Effects of Adjuvant Androgen on Anemia and Nutritional Parameters in Chronic Hemodialysis Patients Using Low-dose Recombinant Human Erythropoietin
Article information
Abstract
Background:
Recombinant human erythropoietin (epoetin) has greatly contributed to improvement of the anemia of chronic renal failure patients on hemodialysis. However, the reduced erythropoietic effect to epoetin and its high cost have induced lots of supplementary treatments. Therefore, we performed a prospective study to evaluate the effects of adjuvant low-dose androgen therapy in patients using a lower-dose of epoetin than the commonly recommended dose on anemia and the nutritional parameters.
Methods :
17 patients of hemoglobin (Hgb) less than 9 g/dL even after being treated with 1,000 U epoetin subcutaneously (s.c.) 3 times per week on a stable status for more than 6 months, who were on hemodialysis at our institution were examined. They were injected with the same dose of epoetin s.c. and nandrolone decanoate 100 mg intramuscularly (i.m) weekly for another 6 months. Blood test was performed every month before therapy for 6 months and after therapy for 6 months and the mean values were reviewed for comparison.
Results:
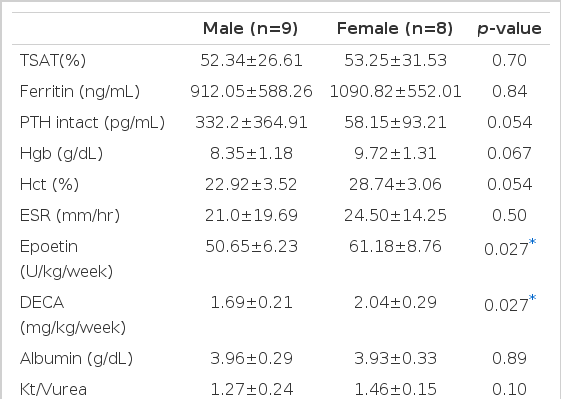
Hgb (7.75±0.9 vs 8.99±1.39 g/dL, p <0.01) and hematocrit (Hct) (23.68±2.85 vs 26.66±3.91%, p <0.01) were apparently changed before and after adjuvant therapy. Hgb and Hct, weekly dose of epoetin were not statistically different in 9 male patients before and after adjuvant therapy. The weekly dose of epoetin was not statistically different in 8 female patients, but Hgb and Hct (8.02±0.6 vs 9.72±1.31 g/dL, 24.54±1.7 vs 28.74±3.06%, p <0.01) were statistically different before and after adjuvant therapy. In comparison between male and female groups, weekly doses of epoetin and nandrolone decanoate were significantly greater in the female group than the male group (epoetin: 50.66±6.23 vs 61.18±8.76 U/kg/week, nandrolone decanoate: 1.69±0.2 vs 2.04±0.29 mg/kg/week, p <0.05).
Conclusion :
Our data show that the adjuvant androgen therapy is effective for the anemia of hemodialysis patients who did not recover from anemia even after being continuously treated with low-dose epoetin.
INTRODUCTION
Recombinant human erythropoietin (called epoetin hereafter) has greatly contributed to improve the anemia of end-stage renal disease (ESRD) patients on hemodialysis.
However, lots of adjuvant therapies are being tried to improve poor erythropoietic response to epoetin and the high cost1). Androgen was the mainstay of nontransfusional methods to correct the anemia of ESRD patients before epoetin appeared in the late 1980s but, after that, the use of androgen was reduced2,3). Androgen is known to directly stimulate erythropoiesis and increase the effect of endogenous erythropoietin (called EPO hereafter)4–6). It is not clear whether androgen increases the erythropoietic response to exogenous EPO7) and the adjuvant androgen therapy is still under discussion. The previous studies have reviewed the effects of adjuvant therapy group and epoetin treatment alone group in independent samples7–9).
Therefore, we performed a prospective study to evaluate effects of low-dose adjuvant androgen on anemia and nutritional parameters in chronic hemodialysis patients using low-dose epoetin, which is lower than the commonly recommended dose.
