenation (O1903, O1904), and partial extracorporeal circulation-extracorporeal membrane oxygenation-interventional lung-assist membrane ventilator (O1905, O1906).
In Korea, patients can receive financial support through copayment assistance policies for rare and intractable diseases. Additionally, IPF was registered as a rare and intractable disease in May 2009 with a disease code of V236. We only included patients who had been diagnosed with IPF since 2011 under both codes of J84.1 and V236. Patients diagnosed in 2020 were excluded due to incomplete survival data. Consequently, patients diagnosed with IPF between January 2011 and December 2019 were included in this study.
Comorbidzities and outcomes
The first day of mechanical ventilation after IPF diagnosis was set as the index date. For patients with multiple applications of mechanical ventilation, only data from the first mechanical ventilation were collected. Baseline characteristics on the index date, annual number of patients with IPF placed on mechanical ventilation, annual number of patients with IPF, and clinical outcomes of patients with IPF placed on mechanical ventilation each year were collected. Baseline characteristics included age, sex, and the Charlson comorbidity index (CCI). Comorbidities were identified using ICD-10 codes (Supplementary Table 1). The main outcome was hospital mortality during the index hospitalization. The length of hospital stay and mortalities (30-day and 90-day) were assessed based on index hospitalization and index date, respectively.
Statistical analysis
Categorical variables are expressed as numbers (percentages) and continuous variables are expressed as means ± standard deviations. Simple linear regression was used to assess annual trends in patient numbers, baseline characteristics, and length of hospital stay. The annual rate of patients with IPF placed on mechanical ventilation and their mortality each year were analyzed using the Cochran-Armitage trend test. To investigate whether or not the year of mechanical ventilation was associated with hospital mortality, a multivariable logistic regression analysis was performed incorporating variables with a p value < 0.1 in the univariable analysis and the year of mechanical ventilation. All tests were two-sided and a p value < 0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and R version 3.3.3 (R Foundation for Statistical Computing).
