Comparison of the 13C-urea breath test and the endoscopic phenol red mucosal pH test in the quantification of Helicobacter pylori infection loading
Article information
Abstract
Background/Aims
The 13C-urea breath test (UBT) is a semiquantitative test for measuring Helicobacter pylori infection loading. H. pylori produces ammonia, which elevates the pH of the gastric mucosa and is detectable via endoscopy using a phenol red indicator. We evaluated whether this test could be used to diagnose H. pylori infection and whether phenol red staining was correlated with 13C-UBT results.
Methods
One hundred and twenty-three patients participated. The UBT was performed after ingestion of a capsule containing urea. A change in 13C-UBT >2‰ was selected as the cutoff value for diagnosing infection. After spraying evenly with a 0.1% phenol red solution, the pH of the gastric mucosal surface was measured using an antimony electrode through the biopsy channel.
Results
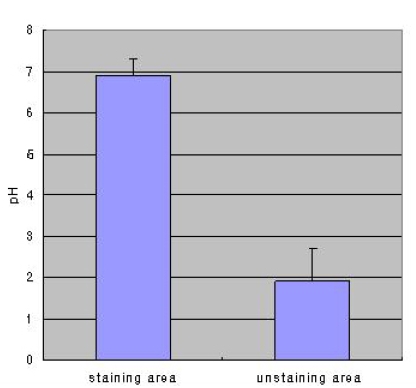
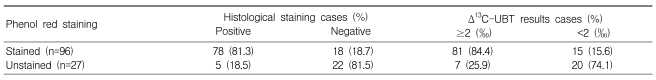
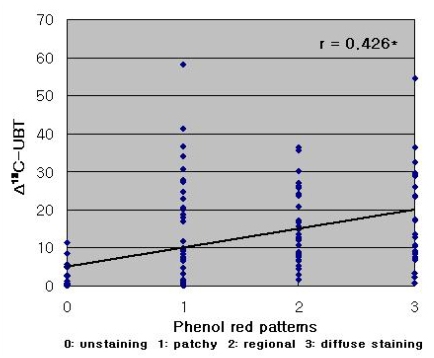
The pH of stained mucosa (6.9±0.4) was significantly higher than that of unstained mucosa (1.9±0.8; p<0.001), and the H. pylori detection rate confirmed via histology was higher in stained versus unstained mucosa (p<0.01). Extensive mucosal staining resulted in a higher detection rate (p<0.001). The UBT produced results were very similar to those obtained via histological detection in stained mucosa (p<0.001). The extent of staining, expressed as a staining score, was positively correlated with the change in 13C-UBT (r=0.426, p<0.001). A significant correlation was also observed between the histologically determined H. pylori density and 13C-UBT results (r=0.674, p<0.001).
Conclusions
H. pylori infection elevates gastric mucosal surface pH, and endoscopic phenol red staining may be an alternative method for the diagnosis of H. pylori infection.
INTRODUCTION
Helicobacter pylori infection is associated with chronic active gastritis, peptic ulcer disease, gastric adenocarcinoma, and mucosa-associated lymphoid tissue lymphomas1-3). Many noninvasive and invasive methods have been developed for the diagnosis of H. pylori infections, including direct culture, histology, rapid urease testing, polymerase chain reaction (PCR) bacterial DNA amplification, the use of H. pylori-specific antibodies, the urea breath test (UBT) using 13C- or 14C-labeled urea, and stool antigen detection4). The 13C-UBT measures intragastric urease activity and is generally considered to be an accurate, noninvasive, and semiquantitative method to determine H. pylori loading5, 6). However, the UBT has limitations inherent to noninvasive methods: it cannot pinpoint the position of upper gastrointestinal disease or provide histological information.
H. pylori produces ammonia via the activity of a potent bacterial urease, which is detectable via diagnostic assays such as the CLOtest7). Urease activity can lead to mucosal damage by degrading urea in human tissue8, 9), and the resultant ammonia can elevate mucosal pH at the site of infection. Therefore, we examined whether mucosal surface pH can be determined endoscopically using phenol red as a pH indicator, and whether such a technique is useful in the detection of H. pylori infection. To quantify H. pylori loading, we also examined whether the extent of the affected region was correlated with UBT values. In addition, we documented several noteworthy points in the phenol red spraying procedure and in the interpretation of results.
