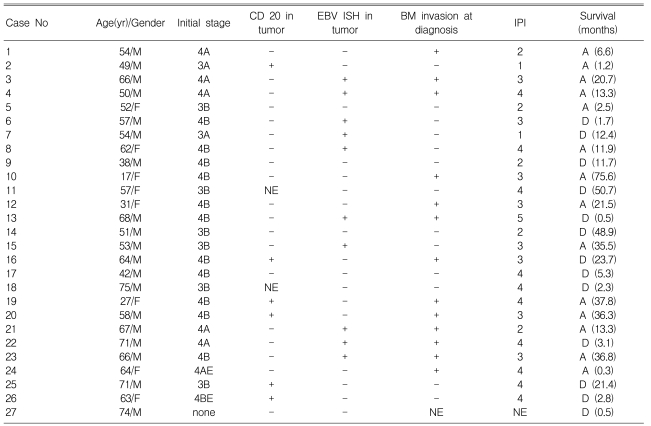
Epstein-Barr Virus-Positivity in Tumor has no Correlation with the Clinical Outcomes of Patients with Angioimmunoblastic T-cell Lymphoma
Article information
Abstract
Background/Aims
Epstein-Barr virus (EBV) is involved in the pathogenesis of angioimmunoblastic T-cell lymphoma (AILT), but its precise role and prognostic impact are not clear. This study aimed to evaluate the incidence of EBV-postitivity in the tumor and bone marrow (BM) samples from AILT patients, and their correlations with the clinical variables and patient survival.
Methods
Seventy AILT cases were identified over a period of 8 years. Twenty seven cases were investigated for their EBV tumor status, and 10 BM samples of these patients were investigated for their EBV status with using in situ hybridization (ISH). EBV PCR was performed for the BM mononuclear cells in 8 cases.
Results
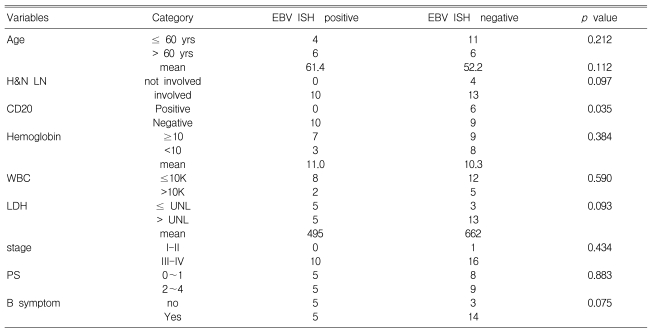
Among the 27 tumor specimens, ten (37%) were EBV-positive. Only CD20-negativity in tumor correlated with the EBV-positivity (p=0.035). In 13 (48%) patients, gross tumor involvement was recognized by hematoxylin-eosin staining at the time of diagnosis. Among the 10 patients who had additional BM slides available, there were 3 with BM involvement, and none showed EBV positive results on ISH. EBV PCR of the BM mononuclear cells revealed one-positive case among 8 patients. This patient was negative for both BM involvement and EBV ISH. The median overall survival of the 25 treated patients was 48.9 months (95% CI: 18.6~79.2 months). Neither overall survival nor progression-free survival was related with EBV-positivity of the tumor.
Conclusions
EBV-positivity of tumor had no impact on the prognosis of AILT patients.
INTRODUCTION
More than 90% of adults worldwide are infected with Epstein-Barr virus (EBV) and they carry the virus as a life-long persistent infection, with latent infection of their B lymphocytes and viral production into their saliva1, 2). EBV is associated with the pathogenesis of several different types of aggressive non-Hodgkin's lymphoma (NHL) and Hodgkin's lymphoma. In 1976, screening 27 cases of lymphoid tissue lesions for EBV-DNA with using nucleic acid reassociation kinetics led to the detection of one virus-positive case of angioimmunoblastic T-cell lymphoma (AILT)3). Many subsequent studies have demonstrated the presence of EBV in 58~97% of all AILT cases in either the T or B cells4-7). The clinical presentation of AILT is usually systemic disease with B symptoms, while the pathology reveals a polymorphous infiltrate involving the lymph nodes with a prominent proliferation of endothelial and dendritic cells8). Some patients go on to develop a secondary EBV-positive large B-cell lymphoma.