RESULTS
During the study period, 2,536 patients with pulmonary fibrosis were treated with mechanical ventilation. Approximately half of them were excluded because of concomitant CTDs or exposure to injurious agents. A total of 1,227 patients were included in the final analysis (Fig. 1). The annual number of patients (prevalent cases) with IPF who received mechanical ventilation increased from 55 in 2011 to 189 in 2019 (p < 0.001). The annual number of patients with IPF also increased from 1,874 in 2011 to 6,316 in 2019 (p < 0.001). However, the annual rate of patients with IPF receiving mechanical ventilation among total patients over the study period did not change significantly (p = 0.143). Overall, 3.5% of patients with IPF were treated with mechanical ventilation (Fig. 2).
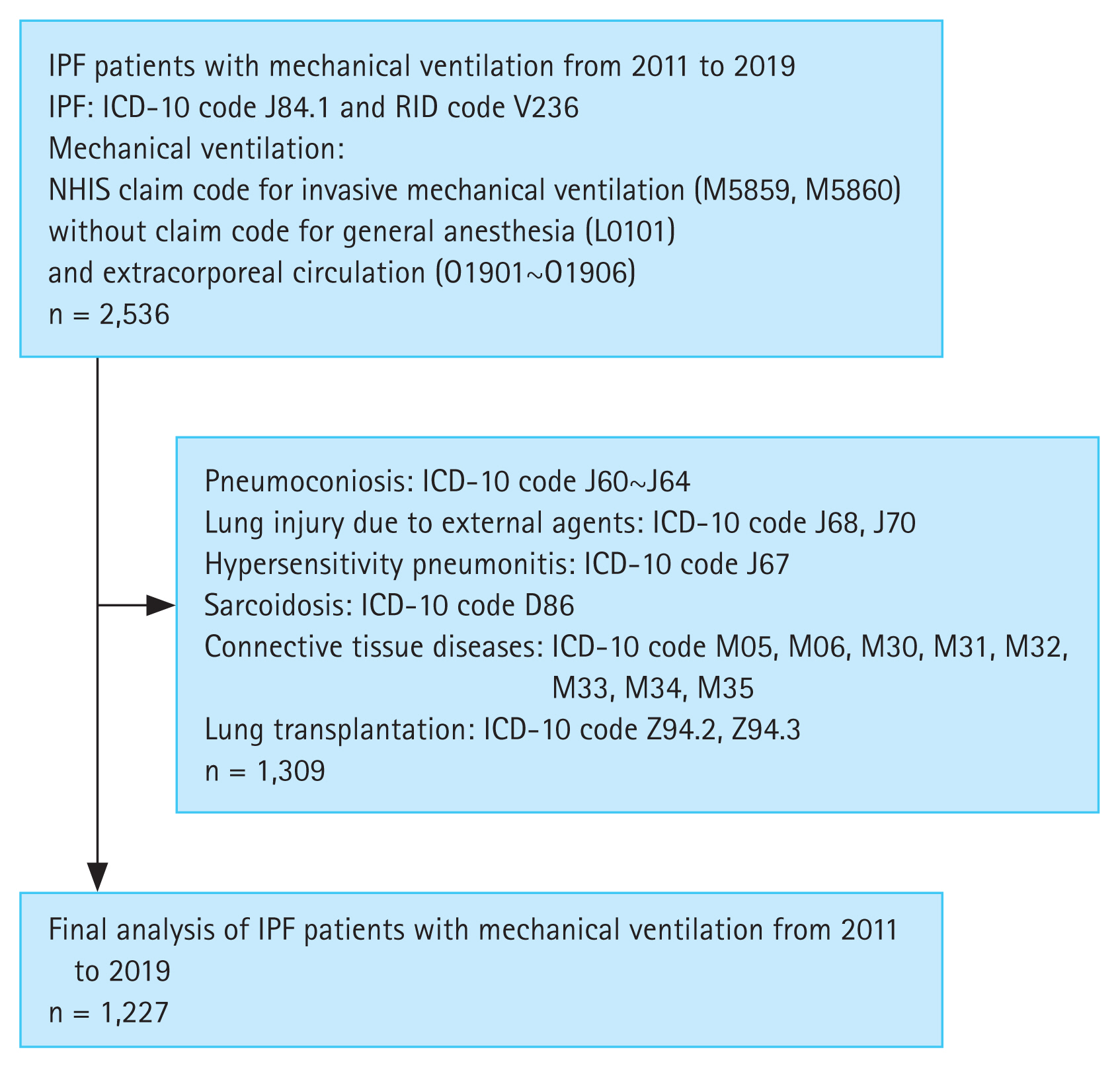
Patient identification flowchart. ICD-10, International Classification of Disease-Tenth Revision; IPF, idiopathic pulmonary fibrosis; NHIS, National Health Insurance Service; RID, rare and intractable disease.

(A) Annual number of patients with idiopathic pulmonary fibrosis (IPF) treated with mechanical ventilation. (B) Annual number of patients with IPF. (C) Annual rate of patients with IPF treated with mechanical ventilation among total patients with IPF.
Baseline characteristics
Table 1 displays baseline characteristics of patients with IPF placed on mechanical ventilation by year. Overall, 82% of patients were males. The mean age of all patients was 73.5 years. The annual mean age increased from 71.9 years in 2011 to 74.7 years in 2019 (p < 0.001). The proportion of patients aged ≥ 80 years increased from 21.8% in 2011 to 26.5% in 2019, although the increase was not statistically significant (p = 0.056). Overall, the CCI was ≥ 3 in approximately 70% of patients. The distribution of comorbidities changed over time. The proportion of patients with congestive heart failure increased from 20.0% in 2011 to 31.8% in 2019 (p = 0.006). Proportions of patients with dementia and metastatic solid tumor also increased from 3.6% in 2011 to 14.3% in 2019 (p = 0.031) and from 0% in 2011 to 4.2% in 2019 (p = 0.023), respectively. However, the proportion of patients with peptic ulcer decreased from 54.6% to 36.0% (p = 0.033).