MATERIALS and METHODS
1. Patients
17 patients (9 males and 8 females) with hemodialysis on a stable status of more than 6 months at our institution were selected. They had hemoglobin (Hgb) <9.0 g/dL even after being treated with 1,000 U epoetin (Recormone® Chungwoe Pharmaceutical Co. ROK) subcutaneously (s.c.) 3 times per week. The mean age was 41.9 years and the mean period of hemodialysis was 6.5 years. Patients who experienced transplantation, nephrectomy, upper gastrointestinal bleeding, ischemic heart disease, hepatic dysfunction, infection or chronic inflammation were excluded.
2. Methods
17 patients (9 males and 8 females) were treated with 1,000 U epoetin s.c. 3 times per week and nandrolone decanoate 100 mg intramuscularly (i.m.) weekly for another 6 months regardless of weight. Before adjuvant nandrolone decanoate therapy, prostate specific antigen (PSA), Vitamin B12 (Vit B12), folic acid and aluminium tests were performed. Hgb, Hct, albumin, AST, BUN, creatinine, serum ferritin, serum iron (s-iran), total iron binding capacity (TIBC) and transferrin saturation (TSAT) were evaluated in the beginning of every month and intact-PTH, ESR test and Kt/Vurea, normalized protein catabolic rate (nPCR) were evaluated every 3 months. As to the laboratory findings, the average for 6 months before adjuvant therapy was a reference value before therapy and the average of the 5th month and the 6th month test outcomes after adjuvant therapy was a reference value after therapy. The indirect evaluation of nutritional parameters was performed by serum albumin and nPCR.
3. Statistics
Every data was explained in mean ± standard deviation. SPSS 9.0 for windows/PC was used for the statistical treatment and paired t-test, Wilcoxon signed rank test, Mann-Whitney U test were performed for comparison between groups. p<0.05 was recognized as statistically significant.
RESULTS
The number of patients were total 17 (9 males and 8 females). Age was 41.9±9.7 years (male; 39.1±10, female; 45±8.9) and the period of hemodialysis was 78.12±49.13 months. Vit B12 was 894.4±432.5 pg/mL (normal; 160–970 pg/mL), folic acid 15.55±5.45 ng/mL (normal; >1.5 ng/mL), aluminium 18.84±8.59 μg/L (normal; 11–44 μg/L). PSA in male patients showed 0.75±0.62 ng/mL (normal; 0–4 ng/mL) (Table 1).
Comparison of mean values between before and after adjuvant androgen therapy for 6 months displayed s-iron (101.9±51.4 vs 104.9±57.1 μg/dL), TIBC (209.7±32.02 vs 202.8±33.8 μg/dL), s-ferritin (961.3±597.3 vs 996.2±561.1 ng/dL), Ca (8.92±0.67 vs 9.1±0.76 mg/dL), P (4.87±1.5 vs 5.0±1.37 mg/dL), intact PTH (230.9±321.6 vs 203.2±300.4 pg/mL), ESR (26.8±23.3 vs 24.6±16.5 mm/hr), epoetin (56.6±10.2 vs 55.6±9.07 U/kg/week) ALT (25.2±11.5 vs 24.9±17.7 U/L), s-albumin (3.95±0.37 vs 3.95±0.3 g/dL), nPCR (0.99±0.23 vs 0.95±0.16 g/kg/day), Kt/Vurea (1.38±0.19 vs 1.36±0.21), which meant no statistical difference in every index before and after adjuvant therapy. But the mean Hgb apparently increased from 7.75±0.9 g/dL before therapy for 6 months to 8.99±1.39 g/dL (p<0.01) after therapy and Hct from 23.68±2.85% before therapy to 26.66±3.91% (p<0.01, Table 2, Figure 1). The effect of the adjuvant therapy on sex was examined. In 9 male patients, mean Hgb 7.5±1.07 g/dL, Hct 22.92±3.52%, epoetin 51.01±5.86 U/kg/week before therapy changed into Hgb 8.35±1.18 g/dL, Hct 24.82±3.78%, epoetin 50.66±6.23 U/kg/week after therapy, which showed no statistical difference. In 8 female patients, the weekly dose of epoetin changed from 62.79±10.81 U/kg/week before therapy to 61.18±8.76 U/kg/week after therapy, which displayed no statistical difference. However, Hgb and Hct apparently increased from 8.02±0.6 g/dL and 24.54±1.7% before therapy to 9.72±1.31 g/dL and 28.74±3.06% (p<0.01) after therapy (Table 3, 4, Figure 2), respectively. There was no difference between male and female groups in s-ferritin, TSAT, intact PTH, ESR, ALT, albumin, Kt/Vurea, nPCR before and after adjuvant androgen therapy combined with epoetin. But, as to weekly dosage of epoetin and DECA dose, rHuEPO was 50.66±6.23 U/kg/week, DECA 1.69±0.2 mg/kg/week in the male group, while epoetin was 61.18±8.76 U/kg/week, DECA 2.04±0.3 mg/kg/week in the female group, which represented that they were significantly greater in the female group than the male group (p<0.05, Table 4, Figure 3). Also, the weekly dosage of epoetin before adjuvant therapy was significantly greater in the female group than the male group (51.01±5.86 vs 62.79±10.81 U/kg/wee, p<0.05). There were no side effects from androgen treatment for 6 months, except muscle pain only.