MATERIALS AND METHODS
Patients
From March to November 2006, 123 patients undergoing routine upper gastrointestinal endoscopy for dyspeptic symptoms were recruited into this study (46 men and 77 women aged 19 - 79 years). These patients suffered from nonulcer dyspepsia (n=109), duodenal ulcers (n=8), or gastric ulcers (n=6). The 13C-UBT was performed 1 day after the patients had fasted for at least 8 h. On the second day, the endoscopic phenol red test was performed after at least an 8-h fast. In addition, two to three gastric mucosal biopsies were taken at stained and unstained sites in the antrum and the body histological analysis, which remains the gold standard for the diagnosis of H. pylori infection. Exclusion criteria included previous eradication therapy or the use of bisthmus compounds, proton pump inhibitors, antibiotics, or anti-secretory drugs within the previous 2 months. Patients with hepatobiliary, pulmonary or metabolic diseases, or previous gastric surgery were also excluded. Written informed consent was obtained from all patients. The protocol was approved by the institutional review board of the Catholic University of Korea.
13C-Urea Breath Test
All patients were subjected to the 13C-UBT on the first day of the study after an overnight or 8-h fast. A baseline breath sample (normal exhalation for 4 s) was collected into a collection tube. A capsule containing 38 mg 13C-urea in 50 mL of water (HeliFinder; Isodiagnostika, Edmonton, AB, Canada) was then administered orally. A second breath sample was collected 20 min later. Breath samples were then analyzed via mass spectrometry (HeliView; MediChems, Seoul, Korea) to determine the 13C to 12C ratio, which was expressed per mil (‰). The change in 13C relative to the baseline value was expressed as Δ13C. A positive result was defined as a positive Δ13C >2‰.
Endoscopic Phenol Red Test
The distribution of pH changes indicative of H. pylori infection in the gastric mucosa was examined via endoscopic phenol red spraying. Patients were subjected to gastroscopy after first receiving a local oral anesthetic (10% xylocaine) and an intramuscular injection of 20 mg of scopolamine butylbromide (Buscopan; Boehringer Ingelheim Company Ltd., Seoul, Korea) to reduce gastric motility. A videoendoscope was inserted, gastric juice was aspirated to improve visibility during endoscopy, and the interior of the stomach was inspected. A 0.1% solution of phenol red was sprayed evenly over the entire surface of the gastric mucosa using a spray catheter (PW-5L-1; Olympus Co., Tokyo, Japan) passed through the biopsy channel of the endoscope. A yellow to red color change occurred 2 - 3 min after applying the dye and persisted for at least 15 min. We classified the staining patterns into four types based on the classification described by Kohli et al10). The extent of staining was scored on a scale of 0 to 3: 0, no staining; 1, patchy staining in which red areas were observed in one part of the stomach, such as the antrum, body, or cardia; 2, regional staining in which red areas were observed in two parts of the stomach, such as the antrum and body or the body and cardia; 3, diffuse staining in which red areas were observed in all regions. An antimony pH electrode catheter (catalog #9012P3001; Medtronic A/S, Køpenhavn S, Denmark) connected to an ambulatory pH meter (Digitrapper Mk III; Synetics Medical AB, Stockholm, Sweden) was passed through the biopsy channel of the endoscope. The tip was gently advanced under direct visualization until it abutted with the mucosa, at which point it was held steady to provide a stable mucosal reading in the red and yellow areas. In total, surface pH measurements were obtained for 25 randomly selected stained areas (regional or diffuse staining) and 20 randomly selected unstained sites.