Clinical outcomes
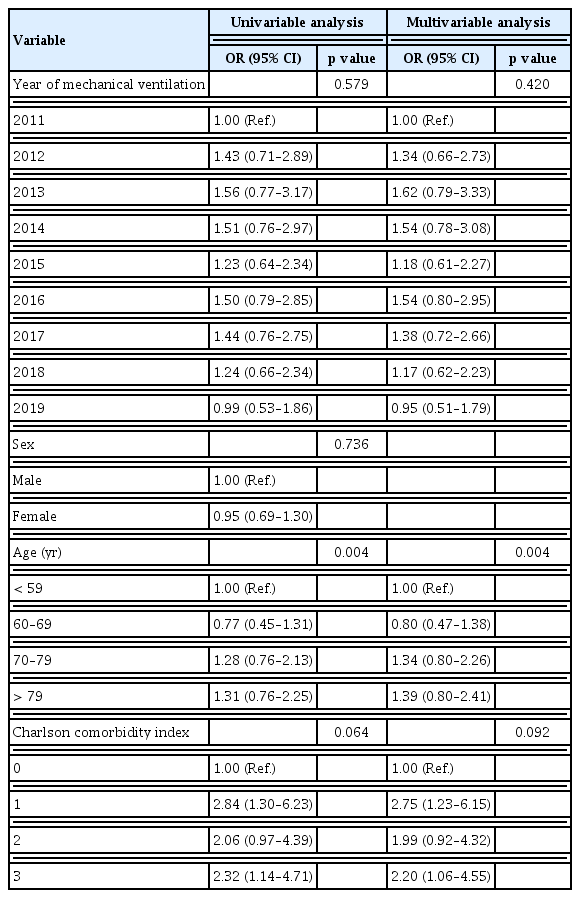
Supplementary Table 2 and Figure 3 present clinical outcomes. The overall hospital mortality rate during the study period was 69.4%. The annual hospital mortality rate did not change over the study period (p = 0.277). The overall length of hospital stay was 16.4 days, without showing a significant change over the study period (p = 0.521). Moreover, the overall 30-day mortality rate was 68.7%, which did not change significantly either (p = 0.821). In contrast, the 90-day mortality rate improved from 90.9% in 2011 to 83.1% in 2019 (p = 0.028). The overall 90-day mortality was 85.3%. Table 2 displays results of the logistic regression analysis of variables associated with hospital mortality. The year of mechanical ventilation was not associated with hospital mortality in either the univariable analysis (p = 0.579) or the multivariable analysis (p = 0.420). Age was associated with increased hospital mortality in the multivariable analysis (p = 0.004). Furthermore, hospital mortality tended to increase with a higher CCI, although statistical significance was not achieved (p = 0.092).
DISCUSSION
In this study, we investigated patients with IPF treated with mechanical ventilation between 2011 and 2019 in Korea. The number of patients with IPF treated with mechanical ventilation increased over time. Several changes in baseline characteristics including age and comorbidities were observed. However, their ratios to all patients with IPF and their mortality did not change significantly.
