Hepatopulmonary Syndrome in Poorly Compensated Postnecrotic Liver Cirrhosis by Hepatitis B Virus in Korea
Article information
Abstract
Background
Hepatopulmonary syndrome (HPS) refers to the association of hypoxemia, intrapulmonary shunting and chronic liver disease. But there is no clear data about the prevalence of HPS in postnecrotic liver cirrhosis by hepatitis B virus (HBV), the most common cause of liver disease in Korea. The aim of this study was to investigate the prevalence of HPS in poorly compensated postnecrotic liver cirrhosis by HBV, and the correlation of the hepatopulmonary syndrome with clinical aspects of postnecrotic liver cirrhosis by HBV.
Methods:
Thirty-five patients underwent pulmonary function test, arterial blood gas analysis and contrast-enhanced echocardiography. All patients were diagnosed as HBV-induced Child class C liver cirrhosis and had no evidence of intrinsic cardiopulmonary disease.
Results:
Intrapulmonary shunt was detected in 6/35 (17.1%) by contrast-enhanced echocardiography. Two of six patients with intrahepatic shunts had significant hypoxemia (PaO2 < 70 mmHg) and four showed increased alveolar-arterial oxygen gradient over 20 mmHg. Only cyanosis could reliably distinguish between shunt positive and negative patients.
Conclusions:
The prevalence of intrapulmonary shunt in poorly compensated postnecrotic liver cirrhosis by HBV was 17.1% and the frequency of hepatopulmonary syndrome was relatively low (5.7%). ‘Subclinical’ hepatopulmonary syndrome (echocardiographically positive intrapulmonary shunt but without profound hypoxemia) exists in 11.4% of cases with poorly compensated postnecrotic liver cirrhosis by HBV. Cyanosis is the only reliable clinical indicator of HPS of HBV-induced poorly compensated liver cirrhosis. Further studies are required to determine if the prevalence and clinical manifestations of HPS varies with etiology or with geographical and racial differences.
INTRODUCTION
The hepatopulmonary syndrome (HPS) is characterized clinically by the triad of pulmonary vascular dilation, systemic hypoxemia and the setting of advanced liver disease1–7). In 1956, a case report by Rydell and Hoffbauer8) concerning a 17-year-old man with hypoxemia and juvenile cirrhosis provided the first clinical and postmortem documentation of what was to be later termed HPS by Knudsen and Kennedy in 19797). The postmortem study demonstrated both precapillary dilations and direct arteriovenous communications after vascular injections with a plastic vinyl acetate solution9,10).
The definition of hypoxemia may vary, but several studies consider PaO2 less than 70 mmHg in patients with liver disease as abnormal. An increased alveolar-arterial oxygen gradient (A-aDO2: greater than 20 mmHg) represents a more sensitive but less practical measure of abnormal oxygenation. Patients with HPS frequently demonstrate oxygenation that become worse as one moves from the supine to standing position (orthodoxies) breathing either room air or 100% inspired oxygen. A poor correlation exists between PaO2 determined while breathing room air and PaO2 determined while breathing 100% oxygen; the latter may be of additional prognostic significance. Research studies using a multiple inert gas elimination technique (MIGET) have shown the hypoxemia of HPS to be a result of shunt, diffusion-perfusion defect and excess perfusion for a given ventilation (low V/Q)11).
Most studies about hepatopulmonary syndrome have focused on alcoholic liver disease12–17). There is no clear data about the prevalence of HPS in postnecrotic liver cirrhosis by hepatitis B virus (HBV). And it is unclear how the presence of HPS relates to the clinical aspects of HBV induced liver cirrhosis. So this study was done to investigate the prevalence of HPS in poorly compensated postnecrotic liver cirrhosis by HBV and the correlation of HPS with clinical aspects of HBV induced poorly compensated postnecrotic liver cirrhosis.
MATERIALS AND METHODS
1. Patients
Thirty-five cirrhotic patients were randomly recruited, from both the Gastroenterology Ward and the Gastroenterology Out-patients in Seoul Municipal Boramae Hospital (Seoul National University Hospital Affiliated Hospital), Seoul, Korea. The inclusion criteria were the followings:
Liver cirrhosis was diagnosed with histological findings (85%), and with conventional clinical (positive evidence of chronic liver disease stigmata and physical findings of liver cirrhosis), ultrasonographic (coarse liver surface and shrunken liver on ultrasonography) and biochemical criteria (abnormal blood liver function test), and clinical presentations of portal hypertension (15%). All patients showed serum HBsAg positive and Child C clinical findings, with absence of cardiac or pulmonary disease and absence of pulmonary vascular abnormalities not related to liver disease.
