Prevalence and predictors of multidrug-resistant bacteremia in liver cirrhosis
Article information
Abstract
Background/Aims
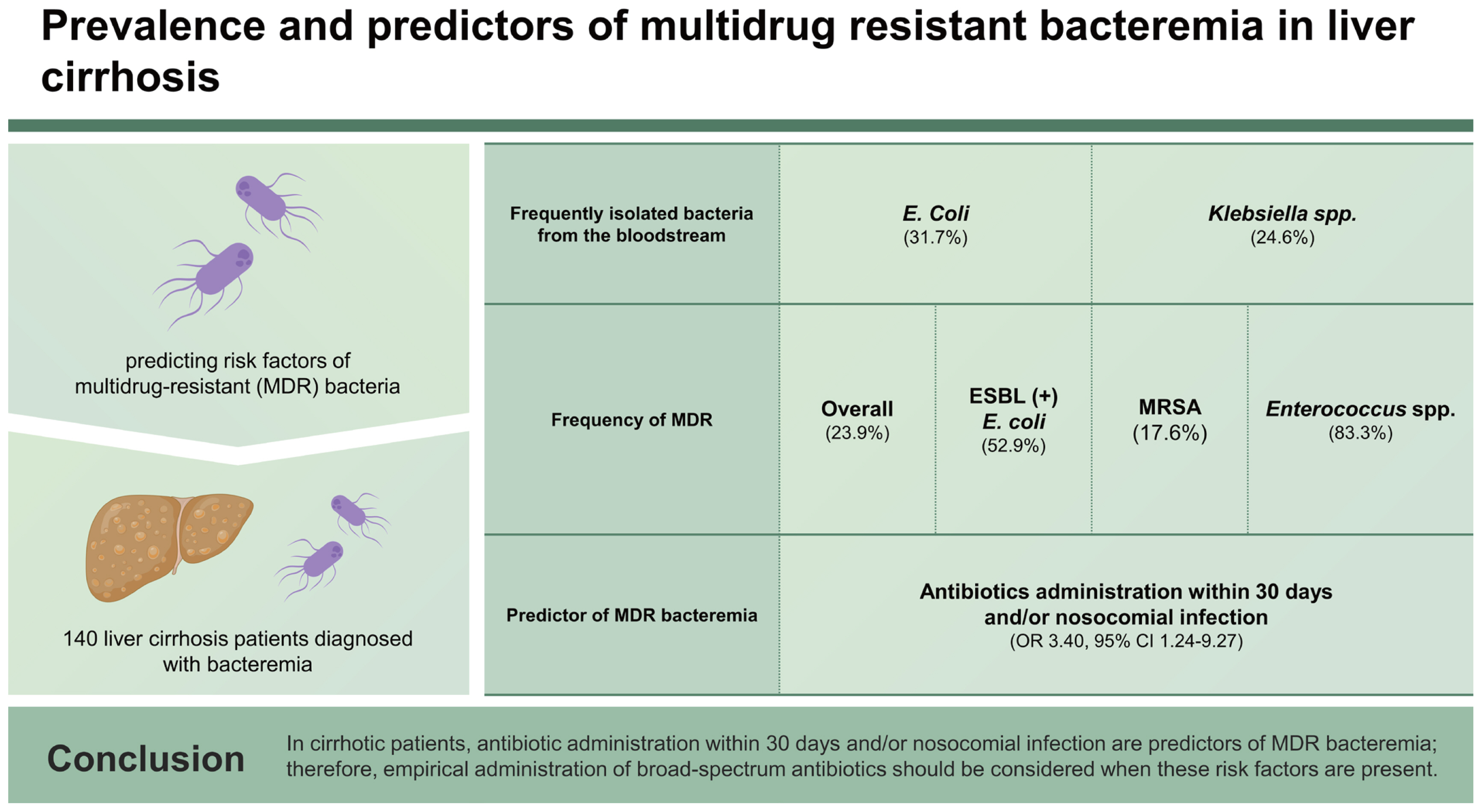
Improved knowledge of local epidemiology and predicting risk factors of multidrug-resistant (MDR) bacteria are required to optimize the management of infections. This study examined local epidemiology and antibiotic resistance patterns of liver cirrhosis (LC) patients and evaluated the predictors of MDR bacteremia in Korea.
Methods
This was a retrospective study including 140 LC patients diagnosed with bacteremia between January 2017 and December 2022. Local epidemiology and antibiotic resistance patterns and the determinants of MDR bacteremia were analyzed using logistic regression analysis.