Recent studies on large cohorts using administrative data from the United States have revealed that hospital mortality of patients with IPF requiring mechanical ventilation ranges from 39.4 to 55.7%, much lower than those reported in previous studies. These studies have also revealed that hospital mortality has declined over time [10,14,15]. These aforementioned results are in contrast to the high hospital mortality demonstrated in our study (nearly 70%). In previous studies, patients who had ILD other than IPF, secondary pulmonary fibrosis, or who underwent surgery were included. In our study, we used both the ICD-10 and rare and intractable disease codes to identify only patients with IPF. As rare and intractable disease code was closely related to financial concerns of patients, health service providers, and government agencies, the registration of the code was performed more strictly. The diagnostic validity was higher in rare and intractable disease claim data than in general disease claim data [16]. In addition, we excluded patients with secondary pulmonary fibrosis or ILD and those who underwent surgery. Acute respiratory failure in other ILDs has demonstrated a better prognosis than that in IPF [17–19]. In patients with IPF who are mechanically ventilated, postoperative respiratory failure is associated with a lower hospital mortality [20]. Therefore, mortality rates of our study and previous studies seem to be different due to difference in the study population. Indeed, our results are similar to those of a study using clinical data of patients with IPF between 2015 and 2017 in Korea [21]. Furthermore, in contrast to previous studies, our study observed no significant temporal changes in mortality among patients with IPF. Acute exacerbation is the most common reason for mechanical ventilation in patients with IPF [22–24]. While guidelines recommend steroid administration for acute exacerbation of IPF, the quality of evidence is very low [3,5]. Recent trials evaluating the efficacy of agents other than steroids have also failed [25,26]. Given the absence of advancement in treatment for acute exacerbation of IPF, the stagnation in prognosis is not surprising.
In our study, the 90-day mortality declined during the study period, although the overall mortality rate remained high at 85.3%. Approximately half of the patients who survived in the hospital died within approximately two months. In pooled analysis of past data, the hospital mortality was 87% and the 3-month mortality was 94% [4]. When compared to pooled analysis results, the 90-day mortality demonstrated less improvement and the difference between hospital and 90-day mortalities widened in our study. This suggests that even if patients were discharged alive, they would still receive life-sustaining treatment rather than recover and return to their daily lives.
In the multivariable analysis, the year of mechanical ventilation was not associated with mortality. Age was the only independent risk factor for increased mortality. Comorbidity appeared to be associated with increased mortality, although such association was not statistically significant. This again demonstrated that there was little improvement in the prognosis of patients with IPF with respiratory failure over time.
The annual rate of patients with IPF receiving mechanical ventilation did not change significantly over the study period. This might reflect the disease course of IPF, wherein a certain proportion of patients would experience respiratory failure annually.
Strengths of our study were the utilization of NHIS data from the last 9 years and the rigorous case definition. These strengths enabled a large sample size, detection of annual changes, and precise selection of patients with IPF on mechanical ventilation.
This study had several limitations. First, this study was not free of inherent weaknesses resulting from the utilization of administrative data, including selection bias and accuracy of diagnosis codes [27]. However, selection bias would be low because the NHIS provides universal coverage to 97% of Koreans [13]. The requirement of rare and intractable disease codes for the case definition could compensate for the shortcomings of the ICD-10 codes and improve the accuracy of diagnosis. Second, the diagnostic criteria for IPF were revised twice (in 2011 and 2018) during the study period [5,28]. Patient characteristics such as chest computed tomography findings and frequency of patients who underwent surgical lung biopsy might differ. Third, information on causes of respiratory failure in patients requiring mechanical ventilation was not provided. Several studies have demonstrated that the most common cause of respiratory failure is acute exacerbation, followed by respiratory tract infection, and that the prognosis is worse in patients with acute exacerbation than in those with respiratory tract infection [22–24]. Another study has also reported that the prognosis of patients with ILD and infection is better than that of patients with acute exacerbation. In addition, the prognosis of patients with ILD exacerbation triggered by infection is better than that of patients with idiopathic or non-infection-trigged exacerbation [29]. For a more accurate analysis, the annual