Clinical Features of Eosinophilic Bronchitis
Article information
Abstract
Background
Eosinophilic inflammation of the airway is usually associated with airway hyper-responsiveness in bronchial asthma. However, there is a small group of patients which has the eosinophilic inflammation in the bronchial tree with normal spirometry and no evidence of airway hyper-responsiveness, which was named eosinophilic bronchitis. The objectives of this study are 1) to investigate the incidence of eosinophilic bronchitis in the chronic cough syndrome and 2) to evaluate the clinical features and course of eosinophilic bronchitis.
Methods
We evaluated 92 patients who had persistent cough for 3 weeks or longer. In addition to routine diagnostic protocol, we performed differential cell count of sputum. Eosinophilic bronchitis was diagnosed when the patient had normal spirometric values, normal peak expiratory flow variability, no airway hyper-responsiveness and sputum eosinophilia (>3%).
Results
The causes of chronic cough were post-nasal drip in 33%, cough variant asthma in 16%, chronic bronchitis in 15% and eosinophilic bronchitis in 12% of the study subjects. Initial eosinophil percentage in the sputum of patients with eosinophilic bronchitis was 26.8±6.1% (3.8–63.7%). Treatment with inhaled steroid is related with a subjective improvement of cough severity and a significant decrease of sputum eosinophil percentage (from 29.1±8.3% to 7.4±3.3%). During the follow-up period, increase in sputum eosinophil percentage with aggravation of symptoms were found.
Conclusion
Eosinophilic bronchitis is one of the important cause of chronics cough. Assessment of airway inflammation by sputum examination is important in investigating the cause of chronic cough. Cough in eosinophilic bronchitis is effectively controlled by inhaled corticosteroid, but may follow a chronic course.
INTRODUCTION
Cell fraction analysis of sputum has been reported in an indirect but reliable test to estimate the severity of airway inflammation in bronchial asthma and some airway diseases1, 2) and is emphasized for its importance. Especially, in case of bronchial asthma, it reveals various degrees of eosinophilia in sputum examination and they are known to be strongly associated with airway hyper-responsiveness3). But some patients with chronic cough have normal radiologic findings in lung parenchyma, but show eosinophilia in sputum without airway hyper- responsiveness or airflow limitation. these disease entities were defined as eosinophilic bronchitis and are being reported to be an important cause of chronic cough4). The reason why eosinophilic bronchitis is clinically important is that chronic cough due to eosinophilic bronchitis is effectively controlled by inhaled steroids in contrast to the one resulting from other causes, such as postnasal drips or gasrtroesophageal reflux. But, as the cell fraction analysis of sputum was not actively performed until recently little is known at all about frequency or clinical and therapeutic progression of eosinophilic bronchitis among chronic cough syndromes. This study aimed to identify the frequency and the clinical status of chronic cough patients with eosinophilic bronchitis by performing the cell fraction analysis of sputum in addition to general diagnostic methods for chronic cough and to observe changes in airway hyper-responsiveness and eosinophilic inflammation of the airway during the follow-up period.
MATERIALS AND METHODS
Ninety-two (male:female=25:67) patients who had been coughing for more than 3 weeks and referred to the Division of Allergy and Respiratory Medicine, Soonchun-hyang Univ. Hospital from Jan. 1998 to Jun. 1999 were evaluated. After questionnaires about respiratory symptoms were obtained, physical examination, pulmonary function test, skin prick test for common aeroallergens, bronchial methacholine challenge test, X-rays of chest and sinus, sputum cell fraction examination and self-monitoring of PEFR (Peak expiratory flow rate) for daily change were performed for all the patients.
1. Questionnaires
Basic questionnaires were checked for the presence of underlying respiratory diseases, smoking, drug history, cough, sputum, respiratory abnormalities suggestive for asthma including wheezing and dyspnea, postnasal drip, gastroesophageal reflux symptom with heartburn pain. Cough was expressed in scores according to subjective symptoms of patients through the questionnaire examination and it was observed for follow-up. Cough scores were divided into 4 grades by day and night, by which the worst case got 3 points and no symptom got 0 point. Each day and night score was summed up on a maximum of 6 points.