A recent study demonstrated that the presence of EBV-DNA obtained by performing real-time quantitative PCR and Southern blotting analysis of tumor tissue had no significant effect on the clinical outcome of AILT patients9). As for the patients with nasal NK/T-cell lymphoma, a EBV-positive tumor is a prerequisite for making the diagnosis10). A recent study proved that minimal EBV involvement in the bone marrow (BM) was a powerful prognostic marker11).
Therefore, we performed this study to evaluate the rate of EBV-positivity in tumor and BM and their prognostic values for the clinical outcome of AILT patients.
MATERIALS AND METHODS
Patients
Over a period of 8 years (from August 1998 to July 2005), 70 AILT cases from 8 medical centers were identified based on the histological and immunohistochemical criteria with using the REAL and/or WHO classifications. We enrolled 27 patients who had additional tissue slides for EBV in situ hybridization (ISH). Ten patients' BM slides were also analyzed in this study. Eight patients had their cryopreserved BM mononuclear cells for performing EBV DNA PCR.
All the patients were staged according to the Ann Arbor staging system. The complete staging procedures included physical examination, chest X-ray, complete blood cell counts, blood biochemistry, computed tomography of the thorax, abdomen and pelvis, and BM aspiration and biopsy. The clinical and laboratory records were reviewed for all the patients.
Treatment
The chemotherapy regimens included CHOP (cyclophosphamide, Hydroxydaunomycin, vincristin and prednisone), CHOP-E (etoposide) or ESHAP (etoposide, solumedrol, cytarabine and cisplatin).
ISH for EBV in the tumor and BM biopsy12)
We performed EBV ISH on 27 tumor specimens and 10 BM biopsy specimens. The paraffin-embedded sections of the biopsy specimens were deparaffinized with xylene, and this was followed by treatment with proteinase K. The sections were then hybridized with fluorescein isothiocynanate-conjugeted EBV oligonucleotides (Novocastra, Newcastle, U.K.) that were complementary to the nuclear RNA protein of the EBER1 and EBER2 genes. Positive labeling was identified only when the cells showed nuclear staining with EBV oligonucleotide. As negative controls, we used EBV negative lymphoid tissues and a hybridization mixture without the EBV oligonucleotides. As the internal positive control, the tumor and BM slides of patient with NK-cell leukemia were used.
Sample DNA preparation and EBV PCR11)
The BM mononuclear cells from 8 patients were studied by performing EBV PCR. EBV DNA was extracted from the patients' BM aspiration samples (frozen monocytes) with using the QIAmp DNA Blood Mini Kit (Qiagen, USA). Approximately 100ng of DNA was used for PCR reaction. The PCR primer sequences were 5'-GACGAGGGGCCAGGTACAGG-3' and 3'-GCAGCCAATGCAACTTGGACGTTTTAGG-5', respectively. The PCR reaction was performed using the primer set (Maxim biotech, Inc, USA). The PCR mixture contained 250 µL of the pre-mixed primers and 750 µL of optimized PCR buffer. The PCR was preformed in a total volume of 50 µL, which contained 10.0 L of DNA, 39.8 L of the PCR mixture and 0.2 µL of Taq DNA polymerase. The amplification reaction was performed for 37 cycles under the following conditions: initially, 96℃ for 1min for activation (one cycle), followed by a denaturation phase at 94℃ for 1min, an annealing phase at 5 8℃ for 1min and an extension phase at 72℃ for 1min (35 cycles). Another 10 min extension phase at 72℃ was performed at the end of each round of PCR. Ten microliters of the PCR products were subjected to electrophoresis (100V, 25min) on a 2% agarose gel, and the products were stained with ethidium bromide. The presence or absence of specific PCR targets was observed under UV illumination.
Statistical analysis
Overall survival was measured from the time of diagnosis to death or to the last follow-up. The survival curves were calculated using the Kaplan-Meier method and they were then compared using the log-rank test. Other clinical factors were compared using chi-square tests or t-tests. Differences were considered statistically significant if the p value was <0.05. All statistical analyses were performed using the SPSS for Windows 13.0 statistical package (SPSS Inc., Chicago, IL).