Patients were informed about the intended procedures and the aim of the study, and consent was obtained in every case, according to the specifications guidelines of the 1975 Declaration of Helsinki. Transthoracic contrast echocardiography (TTCE) was performed in all cirrhotic patients.
2. Transthoracic contrast echocardiography (TTCE)
We used a standard echocardiograph (Acuson Computerized Sonography 128XP, USA), with a 3.5 MHz transthoracic probe. Studies were recorded on videotape for further analysis. For TTCE, a previously published method2 was closely followed. Four-chamber apical image was obtained through a transthoracic approach. Then, 10 mL of saline with 0.5 mL room air, were injected through the intravenous line. The second and third injection followed. A positive result was defined when microbubbles were observed in the left atrium in one or more of the three injections. The grading method was validated by examination of its reproducibility. First, the intrinsic reproducibility was examined by comparing the results obtained after the first and the second injection of each substance. Second, interobserver reproducibility was measured by assessing the concordance resulting from a two-step blind review of each register performed by the same observer. Third, interobserver reproducibility was assessed by blind comparison of the results obtained by both observers in the interpretation of each register. We did not compare two studies of the same patient performed at different times for ethical reasons.
3. Arterial blood gas analysis (ABGA)
A sample of arterial blood was obtained in each patient at the time of the echocardiography study by puncture of the radial artery of the left arm, following the standard technique, in the supine position and breathing room air. PaO2 and PaCO2 were determined by selective electrodes (IL 16/40 ph/blood gas analyzer. Instrumentation laboratory SpA, Milano, Italy). PaO2 < 70 mmHg was considered as hypoxemia. PaCO2 <35 mmHg was considered as hypoxemia.
4. Pulmonary function test
We used a standard pulmonary function test machine (Sensor Medic Model 2200, USA).
5. Diagnosis of HPS
Using saline-TTCE, we considered clinical HPS to be present in cases with PaO2 < 70 mmHg with bubbles in left cardiac chambers, ‘sub clinical’ HPS in A-aDO2 greater than 20 mmHg with bubbles in left cardiac chambers.
6. Statistical analysis
Estimation of Kappa heavy index allowed determination of the reproducibility of the procedure and intra- and inter-observer variability. Wilcoxon’s test was chosen to compare results of TTCE, and correlation between ordinal variables was determined by Spearman’s test. Correlation between continuous and ordinal variables was determined by ANOVA and post-hoc test (Bonferroni). The limit of significance was set at a p<0.05. For analysis purposes, we used the SPSS.
RESULTS
Thirty-five patients were studied (13 women and 22 men: mean age, 53.1 ± 14.5 years) All patients were classified as Child’s C. (Child’s classification included assessment of total bilirubin, serum albumin, clinical ascites, nutrition and existence of encephalopathy; Child’s A classification represented minimal disease and Child’s C classification represented the most severe liver disease). The etiologies of liver diseases of all the patients were postnecrotic liver cirrhosis by hepative B virus.
1) Symptoms and signs of study population (Table 1)
Splenomegaly and ascites were seen in all 35 patients (100%), and spider angiomas were seen in 21/35 patients (60%). Cyanosis was observed in 2 patients (6%). Esophagogastroduodenoscopy was performed before the echocardiography studies. Thirty-two patients had esophageal varies, nine of which (25.7%) were grade I, twenty of which (57.1%) were grade II, three of which (8.6%) were grade III. Japanese portal hypertension study group classification was used for esophageal varies grading (Table 2).
2) Pulmonary function test (Table 3) and arterial blood gas analysis (Table 4)
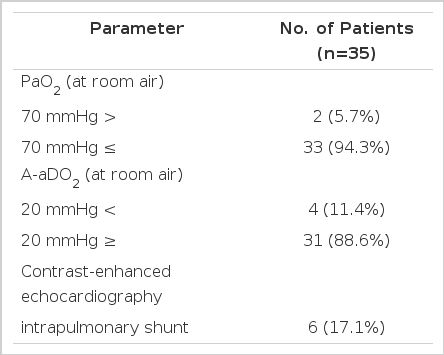
Mild ventilatory defect of the restrictive type (65–79% of predicted value) was found in 7/35 patients (20%) and decrease in diffusing capacity of carbon monoxide (below 70% of predicted value) was found in 4/35 patients (11%). Significant hypoxemia (PaO2 <70 mmHg) was found in 2/35 patients (5.7%) and PaO2 was below 80 mmHg in 4/35 patients (11%) and below 90 mmHg in 10/35 patients (29%), and the PaO2 value of the remaining 19/35 patients (54%) was above 90 mmHg. A-aDO2 were below 20 mmHg in 31/35 (88.6%) and above 20 mmHg in 4/35 patients (11.4%).