2. Pulmonary function test
For basal ventilatory function test, FVC (forced vital capacity), FEV1 (volume of air expired in the first second), FEV1/FVC, FEF25–75 (forced midexpiratory airflow) and PEFR were measured by using Sensor Medics Mvax 22 spirometer (Cardiopulmonary ere companyTM, Yorba Kinda, California)
3. Methacholine bronchial provocation test
Normal saline containing various concentrations of methacholine was made into aerosol by using pressure type nebulizer (De Vibliss series No. 646), then it was inhaled for 2 minutes with normal tidal breathing. After measuring basal FEV1, normal saline was inhaled and then FEV1 was measured again after 60 & 120 seconds and higher values were defined as a control FEV1. Methacholine concentration 0.075 mg/mL, 0.15 mg/mL, 0.31 mg/mL, 0.62 mg/mL, 1.25 mg/mL, 2.5 mg/mL, 5 mg/mL, 10 mg/mL, 25 mg/mL were inhaled sequentially by 5 minute intervals. FEV1 was measured 60 seconds after inhaling each concentration of methacholine. If FEV1 decreased over 20% compared to the FEV1 after normal saline inhalation, the test was stopped. In case of not decreasing over 20%, the test was continued until the final methacholine concentration of 25 mg/mL. Airway hyper-responsiveness was demonstrated as PC20 of methacholine concentration, which is decreased by 20% compared to control FEV1. When PC20 was below 8 mg/mL, it was defined as positive.
4. Allergic skin prick test
Skin prick test was performed for 50 common aeroallergens (Bencard Co. England) in Korea. At the same time, 1 mg/mL histamine was used as a positive control and normal saline as a negative control for comparison. After 20 minutes of prick test, wheals produced by allergen were measured for long and short diameters. When their average was the same or bigger than one by histamine, it was defined as positive.
5. Induction of sputum and analysis of cell fraction in sputum
Sputum was induced by an inhalation of hypertonic saline aerosol as described by Pin and coworkers2). After the subjects inhaled two puffs of salbutamol, increasing concentration of saline (3, 4 and 5%) was inhaled for 7 minutes each through a mouthpiece. After each inhalation, FEV1 was measured, then the patients were asked to cough sputum into a container. If the FEV1 fell by >20% or if troublesome symptoms occurred, nebulization was discontinued. The samples were examined within 2 h. Processing of freshly expectorated sputum was done as described by Pizzichini and colleagues1). Briefly, the expectorated sputum was poured over a paper towel. More opaque and gelatinous portions than saliva were carefully selected by using a blunt ended forceps and were placed in a pre-weighed Eppendorf tube and weighed. The pellet was treated by adding four volumes of 0.1% Dithiothreitol (DTT) (Sputolysin; Calbiochem Corp., San Diego, CA) freshly prepared in Dulbeccos phosphate buffered saline (D-PBS) and followed by adding a further four volumes of D-PBS. The mixture was gently agitated in a shaking water bath at 37°C for 15 minutes, filtered through mesh gauze with a pore size of 48 um (BNSH Thompson, Scarborough, ON, Canada) to remove debris. Total cell number and viability were measured using a standard hemocytometer and trypan blue dye exclusion. Homogenized sample was spun in a cytocentrifuge and stained using Diff-Quik solution (American Scientific Products, Chicago, IL, USA). Two investigators, who were blind to the subject classification, did differential count of 500 cells. The sputum sample was considered acceptable for examination if it contained less than 10% of squamous epithelial cells.