RESULTS
Clinical features
One patient among the 27 AILT patients refused any further staging work-up. The clinicopathologic data of the 27 patients are summarized in Table 1. This series included 19 men and 8 women with ages from 17 to 75 (median age: 57 years-old). All the patients except one had stage III or IV disease. Anemia was detected at diagnosis in 10 patients; two of them had autoimmune hemolytic anemia. Twenty five patients received chemotherapy, including 23 patients who received CHOP, 1 patient who received CHOP-E and 1 patient who received ESHAP, while 2 patients received only supportive care.
EBV ISH in the tumor and its clinical impact
Among the tumor specimens obtained at the time of the initial diagnosis from the 27 AILT patients, 10 were positive for EBV ISH. The correlations of the clinical status, such as the international prognostic index (IPI) score, age, stage and other laboratory findings, with the EBV status of tumor are summarized in Table 2. Only CD20 negativity in the tumor was significant correlated with EBV-positivity in the tumor (p=0.035).
The median overall survival and progression-free survival of the 25 treated patients were 48.9 months (95% CI: 18.6~79.2 months) and 32.3 months (95% CI: 11.5~53.1 months) (Figure 1A). There were no significant correlations between EBV-positivity in tumor and the overall or progression-free survival.

Kaplan-Meier curves for overall (solid line) and progression free survival (dotted line) for the 25 treated patients with angioimmunoblastic T-cell lymphoma (A). Comparison of the overall survival (B) and progression-free survival (C) between the patients with bone marrow involvement (solid line) and those without BM involvement (dotted line)
BM involvement by Hematoxylin-eosin staining, ISH or PCR
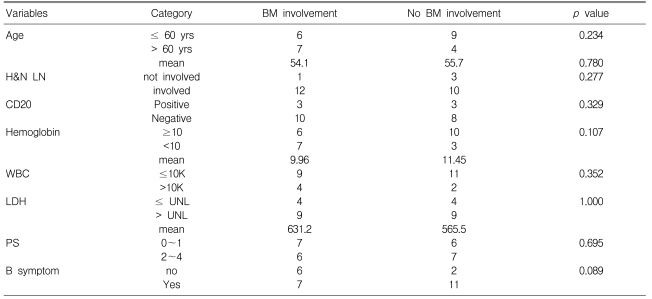
In 13 of the 26 AILT patients, gross tumor involvement was recognized in the initial BM sample by hematoxylin-eosin staining (H-E stain). None of the 10 patients who had additional BM slides available revealed EBV-positivity on BM ISH. Among these 10 patients, 3 patients showed BM involvement by H-E staining, but this was not identified on EBV ISH (Figure 2) or PCR. Yet one patient among the 5 patients without BM involvement showed a positive band on PCR (Figure 3). BM involvement by H-E staining was not related with the clinical status as defined by the IPI, age, stage and laboratory findings (Table 3). The overall survival and progression-free survival for the treated patients among the 13 patients with BM involvement according to H-E staining, were longer than those for the patients without BM involvement (p=0.054 and p=0.005, respectively; Figure 1B, 1C).
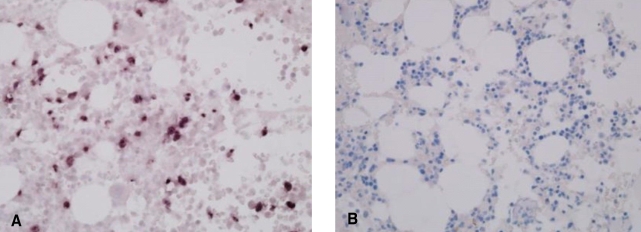
In situ hybridization for EBV in the bone marrow biopsies. Positive staining for EBV in a case with NK/T-cell leukemia (A), and negative staining in a patient with angioimmunoblastic T-cell lymphoma (B)
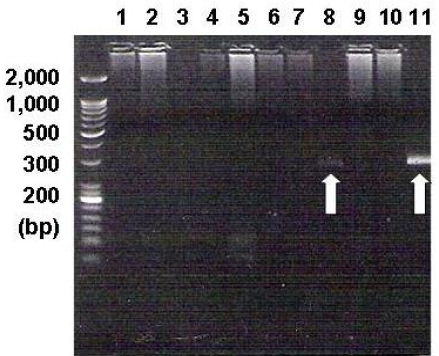
PCR analysis showed the EBV-specific DNA sequences. The EBV DNA was detected in the BM mononuclear cells of one patient (white arrow in line 8). Lines 1~8 are for the BM samples of the angioimmunoblastic T-cell lymphoma cases, lines 9~10 are for the BM samples of NK/T-cell lymphoma cases, and line 11 is for the positive control in the EBV PCR kit (240 base pairs)
Clinical factors affecting survivals
On univariate analysis, older age, male gender, pulmonary parenchymal involvement, the presence of edema and negative BM were adverse factors for progression-free survival and overall survival. Male gender (p=0.041), pulmonary parenchymal involvement (p=0.039) and negative BM (p=0.042) all significantly shortened the progression-free survival on multivariate analysis. Among the significant factors for overall survival, male gender (p=0.078), pulmonary parenchymal involvement (p=0.044) and negative BM (p=0.080) also had modest significance on multivariate analysis.