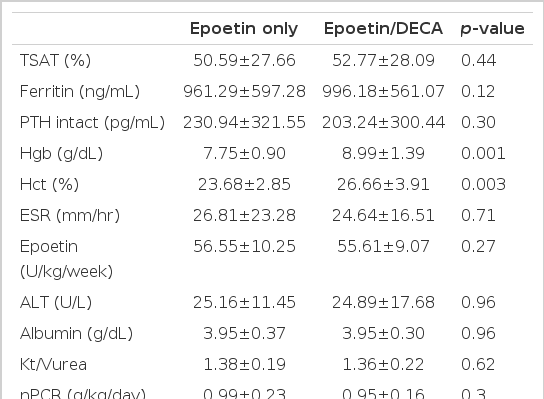
Laboratory data at epoetin only and at 24 weeks after epoetin combined with nandrolone decanoate in total 17 patients
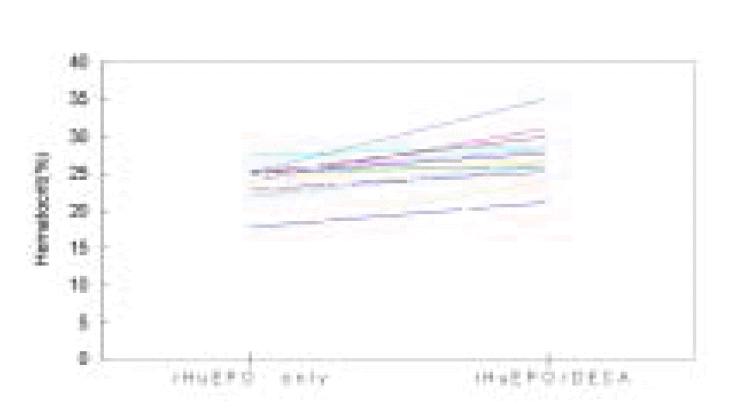
Individual patient responses at recombinant human erythropoietin [epoetin] only and at epoetin combined with nandrolone decanoate [DECA]
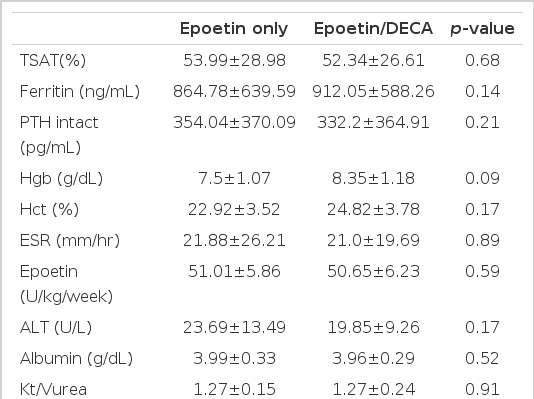
Laboratory data at epoetin only and at 24 weeks after epoetin combined with nandrolone decanoate in males (n=9)

Laboratory data at epoetin only and at 24 weeks after epoetin combined with nandrolone decanoate in females (n=8)

Hct levels at recombinant human erythropoietin [epoetin] and at epoetin combined with nandrolone decanoate [DECA], respectively of male and female group. m, male; f, female
DISCUSSION
Epoetin can correct the anemia of ESRD10), but a lot of studies are being made to lower epoetin dose in the light of reducing expense7). There are various s.c. like s.c. injection of epoetin11,12), i.v. administration of iron to maximize iron supplement13), L-carnitine replacement therapy14) and adjuvant androgen therapy combined with epoetin8,9). Androgen was the mainstay for the anemia of hemodialysis patients before the widespread availability of epoetin2,3).