6. Diagnosis for causes of chronic cough
According to the above findings, the causes of chronic cough were discriminated with the following criteria. When mucous or purulent discharge observed at the oropharynx by oral inspection or cobblestone-shaped change of mucous membrane and improved by antihistamine treatment, it was diagnosed as postnasal drip syndrome. Cough variant asthma was diagnosed when the main complaint was an isolated symptom of cough and PC20 methacholine was below 8 mg/mL. Chronic bronchitis was diagnosed when a patient had smoked and been coughing more than 3 months a year and for more than 2 years continuously. A patient with no postnasal drip and no airway hyper-responsiveness who belonged to the normal number range of eosinophil according to sputum cell analysis and showed improvement by conservative medications, like expectorants or anti-tussives, was diagnosed as chronic bronchitis. If any improvement is shown by weight loss, diet control and medications, like antacids and H2 receptor inhibitors, it was defined as a chronic cough by gastroesophageal reflux. Eosinophilic bronchitis was diagnosed in case of eosinophilic fraction over 3% in sputum, PC20 over 8 mg/mL, PEFR variation below 20%, FVC over 80% at the basal lung function test and FEV1 and FEV1/FVC over 75%. Once it was diagnosed, eosinophilic bronchitis was observed with administration of 800 μg inhaled budesonide a day.
7. Statistics
SPSS 8.0 (for windows) was used for statistical analysis. It was verified by Nonparametric Spearman’s correlations, Wilcoxon Signed Ranks Test. A p value of less than 0.05 was considered statistically significant.
RESULTS
1. Causes of chronic cough syndrome (Figure 1)
Causes of chronic cough were listed as postnasal drip syndrome in 30 patients (33%) as the most, cough variant asthma (16%) and bronchitis (15%) in order. The frequency of eosinophilic bronchitis revealed 11 cases (12%) out of 92 patients.
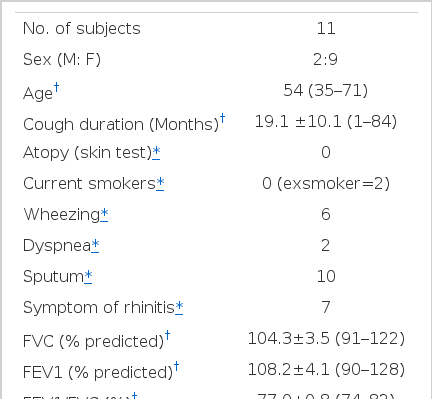
2. Clinical features of eosinophilic bronchitis (Table 1)
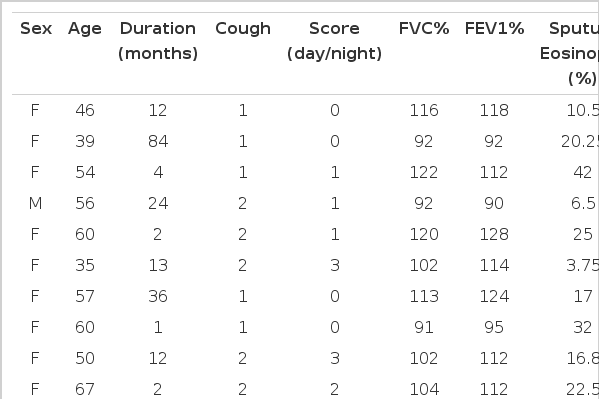
Among 11 patients, sex rate was 2:9 for males and females, in which females were more. The average age was 54 year (range: 35 to 71 years). The mean cough score was 2.45 (range: 1–5 grades) and there was no difference between day and night. Cough duration was 19.1±10.1 months (range: 1 month–84 months) and there were no smokers. Only 6 cases had a history of wheezing and 10 cases complained of sputum, which was mucous usually in the morning, 7 cases showed symptoms of rhinitis, including rhinorrhea and sneeze. The pulmonary function test predicted 104±3.5% and 108.2±4.1% for FVC% and FEV1%, respectively, and 77.0±0.8% for FEV1/FVC. Blood total IgE was normal as 46.2±15.0 IU/mL (0–117 IU/mL) and the number of blood eosinophil was 308±86/mm3 (0–1000/mm3). Only 1 case was over 500/mm3. Every case appeared negative on the skin test for 50 inhaled allergies. Sputum eosinophil % increased as 26.8±6.1% ranging 3.75–63.7%. But there was no relation between the degree of eosinophilic inflammation in the airway, the degree of coughing expressed by cough score, duration of coughing and pulmonary function (Table 2).