hospital mortality rate needs to be adjusted to the cause of respiratory failure each year. Fourth, other clinically important variables including general ICU prognostic measures and IPF-related parameters such as the extent of fibrosis on chest computed tomography, pulmonary function test results, and blood gas analysis were not provided. They are presumed to be closely associated with clinical outcomes. However, they could not be extracted from the NHIS database. We did not provide data on medications usage, particularly antifibrotic agents. The use of antifibrotic agents has recently increased in patients with IPF and improved the prognosis [30,31]. Moreover, antifibrotic agents are associated with a reduced risk of acute exacerbation and mortality after exacerbation [32]. Therefore, the prevalence and prognosis of patients with IPF requiring mechanical ventilation could be affected. In Korea, health insurance coverage for the antifibrotic drug, pirfenidone, was commenced in 2015. However, hospital, 30-day, and 90-day mortality rates of patients with IPF placed on mechanical ventilation did not differ significantly between 2011–2014 and 2015–2019. Short-term mortality did not change meaningfully from 2015 to 2019 either (data not displayed). Thus, despite an overall increase in the use of antifibrotic agents, mortality rates of patients with IPF receiving mechanical ventilation in Korea did not change significantly. Fifth, instead of utilizing individual comorbidities for mortality adjustments, we relied on the CCI. However, it is worth noting that within the CCI, both congestive heart failure and peptic ulcer disease are assigned a score of 1. Nevertheless, intuitively, congestive heart failure should have a more detrimental impact on mechanically ventilated patients than peptic ulcer disease. The increase in number of patients with dementia or metastatic solid tumor might have a negative impact on prognosis. Moreover, by treating CCI ≥ 3 as a single variable, we were unable to discern changes in the distribution of scores within 3 or higher range or their potential impact on mortality. The decline in the prevalence of peptic ulcer disease warrants further consideration. In 2011, 2015, and 2022 guidelines, suggestions for antiacid treatments such as proton pump inhibitors (PPIs), changed slightly [5,6,33]. Therefore, the use of PPIs might have changed over the study period. The requirement of a diagnostic code of peptic ulcer disease for prescribing PPIs introduces the possibility that changes in prescribing behavior might have influenced the recorded frequency of peptic ulcer disease irrespective of the true prevalence of the condition. In this case, the CCI score might also have been affected. Sixth, this study’s results cannot be applied to patients who have undergone lung transplantation or placed on extracorporeal life support. Considering the increasing number of lung transplantation cases in Korea, the prognosis for all patients with IPF who developed respiratory failure might have improved over time [34]. Finally, we did not provide information on conditions of patients who were discharged alive, such as performance status, dependency on mechanical ventilation, or residency in the nursing care facility.
In summary, in patients with IPF treated with mechanical ventilation, hospital and 30-day mortality rates did not change significantly during the study period. Although the 90-day mortality rate decreased over time, it remained high. Despite improvements in ICU care and ventilator management in the general population, the prognosis of patients with IPF receiving mechanical ventilation was not much improved. Given the unfavorable prognosis and the limited medical resources, the use of mechanical ventilation in this population should be approached cautiously.
KEY MESSAGE
1. The hospital mortality rate for patients with idiopathic pulmonary fibrosis receiving mechanical ventilation in Korea has not improved over the past 9 years.
2. The 90-day mortality rate for patients with idiopathic pulmonary fibrosis receiving mechanical ventilation in Korea decreased but remained high above 85%.
3. The decision to treat patients with idiopathic pulmonary fibrosis using mechanical ventilation should be made with careful deliberation.
Supplementary Materials
Acknowledgments
The authors used generative artificial intelligence (ChatGPT) to improve readability and language when writing a draft. The final manuscript was written with the assistance of a professional editing service.
Notes
CRedit authorship contributions
Jae Kyeom Sim: conceptualization, investigation, data curation, formal analysis, writing - original draft; Seok Joo Moon: data curation, formal analysis, writing - review & editing; Juwhan Choi: investigation, writing - review & editing; Jee Youn Oh: investigation, writing - review & editing; Young Seok Lee: investigation, writing - review & editing; Kyung Hoon Min: investigation, writing - review & editing; Gyu Young Hur: investigation, writing - review & editing; Sung Yong Lee: investigation, writing - review & editing; Jae Jeong Shim: conceptualization, investigation, data curation, writing - original draft, supervision
Conflicts of interest
The authors disclose no conflicts.
Funding
None