Androgen in current use is nandrolone decanoate as a derivative of 19-nortestosterone ester15). The effect of androgen on erythropoiesis partly helps formation of endogenous EPO and increases sensibility of erythroid progenitor to erythropoietic factor4,5). But some studies have reported contrary outcomes about the effect of androgen on exogenous EPO in patients on maintenance hemodialysis. Ball et al8). reported in a prospective study with 15 male patients that Hct of 7 patients with 2,000 U epoetin i.v. 3 times per week changed from 25.4±0.8% to 27.4±1.5% after 12 weeks, while Hct of 8 patients with adjuvant therapy of 2,000 U i.v. 3 times per week and nandrolone decanoate 100 mg i.m. weekly increased from 24.4±1.4% to 32.9±1.8% after 12 weeks, which represented a statistical significance between the two groups. And Gaughan et al7). performed a long-term prospective study for 26 weeks with 19 stable patients. In this study, Hct of 10 patients (4 males and 6 females) with 1,500 U epoetin i.v. 3 times per week changed from 24.8±1.4% to 28.3±2.8%, while Hct of 9 patients (7 males and 2 females) with adjuvant therapy of 1,500 U epoetin i.v. 3 times per week and 100 mg nandrolone decanoate weekly increased from 25.1±1.5% to 33.2±4.5%, which displayed a significant increase in the adjuvant therapy group. But, on the contrary, Berns et al9). reported in a prospective study with 12 patients (7 males and 5 females) for 16 weeks that Hct in the group with epoetin 40 U by weight 3 times per week increased 23.5±0.7% to 23.82±0.81% and Hct in the adjuvant therapy group with the same dose of epoetin 3 times per week and nandrolone decanoate 2 mg by weight weekly increased from 23.3±0.8% to 23.67±0.91%, which represented no statistical significance between the two groups. This study investigated 17 patients (9 males and 8 females) who had been on a stable status for more than 6 months on hemodialysis and treated with 1,000 U epoetin s.c. 3 times per week only to have less than 9.0 g/dL of Hgb. It is known to require about 5 months for the effect of androgen to appear3). Therefore, in this study, patients were treated with the same dose of epoetin s.c. 3 times per week and nandrolone decanoate 100 mg i.m. weekly for another 6 months. There were no statistical differences in TSAT, ferritin, intact PTH, ESR, weekly dose of epoetin, Kt/Vurea and nPCR between before therapy for 6 months and after adjuvant therapy. Hct apparently increased from 23.68±2.85% before therapy to 26.66±3.91% after adjuvant therapy in total group (p<0.01). It was the same as the results of Gaughan et al7). and Ballal et al8), Adjuvant therapy with nandrolone decanoate displayed a significant increase in Hgb and Hct in the female group, but not in the male group. Hct of male patients did not show a statistical difference from 22.92±3.52% before therapy to 24.82±3.78% after therapy. However, the Hct of female patients was statistically significant from 24.54±1.70% to 28.74±3.06%. Hct, Hgb, TAST, ferritin intact PTH, ESR, albumin, Kt/Vurea d nPCR were not statistically different between the male and female groups, but epoetin 50.65±6.23 U/kg/week, nandrolone decanoate 1.69±0.21 mg/kg/week were administered in male group and epoetin 61.18±8.76 U/kg/week, nandrolone decanoate 2.04±0.29 mg/kg/week were administered in female group, which meant epoetin and nandrolone decanoate were administered significantly greater in the female group than in the male group. But, the dose of epoetin in the male group before therapy was 51.01±5.86 U/kg/week, while 62.79±10.81 U/kg/week in the female group, which explained that more dose was administered in the female group than in the male group. As there was no change in epoetin dosage between before and after the adjuvant therapy, we assumed that androgen has a major effect on the outcome. Though we did not check the blood level of androgen, androgen is thought to be higher in male than in female. Higher androgen level in male may reduce the effect of adjuvant nandrolone decanoate therapy in the male group. In this study, a lower-dose of epoetin than the commonly recommended dose was treated, but adjuvant therapy combined with nandrolone decanoate was effective to improve the anemia and especially, more effective in the female group. It suggests that and that nandrolone decanoate can enhance the effect of exogenous EPO in patients on maintenance hemodialysis.