3. Clinical follow-up in patients with eosinophilic bronchitis
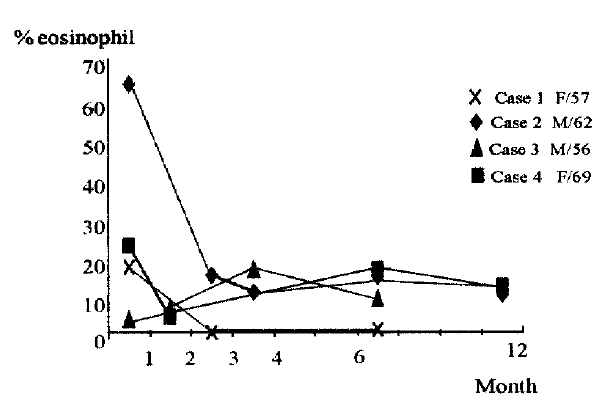
Eight patients out of 11 with eosinophilic bronchitis were administered with 800 μg inhaled budesonid in the morning and at night for follow-up. Follow-up sputum analysis was available with 8 patients for 6.8±4.6 weeks later and sputum eosinophilic % remarkably decreased from 29.1±8.3% before therapy to 7.4±3.3% after therapy (Figure 2). Also, cough score that patients complained of improved. To investigate any relation between eosinophil decrease and pulmonary functional decrease, even though the initial pulmonary function finding was normal, this study performed the sputum analysis for responses to pulmonary function test and bronchodilator, as well as the follow-up test. As a result, there was norelation between pulmonary function or bronchodilator response and changes in sputum eosinophil % (Figure 3). Follow-up of 4 cases for more than 6 months showed a decrease in coughing and improvement of eosinophilic inflammation after inhaled steroid therapy. However, eosinophilic fraction in only 1 case decreased and was maintained normally, whereas 3 cases did not show a decrease to the normal range in eosinophilic fraction; rather they had worse coughing with lapse of the time and temporary increases in eosinophilic fraction were observed at the same time (Figure 4).
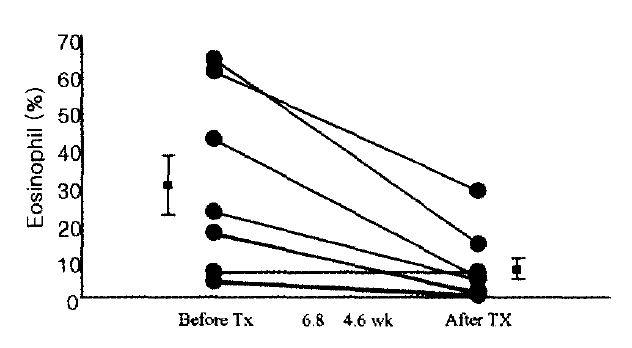
Sputum eosinophil % in patients with eosinphilic bronchitis before and after inhaled steroid therapy (n=8, p<0.05). Duration of mean follow-up is 6.8±4.6 weeks.
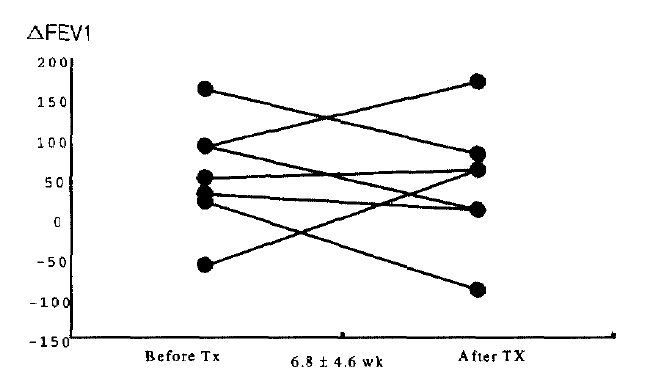
Bronchodilator responses in patients with eosinophilic bronchitis before and after inhaled steroid therapy (n=7, p>0.05). ⊿ FEV1 (mL)=post-bronchodilator FEV1-pre-bronchodilator FEV1.