Histology
To examine the relationship between positive phenol red staining and the presence of H. pylori, endoscopic biopsies were taken at unstained and stained (patchy, regional, or diffuse) sites. Silver staining was used to histologically confirm the presence of H. pylori bacilli, irrespective of color change, staining patterns, or location (antrum or body). To quantify H. pylori loading, we selected the densest H. pylori infection site for comparison with UBT values. The specimens were fixed separately in 10% formalin, embedded in paraffin wax, and 5 µm sections were stained with hematoxylin and eosin and the Warthin.Starry silver technique. Two pathologists, who were unaware of the clinical, endoscopic, and Δ13C-UBT results, evaluated the sections. The H. pylori bacterial load (H. pylori score) was classified according to the Updated Sydney System10).
Statistical Analysis
Spearman's rank correlation test was used to detect any correlation between Δ13C-UBT values and H. pylori bacterial loading, gastric histological variables, and endoscopic phenol red scoring. Student's t-test was used to compare gastric surface pH values between stained and unstained mucosal sites, and chi-square tests were applied to compare the positive rate of H. pylori infection between stained and unstained mucosal sites. The data were analyzed using SPSS (version 10.0; SPSS Inc., Chicago, IL, USA).
RESULTS
Phenol Red Staining Pattern and Mucosal Surface pH
Phenol staining was classified into four types as described in the Materials and Methods (Figure 1). Table 1 shows the distribution of staining patterns, of which patchy staining (score of 1) was the most common. The surface pH of stained mucosa (6.9±0.4) was significantly higher than that of unstained (yellow) gastric mucosa (1.9±0.8, p<0.001) (Figure 2).
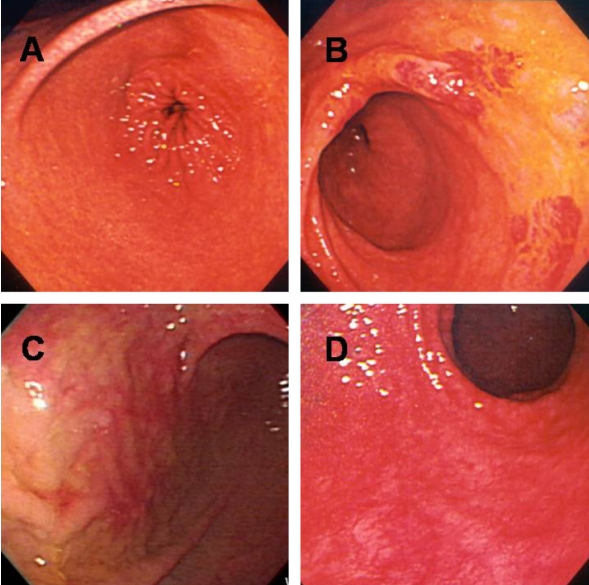
After spraying the gastric mucosa with phenol red, mucosal staining patterns were classified into four types: diffuse (A), regional (B), patchy (C), or unstained (D).
Phenol Red Staining and UBT Compared with Histopathology
Seventy-eight of 96 patients showing patchy, regional, or diffuse staining were also histologically positive for H. pylori (sensitivity, 81.3%), whereas 22 of 27 people with unstained mucosa were histologically negative for H. pylori (specificity, 81.5%; Table 2). In comparison to UBT results, phenol red staining also showed fair sensitivity (81/96 or 84.4%), but relatively low specificity (20/27 or 74.1%; Table 2). Among staining patterns, the H. pylori detection rate was higher at sites showing broad staining (regional and diffuse pattern) compared to patchy or no staining (Table 3), and H. pylori detection was most sensitive in sites showing regional staining (Table 3). The UBT and histological analysis showed very similar results (Table 3).
Correlation of UBT with Phenol Red Staining Patterns or Histology Results
Phenol red staining scores were correlated with Δ13C-UBT results (r=0.426, p<0.001; Figure 3). However, a stronger correlation was observed between Δ13C-UBT and the histologically determined H. pylori density (r=0.674, p<0.001) (Figure 4).
DISCUSSION
Numerous tests are available for H. pylori detection4), and noninvasive procedures, such as the UBT and serologic examination, are as accurate in predicting H. pylori status as invasive tests in untreated patients12). Previous studies have indicated that the high incidence of gastric cancer is closely associated with the high rate of H. pylori infection, particularly in Korea13-17). Therefore, it is important to use simultaneous invasive endoscopic observation and direct histological evaluation of H. pylori status to detect gastric cancer and develop a treatment strategy.