Results
The most frequently isolated bacteria, from the bloodstream, were Escherichia coli (n = 45, 31.7%) and Klebsiella spp. (n = 35, 24.6%). Thirty-four isolates (23.9%) were MDR, and extended-spectrum beta-lactamase E. coli (52.9%) and methicillin-resistant Staphylococcus aureus (17.6%) were the most commonly isolated MDR bacteria. When Enterococcus spp. were cultured, the majority were MDR (MDR 83.3% vs. 16.7%, p = 0.003), particularly vancomycin-susceptible Enterococcus faecium. Antibiotics administration within 30 days and/or nosocomial infection was a significant predictor of MDR bacteremia (OR: 3.40, 95% CI: 1.24–9.27, p = 0.02). MDR bacteremia was not predicted by sepsis predictors, such as positive systemic inflammatory response syndrome (SIRS) or quick Sequential Organ Failure Assessment (qSOFA).
Conclusions
More than 70% of strains that can be treated with a third-generation cephalosporin have been cultured. In cirrhotic patients, antibiotic administration within 30 days and/or nosocomial infection are predictors of MDR bacteremia; therefore, empirical administration of broad-spectrum antibiotics should be considered when these risk factors are present.
INTRODUCTION
Sepsis has been the leading cause of the increase in liver-related mortality among patients with liver cirrhosis (LC) over the past decade [1]. Patients with LC have a 10-fold higher incidence of bacterial infections [2] compared to the general population, which is attributable to their dysregulated immune function, changes in the microbiome, and increase in bacterial translocation from the gut to the systemic circulation [3,4]. Bacterial infections are associated with prolonged hospitalization, decompensation events, including hepatorenal syndrome and hepatic encephalopathy, acuteon-chronic liver failure, and a fourfold increase in the risk of mortality [5]. Therefore, it is critical to administer timely and appropriate empirical antibiotic therapy to manage bacterial infections.
However, in recent years, empirical antibiotic therapy in clinical settings has become more challenging due to the heterogeneous epidemiology of bacterial infections, particularly the increasing incidence of Gram-positive bacterial pathogens and the emergence of multidrug-resistant (MDR) organisms [4]. MDR infections have been associated with an increased risk of receiving inappropriate empiric therapy, thus resulting in a higher incidence of septic shock and in-hospital mortality [6,7]. There are also significant differences in MDR bacteria profiles among geographical areas as well as in the demographic and clinical characteristics of cirrhotic patients [8–11]. Therefore, improved knowledge of local epidemiology and antibiotic resistance patterns is required to optimize the management of infections
There have been numerous studies examining the risk factors for several types of MDR infections, such as spontaneous bacterial peritonitis or urinary tract infection, in cirrhotic patients [6,12,13]. By contrast, only a few studies on MDR bacteremia in cirrhotic patients have been conducted in Korea. Moreover, patients with cirrhosis who had bacteremia exhibited a higher mortality risk than those who did not have bacteremia [14]. Therefore, this study aimed to investigate local epidemiology and antimicrobial resistance patterns in patients with LC over the past 5 years and to identify the predictors of MDR bacteremia.
METHODS
Study design and population
This is a single center retrospective cohort study of LC patients who were diagnosed with bacterial infections between January 2017 and December 2022, without malignancy, transplantation, or end-stage renal disease with hemodialysis (n = 157). Among them, patients whose antibiotic susceptibility results were not confirmed were excluded (n = 17) and 140 patients were ultimately enrolled in our study. In patients with repeated bacteremia diagnosis, only the first strain, when bacteremia first occurred, was included. The authors followed principles of the Declaration of Helsinki, and the work was approved by the ethics committee of the Institutional Review Board of Samsung Medical Center (approval no.2023-07-077). Informed consent from patients was waived because only de-identified data routinely collected during hospital visits.
Data collection and definitions
Data were extracted from electronic medical records. Data collected at the time of bacterial infection included patient demographics (age, sex), cause of LC, underlying comorbidity (diabetes mellitus, hypertension, and medical history of hospital admission within the previous 3 months), clinical data including vital signs (systolic blood pressure, heart rate, respiratory rate, and body temperature), use of external medical equipment (mechanical ventilation, percutaneous catheter drainage, and central venous line), laboratory values (white blood cell [WBC] count, hemoglobin count, platelet count, prothrombin time [INR], albumin, total bilirubin, aspartate transaminase, alanine transaminase, alkaline phosphatase, blood urea nitrogen, creatinine, C-reactive protein, neutrophil to lymphocyte ratio [NLR]), medication use, and antibiotic use within 30 days of diagnosis of the bacterial infection. The severity of liver disease was estimated by the model for end-stage liver disease (MELD) score and Child-pugh class and albumin-bilirubin (ALBI) grade. [15].