DISCUSSION
To investigate the causes of chronic persistent cough, a well-organized approach using a history of illness, physical examination, chest X-ray and pulmonary function tests are required5–7). Accordingly, lots of diagnostic protocols have been suggested. The frequency of causes of chronic cough by these diagnoses are different according to each research, but they appear as postnasal drip, cough variant asthma and simple bronchitis, in order, which was confirmed again in this study result (Figure 1). But it is reported8) that there is a frequency of idiopathic chronic cough up to 12–26%8, 9), which has not been clarified with any approaches in the above. Also, there was a report10) that sensitivity of cough reflex increased in these patients, of which causes are not clear yet. While airway inflammation has been evaluated in some airway diseases, sputum examination was approved as accurate and useful. Therefore, sputum cell fraction analysis should be included in the diagnostic protocol for chronic cough syndrome. If sputum cell fraction analysis is performed as one of the diagnosis system of chronic cough syndrome, increases in eosinophilic fraction are observed with all the other normal test findings. That means that considerable eosinophilic bronchitis that can be diagnosed only by sputum cell fraction analysis are observed11, 12). Accordingly, it may be assumed that considerable part of chronic coughs with unknown causes can be diagnosed as eosinophilic bronchitis.
As sputum cell fraction analysis is actively being performed, it is supposed that a there are some arguments on methods. First, there is a difference in eosinophilic fraction between researchers to diagnose eosinophilic bronchitis based on the normal range of sputum eosinophilic fraction. Gibson et al.12) judged by 10%, while Brightling et al.4) adopted 3%. This study allowed sputum eosinophilic fraction over 3% as being significantly increased. It was not revised by age, but when sputum cell fraction analysis was performed with spontaneous or induced sputum of people without diseases, 95% revealed sputum eosinophilic fraction below 2.6%, which was used as a background. Second, according to Pizzichini et al.13), there is no difference between sputum cell fractions of induced and spontaneous sputum as a method to collect sputum, but it is reported that contamination of squamous cells in induced sputum is little. This study performed cell fraction analysis with spontaneous or induced sputum according to circumstances. Also, sputum was induced by increasing the concentration of physiologic saline in order until enough for the test. Third, the possibility of nasal discharge contaminating the sputum can not be completely prevented. So, sputum was collected after patients blew their noses. When sputum was induced, it was performed with their noses clogged by device to prevent any contamination. In case of rhinitis, nasal smear test was performed together and patients with eosinophilia underwent the rhinitis therapy first, even if there was eosinophilia in the sputum. Any improvement was classified as postnasal drip. It is assumed that this study did not make a wrong diagnosis of eosinophilic rhinitis.
According to the above method, the frequency of eosinophilic bronchitis was 11.9%, which was almost the same as 13.2% by Brightling et al4). Based on clinical features of each patient, sex rate was 2:9 (M:F), which showed many more females than males. This is similar to the result of Brightling et al4). It is unclear if it is attributed to more incidences of eosinophilic bronchitis in females or poor diagnostic rate in males14) by social elements. Also, it can not be excepted that cough threshold varies by gender, like a higher frequency of asthma in boys15, 16). Males display airway constriction or hyper-responsiveness, whereas females have a lower cough threshold than airway constriction or hyper-responsiveness. Mean Duration of cough was 19.1 months, but the range was wider. Coughing was repeated between worsening or improving. The degree of coughing varies according to patients from 1 to 5 points. There was no difference between day and night. Wheezing appeared as a combined symptom in 6 cases, but it was not proved by examination. It is thought that the accuracy of wheezing complaints is uncertain. This study did not except the diagnosis of eosinophilic bronchitis in case of PC20 methacholine as normal without reversible airway obstruction, even if with previous wheezing complaints. With reversible airway obstruction, wheezing can be occurred intermittently, which causes some debate if a patient with previous wheezing belongs to the entity of eosinophilic bronchitis or not. When there is a intermittent wheezing based on the clinical features of bronchial asthma, if pulmonary function and airway hyper-responsiveness are normal, it is defined as a trivial wheeze, of which clinical meaning or spontaneous progress has not been known. When patients with eosinophilic bronchitis complain of wheezing, but no symptom appears by examination, it predicts that trivial wheeze17) can be occurred in eosinophilic bronchitis. The lab finding was that all cases appeared negative in allergy skin test, which was lower than the usual frequency of atopy in normal persons. Total IgE was normal for all cases, by which it was estimated that thert is no relation between eosinophilic bronchitis and atopy. The number of blood eosinophil increased to 1000/mm3 in one case, but the other cases appeared as normal, which revealed no relation between sputum eosinophilia and the number of blood eosinophil.