3) Clinical and laboratory findings of liver cirrhosis patients with positive shunt (Table 5)
Intrapulmonary shunt was detected by TTCE in 6/35 patients (17.1%) and these cases showed significantly lower PaO2 than in negative intrapulmonary shunt cases (PaO2: 72.2 ± 15.1 vs. 90.2 ± 7.40, p <0.05) and more increased A-aDO2 (43.1 ± 16.2 vs. 22.4 ± 7.43) than in negative intrapulmonary shunt cases.
4) Comparison of clinical and laboratory findings between shunt negative and positive patients (Table 6 and Table 7)
Except cyanosis, any clinical or laboratory findings including spider angioma, esophageal varies, biochemical indicator of hepatic function and parameters of pulmonary function test, including diffusing capacity did not distinguish between positive and negative intrapulmonary shunt patients.
DISCUSSION
The triad of liver disease, arterial hypoxemia and intrapulmonary vascular dilatation has defined an entity commonly referred to as the hepatopulmonary syndrome1,3,10–11,19,26). In the original description by Rydell and Hoffbauer4), lung necropsy specimens studied using plastic vascular casts contained both precapillary/capillary dilatations and distinct anatomic arteriovenous communications which caused severe hypoxemia in the setting of chronic liver disease (juvenile cirrhosis)14,16–18). Hepatopulmonary syndrome is becoming increasingly recognized as one of the most serious complications of chronic liver disease. Different workers have reported incidences of positive air-contrast echocardiography varying from 5 to 47%, and prevalence of HPS between 5 and 29%3,14). Our present study, although small, suggested a relatively lower occurrence of this condition (17.1% positive intrapulmonary shunt, 5.7% hepatopulmonary syndrome) in the Korean population, among whom hepatitis B virus is the most common cause of cirrhosis (100% in the present study), compared with alcohol and hepatitis C virus in Western countries. The results from our study were similar to those from an Indian study that the prevalence of intrapulmonary shunting in hepatitis B virus induced liver cirrhosis was 8.9% but HPS with hypoxemia was only 6.7%2). Further studies are required to determine if the prevalence of HPS varies with etiology of liver disease or with geographical and racial differences.
Advanced hepatic dysfunction, with associated hyperdynamic circulation, has been suggested as being the most probable setting for the development of HPS. However, the condition has also been found in cases of congenital hepatic fibrosis and portal vein thrombosis. This has given rise to the question of whether portal hypertension is a contributing factor1,3). Moreover, if hepatic dysfunction was the only prerequisite, one would expect HPS to occur predominantly among Child’s class C cirrhosis. However, Abrams et al. found 15 of 25 cases of HPS (60%) had Child’s grade A, and only two had grade C3). In this study, most of HPS cases were alcoholic liver cirrhosis and hepatitis C virus (HCV)-induced liver cirrhosis. A common bile duct legation rat model for hepatopulmonary syndrome has been developed and increased pulmonary endothelial nitric oxide syntheses activities and circulating endothelin-1 levels seem to correlate with vascular dilatation and oxygen abnormalities19,26)
In the present study, all cases were HBV-induced Child’s grade C liver cirrhosis and the prevalence of HPS is 5.7%. In this small study, the prevalence of hepatopulmonay syndrome in patients with poorly compensated (Child C) postnecrotic liver cirrhosis by HBV was 5.7%. Two of 35 cases of cirrhosis (5.7%) had positive contrast echocardiography with hypoxemia (PO2 <70 mmHg) and four of 35 case of cirrhosis (11.4%) were ‘subclinical’ cases (positive contrast echocardiography without hypoxemia). Our results suggested that “subclinical hepatopulmonary syndrome” exists and maybe there were some factors, still unknown, to determine definite hepatopulmonary syndrome (hypoxemia with positive contrast echocardiography) and subclinical hepatopulmonary syndrome. This study suggested that there was a wide clinical spectrum of hepatopulmonary syndrome. There was one report about association of esophageal varies and hepatopulmonary syndrome in liver cirrhosis27). But, in this study, there was no association of the grade of esophageal varies and hepatopulmonary syndrome. Cyanosis was the only reliable clinical indicator, and there was no clear relationship with the presence of spider angioma and hepatopulmonary syndrome. Further studies are required to determine if the prevalence of HPS and clinical manifestations of HPS varies with etiology or with geographical and racial differences.