MDR was defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories [16]; in the current study, the following bacteria were considered MDR according to previous reports: extended-spectrum beta-lactamase (ESBL) producing Escherichia coli or Klebsiella pneumoniae, derepressed chromosomic Amp-C beta-lactamase-producing Enterobacteriaceae (Enterobacter or Citrobacter spp.), carbapenem-resistant K. pneumoniae, carbapenem-resistant E. coli, carbapenem-resistant Pseudomonas aeruginosa, carbapenem-resistant Stenotrophomonas maltophilia, carbapenem-resistant Acinetobacter baumanii and Burkholderia cepacia, methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant coagulase-negative staphylococci (MR CoNS), and vancomycin-susceptible and vancomycin-resistant Enterococcus faecium (VSE, VRE) [17],
Systemic inflammatory response syndrome (SIRS) criteria and quick Sequential Organ Failure Assessment (qSOFA) were used to define sepsis [18,19]. SIRS was defined by the presence of at least two of the following: body temperature < 36°C or > 38°C, heart rate > 90 beats per minute, respiratory rate > 20/min, WBC < 4,000/μL or > 12,000/μL, or immature neutrophils > 10% [18]. qSOFA is defined as having at least two of the following: respiratory rate of 22/min or greater, altered mentation, or systolic blood pressure of 100 mmHg or less [19].
Community-acquired bacterial infections were those diagnosed within the first 48 hours of hospitalization whereas those diagnosed 48 hours after admission were classified as nosocomial bacterial infections [20].
Statistical analysis
Values are presented in the form of median (interquartile range) or frequency (percentage). The baseline characteristics of patients with MDR and non MDR bacteremia were compared using the Mann–Whitney U tests for continuous variables or the χ2 test or Fisher’s exact test for categorical variables. Logistic regression analyses were used to identify factors associated with MDR. Multivariable analyses were performed for variables that showed p values < 0.2 in the univariable analyses. All statistical analyses were performed using SPSS software version 27 for Windows (IBM Corp., Armonk, NY, USA). A two-tailed p < 0.05 was considered to reflect a statistically significant result.
RESULTS
Baseline characteristics
Of the 140 patients that were ultimately enrolled, 88 (62.9%) were male, and the median age was 57 years old (49–70 years). Table 1 presents the baseline characteristics of the included patients. Thirty-three (23.6%) patients were infected with MDR bacteria, while 107 (76.4%) were infected with non MDR bacteria. There were no significant differences between these two groups in terms of age, sex, cause of LC, presence of diabetes mellitus or hypertension, vital signs, SIRS score, qSOFA score, presence of septic shock, steroid use within 30 days, use of external medical equipment or laboratory values, including MELD score, Child-pugh class, ALBI grade, and NLR. Patients in the MDR group were more likely to have a history of hospital admission within the previous 3 months (MDR: 60.6% vs. 41.1%, p = 0.049), administered antibiotics within 30 days of bacteremia infection (MDR: 51.5% vs. 30.8%, p = 0.03) or nosocomial infection (MDR: 54.5% vs. 29.0%, p = 0.007). There were no differences in the class, route, and duration of antibiotics administered within 30 days between the two groups (Supplementary Table 1).
Microbiological characteristics
Ultimately, 142 bacteria, from the blood of 140 patients, were cultured; 2 microorganisms from 2 patients were cultured simultaneously. Gram-negative pathogens (n = 91, 64.1%) were more common than Gram-positive pathogens (n = 51, 35.9%), with E. coli (n = 45, 31.7%), Klebsiella spp. (n = 35, 24.6%), S. aureus (n = 26, 18.3%), and Streptococcus spp. (n = 16, 11.3%) being the most frequently identified bacteria. P. aeruginosa was found in two patients (1.4%). The proportion of MDR bacteremia did not significantly differ between Gram-negative pathogens and Gram-positive pathogens (23.1% vs. 25.5%). E. coli was the most frequently isolated MDR pathogen (n = 18/34, 52.9%), and all 18 MDR E. coli were ESBL E. coli. Notably, the majority of Enterococcus spp. were MDR strains (MDR 83.3% vs. 16.7%, p = 0.003) and all five MDR Enterococcus spp. were VSE. By contrast, Klebsiella spp. (MDR 2.9% vs. 97.1%, p = 0.001) and Streptococcus spp. (MDR 0.0 vs. 100.0%, p = 0.013) were mostly non MDR strains (Table 2). There were two incidences of carbapenem-resistant Gram-negative bacteria (CRGNB) among the 34 MDR strains, and they were all A. baumanii.