Ifudu et al16). said that responsiveness to epoetin is related with the amount of dialysis, which meant appropriate dialysis can enhance the erythropoietic effect of epoetin. In this study, Kt/Vurea was not changed as 1.38±0.19 and 1.36±0.22 before and after adjuvant therapy, which explains that dialysis has been properly performed. Therefore, it can not be said that Kt/Vurea directly influenced the change of Hct before and after adjuvant androgen therapy. Some factors known to reduce the response to epoetin are osteitis fibrosa, iron deficiency, chronic inflammation and aluminium storage17). There were no statistical differences in TAST of 50.6±27.7% vs 52.8±28.1 and ferritin of 961.3±597.3 ng/dL vs 996.2±561.1 ng/dL before and after adjuvant therapy in this study, and iron deficiency could be excluded. Chronic inflammation was removed from the study and plasma aluminium was 18.84±8.59 (referred value 11–40 μg/L), by which reduced response of epoetin by aluminium storage could be excepted. Bone biopsy test was not performed in this study that myelofibrosis by secondary hyperparathyroidism could not be excepted. However, we assumed that intact PTH did not have direct influence the outcome of adjuvant androgen treatment because it was not changed before and after adjuvant therapy6,18–20) and it’s blood level is with in upper normal limit.
It is known that androgen can slightly increase serum albumin by anabolism. But this study did not find a statistical difference in 3.95±0.37 g/dL and 3.95±0.3 g/dL before and after adjuvant therapy. Also, nPCR as an index of protein intake, did not show a statistical significance in 0.99±0.23 and 0.95±0.16 before and after adjuvant therapy. nPCR presumed to be the same as the amount of protein intake on a stable status. nPCR is being used on a hemodialysis patient as an objective method to calculate protein intake21) and to check if a patient follows a dietetic treatment well. Lower level of serum albumin in this study is attributed to less intake of protein than the commonly recommended protein intake. It is reported that use of androgen induces liver dysfunction, peliosis hepatis and hepatoma when 17-alkylated androgen was used22–25), but such liver damages were not reported when nandrolone decanoate as a derivative of 19-nortestosterone was used3,8,9,26,27). No finding of liver dysfunction was observed in this study.
Teruel et al18). recommended the use of androgen for male patients over 50 years, because of side effects like hypertrichosis and voice change, and the use of epoetin in female patients instead of androgen. Androgen was used in 8 female patients in this study, but such side effects as hypertrichosis and voice change were not observed and only a mild discomfort at the injected site was found. But it is thought that a longer follow-up may be required to find out a possible production of hypertrichosis and voice change because the observation period of 6 months was rather short, even with a low-dose of androgen, and the treatment should be stopped in its early stage if such side effects occur. It is said that PSA in the normal prostate depends on androgen28) and its concentration in serum is correlated with the size of the prostate29, 30). Serum PSA concentration can be reduced by administering finasteride to competitively inhibit 5-α reductase31), which is related with reduction in the amount of the prostate. Male patients on dialysis were treated with nandrolone decanoate and compared for serum PSA concentration, but they showed no increase in concentration of serum PSA so that the influence of nandrolone decanoate on the prostate in uremic patients was thought to be slight32). However, the role of androgen in expression of prostate cancer during latency period is not clearly explained and there is no study result to show if androgen stimulates prostate cancer to progress from the latency period to the clinical expression33). Therefore, with the use of androgen, the prostate should be periodically observed with serum PSA concentration and through digital rectal examination.
Considering the prices cost, in Korea, the adjuvant treatment of nandrolone decanoate 100 mg (11,114 won) weekly and 1,000 U epoetin (12,500 won) 3 times per week cost 48,614 won every week. On the other hand, the treatment of 2,000 U epoetin (23,000 won) 3 times per week alone costs 69,000, thereby making the adjuvant treatment more economical.
Finally, the adjuvant androgen therapy is effective to improve the anemia in hemodialysis patients who were not recovered with a continuous therapy using a low-dose of epoetin or who did not response to epoetin without any special reason or who could not increase epoetin dosage due to economic reasons. The nutritional parameters like albumin and nPCR were not improved. Side effects by androgen therapy for 6 months were not observed in this study, but a longer follow-up observation and it’s evaluation should be required.