As inhaled steroid therapy improves asthma satisfactoricy, airway eosinophilic inflammation responds well to steroid therapy. It is known that the degree of airway eosinophilic inflammation tested by sputum eosinophilic fraction in bronchial asthma or chronic obstructive bronchitis can predict the response to steroid therapy18, 19). As it is assumed that eosinophilic bronchitis is well controlled by inhaled steroid, this study administered 800 μg inhaled budesonide in case of eosinophilic bronchitis and observed improvement by remarkably decreased cough scores in 8 follow-up cases, even though it was not mentioned in their results. With improvement, eosinophilic fraction decreased during follow-up analysis of sputum cell fraction, which confirmed that the degree of coughing is decided by sputum eosinophil. It is known that airway inflammation is founded in the tissue of patients with chronic cough20) and assumed that epithelial damage by eosinophil and exposure of irritant receptors induce cough in the subject of eosinophilic bronchitis. As every patient was not examined at a regular interval of time for follow-up, it was not determined how fast eosinophilic inflammation is improved by inhaled steroids. However, mean follow-up period was 6.8 weeks and the response came out in 1–2 months. As the sputum test was not re-performed often, the cause and effect relation between the degree of coughing and the period of eosinophilic inflammation was not clarified.
It is still unclear that eosinophilic bronchitis is a disease different from bronchial asthma or a varient type of asthma. A long follow-up report of eosinophilic bronchitis21) is rare and it is hard to make a conclusion. As eosinophilic bronchitis is relatively responsive to the therapy and does not show any severe symptoms, a long-period follow-up test is considered as difficult at tertiary medical facilities. In this study, 3 out of 4 cases available for follow-up over 6 months showed a decrease in symptoms and airway eosinophilic inflammation by initial treatment, but did not become normal completely. Rather, they experienced exacerbations as time went by and the sputum cell analysis performed at that time revealed increases in sputum eosinophilic fraction, which indicated a chronic course. Though the result did not mention, it 1 out of 4 cases became a positive conversion in methacholine bronchoprovocation test after follow-up for 18 months, which implies a possibility of development from eosinophilic bronchitis into bronchial asthma.
It has not been clearly reported about the pathophysiology of eosinophilic bronchitis so far. This study aimed to investigate the relation between airway eosinophilic inflammation, pulmonary function and clinical symptoms in eosinophilic bronchitis. To this end, we performed follow-up analysis of sputum cell fraction, pulmonary function test and bronchodilator response test and compared the degree of airway inflammation, degree and duration of coughing and pulmonary function. As a result, There was no relation between the degree of eosinophilic inflammation and pulmonary function and the degree of coughing. Therefore, we can see that development or loss of eosinophilic bronchial inflammation does not affect the airway function. But, even in case of severe eosinophilic inflammation, all subjects showed normal pulmonary function and it is doubtful that follow-up pulmonary function test can reflect the state of a patient. There are several hypotheses about the reason why eosinophilic bronchitis does not show airway hyper-responsiveness, but it is not known clearly yet. It is thought that more efforts should be necessary to measure the degree of hyper-responsiveness and further studies are needed for activation of eosinophil, evaluation of neurogenic airway inflammation and difference from asthma by airway morphology.