Risk factors for MDR bacterial infections
On univariable logistic regression, antibiotics administration within 30 days and/or nosocomial infections was found to be a significant predictor of MDR, compared to a lack of antibiotics administration within 30 days and community-acquired infections (odds ratio [OR]: 4.31, 95% confidence interval [CI]: 1.78–10.42, p = 0.001) (Table 3). In multivariable logistic regression, adjusting hospital admission within the previous 3 months and use of external medical equipment, antibiotics administration within 30 days and/or nosocomial infection was independently associated with MDR bacteremia (OR: 3.40, 95% CI: 1.24–9.27, p = 0.02). Patients were classified into four subgroups according to nosocomial infection and/or antibiotic history within 30 days (Group 1: no antibiotics administration within 30 days and non-nosocomial infection; Group 2: antibiotics administration within 30 days and non-nosocomial infection; Group 3: no antibiotics administration within 30 days and nosocomial infection; Group 4: antibiotics administration within 30 days and nosocomial infection). Notably, a significantly higher incidence of MDR bacteremia was observed in patients with either antibiotic administration within 30 days and nosocomial infection compared to patients without antibiotics administration within 30 days and community-acquired infection (p = 0.005), respectively. However, there were no significant differences in the incidence of MDR bacteremia between groups 2, 3, and 4 (33.3%, 40.0%, and 34.5%, respectively) (Fig. 1).
DISCUSSION
In the current study, we demonstrated the epidemiology and antimicrobial resistance patterns of bacteremia and risk factors for MDR bacteremia in patients with LC. MDR bacteria were identified in 33 (23.6%) patients, with E. coli being the most frequently isolated MDR pathogen. Antibiotic exposure within the previous 30 days and/or a nosocomial infection was found to increase the risk of MDR bacteremia whereas sepsis predictors including SIRS and qSOFA were shown to be ineffective for predicting MDR bacteremia.
The most commonly isolated bacteria were E. coli and Klebsiella spp.; this distribution is consistent with that reported in the recently published global cross-sectional research of infections in cirrhosis patients [8], indicating that the translocation of intestinal bacteria is an important pathophysiological mechanism for bacteremia in cirrhosis patients [21]. However, the current study found that the proportion of bacteremia caused by Gram-positive bacterial pathogens increased slightly, from 27.7% to 35.9%, compared to the results reported by a previous Korean multicenter study conducted between 2006 and 2009 [22]. This change may be attributed to the widespread use of quinolones for bacterial infection prophylaxis in patients with LC. Notably, a previous study found increased rates of Gram-positive bacterial infections in 77% and 58% of patients in intensive care units and invasive procedure patients, respectively [23]. It is therefore necessary to consider the possibility that Gram-positive bacteria could be the cause of infection, particularly in hospitalized patients. Meanwhile, only two Pseudomonas cases have been isolated, which is similar to the distribution reported in a recent study in China (1.1%) [24]. Given the low prevalence of Pseudomonas, an empiric agent active against Pseudomonas does not seem to be routinely required.
In our population-based study, 34 (23.9%) of the 142 bacteria isolated from the blood of the LC patients were found to be MDR pathogens, which was comparable to the findings of a recent multicenter study in Korea (24.8%) [25]. However, it was slightly lower than the recently reported rate of MDR bacteremia in cirrhosis in the United States (33%) [9] and Europe (43.5%) [10], while it was higher than that in Australia (5.6%) [11]. ESBL-producing E. coli was the most frequently isolated MDR bacteria, followed by MRSA and VSE. In particular, the majority of Enterococcus spp. were resistant to cephalosporins, potentially due to the widespread use of third-generation cephalosporins for prophylaxis of a wide range of infections.
Antibiotic resistance is a major public health issue around the world. Especially, in cirrhotic patients, early recognition of patients at risk for MDR bacteremia is essential for optimizing the choice of empiric therapy, since proper empiric therapy is independently related to enhanced survival in cirrhotic patients with bacteremia [7]. In this study, antibiotic exposure within 30 days and/or nosocomial infection was identified as an important predictor of MDR bacteremia in patients with LC. Increased resistance rates in nosocomial infection can be attributed to human-to-human transmission or the use of infusion medical devices (diuretic catheters, intravascular catheters, or intravascular tubes), but they are commonly linked to the recent use of antibiotics [26]. Therefore, this study conducted an additional subgroup analysis, and the incidence of MDR bacteremia was comparable among patients who had taken antibiotics within the previous 30 days, those with nosocomial infections, or both groups. These findings suggest that empirical antibiotics should be used against MDR bacteremia in patients with LC and recent antibiotic exposure and/or nosocomial infection.