DISCUSSION
EBV DNA was detected in Burkitt's lymphoma tissue in 1964, from non-Hodgkin's lymphoma in the early 1980s and from T-cell lymphoma and Hodgkin's lymphoma in the late 1980s13). Clonotypic proliferation of EBV has been demonstrated in the neoplastic cells of lymphoid malignancies, which suggests a causative role for EBV in their tumorigenesis14). The cells infected with EBV are thought to avoid apoptosis by expressing EBV latent membrane proteins (LMP) 1 (CD 40 homologue) and 2a (BCR engagement). Infected B cells are delivered by binding to helper T cells via the CD40 molecule that presents on the B cell surface, and by binding to the B cell receptor1). The outcome of AILT is poor, with most series reporting a 5-year OS of approximately 30% and a median survival of 3 years15). Several studies have been done to assess how the pathogenesis of EBV contributes to the prognosis. Anagonostopoulos et al7) showed the predominant infection of B cells in the nodular EBV-positive cases and the predominant infection of T cells in the diffusely EBV-positive cases, and this was confirmed by double labeling for EBER and the lineage markers for all the AILT cases. Further, EBV infection of the two cell lineages would appear to be unique to AILT as this has not yet been reported for other malignancies, but the pathway of B- and T-cell infection by EBV in AILT patients is unclear. Our study showed that CD20 negativity of a tumor was significant correlated with EBV ISH positivity (p=0.035).The expression of the CD20 antigen may be down regulated in latently EBV-infected small lymphoid cells in vivo16). Our findings supported this observation. Several other articles showed the contradictory result that AILT often have increased numbers of CD20-positive cells that are also EBV-positive17-19). This discrepancy needs further verification. The relative lower incidence (37%) of EBV-positivity of tumor in this study was lower when compared to that (58~97%) of western studies. We used an adequate positive control, that is, EBV-positive NK/T-cell leukemia. Therefore, using more sensitive methods such as PCR to detect low copy numbers of EBV would be the next step9, 20).
Another aim of this study was to adopt the prognostic model of minimal BM involvement by EBV-positive tumor cells in NK/T-cell lymphoma and apply it to AILT. Unexpectedly, it was difficult to use EBV ISH or PCR for detecting EBV-positive tumor cells in BM, such as those EBV-positive tumor cells in NK/T-cell lymphoma. The EBV ISH or PCR results were all negative for the AILT patients with gross tumor involvement, as determined by H-E staining. This made it impossible to introduce the concept of minimal BM involvement by EBV-positive tumor cells in AILT. Also, the results of performing EBV ISH and/or PCR for tumor or BM had no impact on the outcomes of the AILT patients in our study; this was similar to a recent Japanese study9).
Our study showed a particular result that the overall survival and progression free survival of the AITL patients with BM involvement were significant longer than those of the patients without BM involvement. Of course, the limited number of patients makes it difficult to generalize this finding. However, most AILT patients in this study were at an advanced stage of disease at the time of diagnosis. Except for stage, patient survival was significantly related to age, B cell symptoms, rash/pruritus, edema, ascites, lactate dehydrogenase, hemoglobin and the number of clinical symptoms with excluding the B symptoms of the AILT patients21). The presence of bulky disease (>7.5 cm), an advanced stage and bone marrow involvement did not influence survival of the patients with T-cell lymphoma in the previous studies22, 23). Unlike B-cell lymphoid malignancies such as diffuse large B-cell lymphoma and chronic lymphoid leukemia, BM involvement may not be a poor prognostic factor for T-cell lymphoma. A future study that would include a sufficient number of AILT patients will clarify this particular finding.