Currently, gastric biopsies for the purpose of histological H. pylori detection are generally taken from within the lesser curvature of the stomach, approximately 2~3 cm from the pylorus; this technique yields a relatively high rate of detection (>90%) under the correct orientation11). However, because H. pylori infection is usually unevenly distributed across the gastric mucosa18), sampling errors leading to false negatives are unavoidable. Therefore, it is important to consider the intragastric distribution of H. pylori infection. In this study, we used phenol red staining to detect local pH changes due to ammonium production by urease, which revealed the intragastric distribution of H. pylori. We found that phenol red staining shows high detection sensitivity in stained mucosa (histologically positive rate, 81.3%; UBT, 84.4%) and high specificity in unstained mucosa (histologically negative rate 81.5%; UBT 74.1%). These results demonstrate a higher rate of H. pylori detection in mucosal regions with a high pH (stained mucosa) than in mucosal regions with low pH (unstained mucosa). In addition, phenol red staining may help to select infected biopsy sites, thus decreasing the rate of false negatives for H. pylori infection compared to the current blind biopsy approach. According to staining pattern, phenol red staining showed a significantly higher detection sensitivity in areas of broad staining (regional, 97.1%; diffuse, 83.3%) compared to patchy (65.8%) or unstained areas (18.5%).
The rate of H. pylori detection was lower in patients showing diffuse staining compared to those showing regional staining, which may reflect poor acid secretion due to severe gastric mucosal atrophy. Patchy staining was the most commonly observed pattern, but this may also reflect false positives resulting from localized injury caused by the catheter or by high-pressure spray. Alternatively, patchy staining may result from the leakage of extracellular fluid due to injuries before endoscopy, or the patient may experience bile reflux before or during endoscopy. To avoid false positives, we excluded patients showing bile flow from the duodenum or immediate color changes (i.e., bleeding) indicative of catheter-induced trauma. In addition, interobserver variation may have influenced our results, especially in patients showing patchy staining, which was associated with relatively low sensitivity and specificity. For these reasons, we excluded ten cases that may be been affected by the above uncertainties. However, the remaining three staining patterns presented no difficulty in terms of endoscopic interpretation.
To our knowledge, we are the first to demonstrate that changes in gastric mucosal pH, as determined via the application of a urea-free phenol red solution, can be used to assess H. pylori infection. In addition, phenol red staining scores were positively correlated with Δ13C-UBT values. As such, the development of a device to measure the area of color change may one day allow for the direct quantification of H. pylori loading from phenol red staining. Previous studies have indicated that Δ13C-UBT values are correlated with bacterial density19, 20), although conflicting data have also been reported5, 21). However, Rauws et al.22) reported a positive correlation between UBT values and quantitative antral culture results. In the present study, bacterial density was more strongly correlated with Δ13C-UBT values than phenol red staining scores.
In the clinical setting, Δ13C-UBT values are useful to determine the success of bacterial eradication therapy and to evaluate the efficacy of new antibiotics. Moreover, bacterial load may play an important role in determining the outcome of H. pylori eradication. A high bacterial load appears to be associated with an increased risk of gastroduodenal endoscopic lesions, such as peptic ulcer disease, and with the reduced efficacy of eradication therapy23-25). Based on the correlation between Δ13C-UBT values and phenol red scores, H. pylori eradication may be more challenging in patients with regional or diffuse staining compared to patchy staining. Thus, endoscopists may be able to estimate the efficacy of eradication therapy directly based on endoscopic phenol red spraying, without waiting for UBT results.
Although endoscopic phenol red application and biopsy have some disadvantages in terms of time requirements, misinterpretation of color change or staining patterns, and biopsy-associated complications, this technique offers several advantages in terms of direct detection of the infection site, histological evaluation, potential area-based quantification of H. pylori loading, and little mucosal injury. Moreover, this endoscopic procedure is a relatively easy method of detecting H. pylori infection and is not harmful to humans10). No complication was observed in the course of this study.
Notes
This study has not been previously published or submitted for publication elsewhere. The authors have no conflict of interest to declare.