Third-generation cephalosporins are currently recommended as the first empirical treatment option for most infections in cirrhotic patients [27]. In this study, 105 out of 142 whole bacteria (73.9%) could be treated with third-generation cephalosporins, demonstrating that third-generation cephalosporins are still an appropriate empirical antibiotic for bacteremia in cirrhosis patients. The current guidelines encourage the use of broad-spectrum antibiotics, a combination of carbapenem ± glycopeptide, in individuals with LC who have an infection that is at high risk of MDR. The majority of bacteremia (97.2%) in our cohort could be effectively treated with ertapenem and vancomycin. By contrast, empirical antibiotics such as colistin and linezolid that target CRGNB and VRE are not recommended for use due to the extremely low incidence of CRGNB and VRE.
Previous studies have reported higher mortality in LC patients who develop septic shock [28,29]. Certain guidelines have proposed SIRS or qSOFA scores to quickly identify individuals with suspected infections who are likely to progress to sepsis [19,30]. Meanwhile, in this study, qSOFA negative findings were observed in 52.9% of cirrhosis patients, even with bacteremia. In addition to a recent study in which 36.7% of sepsis patients with cirrhosis were found to have a qSOFA score of less than 2 [31], these results indicate that qSOFA scores cannot detect infection early in cirrhosis patients. Therefore, screening based on a qSOFA score of ≥ 2 has limited value for predicting infection early with LC, and if infection is suspected in an LC patient beyond the qSOFA score, then active examination and close monitoring are needed to prevent the patient’s deterioration. We also found that positive SIRS or qSOFA could not predict MDR bacteremia in cirrhotic patients; therefore, another screening tool is needed to detect MDR infection in LC patients.
This study has some limitations. First, since this is a single-center study that investigated bacteremia epidemiology in cirrhotic patients, its generalizability is limited. Second, because it was a retrospective study using electronic medical records, information on the infection source is limited, so there is yet to be any analysis of the MDR pathogen according to the infection. Therefore, there is a need for further research to determine the epidemiology and risk factors of MDR pathogens while considering the features of each infection type/source. Finally, because only the first bacteremia event was included, there may be bias in the assessment of prevalence in terms of organism type. However, considering that the epidemiological patterns of MDR bacteremia differ substantially between different geographical areas, our study results provide a basis for developing regional guidelines and improving treatment.
In conclusions, more than 70% of strains from blood isolates of patients with LC are susceptible to treatment with a third-generation cephalosporin; however, if risk factors such as a history of antibiotic administration within 30 days and/or nosocomial infection are present, empirical administration of broad-spectrum antibiotics should be considered.
KEY MESSAGE
1. The most prevalent bacteria isolated from the bloodstream of patients with cirrhosis in Korea were E. coli and Klebsiella spp, with more than 70% of strains susceptible to treatment with a third-generation cephalosporin.
2. Thirty-four isolates (23.9%) were MDR bacteria, with Methicillin-resistant S. aureus and extended-spectrum beta-lactamase E. coli as the most frequently identified MDR bacteria.
3. Antibiotic administration within 30 days and/or nosocomial infection were significant predictors of MDR bacteremia; therefore, empirical administration of broad-spectrum antibiotics should be considered when these risk factors are present.
Notes
CRedit authorship contributions
Aryoung Kim: conceptualization, methodology, data curation, formal analysis, writing - original draft, writing - review & editing, project administration; Byeong Geun Song: writing - review & editing; Myung Ji Goh: conceptualization, methodology, data curation, formal analysis, writing - original draft, writing - review & editing, supervision, project administration; Wonseok Kang: writing - review & editing; Dong Hyun Sinn: writing - review & editing; Geum-Youn Gwak: writing - review & editing; Yong-Han Paik: writing - review & editing; Moon Seok Choi: writing - review & editing; Joon Hyeok Lee: writing - review & editing
Conflicts of interest
The authors disclose no conflicts.
Funding
None
Data availability statement
The data supporting the findings of this article cannot be shared publicly, given the privacy of the individuals who participated in the study. The data will be shared upon reasonable request to the corresponding author.