Helicobacter pylori and Telomerase Activity in Intestinal Metaplasia of the Stomach
Article information
Abstract
Background:
Helicobacter pylori (H. pylori) has been considered a definitive carcinogen in gastric cancer. Telomerase is activated in gastric cancer and some premalignant gastric lesions, including intestinal metaplasia (IM). In this study, we evaluated the relationships of both H. pylori infection and telomerase activity with endoscopic and histologic features in IM. The effects of H. pylori eradication on endoscopic, histologic and biochemical changes were evaluated.
Methods:
Endoscopic biopsies were obtained from 43 patients with IM for rapid urease, histologic and telomerase tests. The endoscopic and histologic features, H. pylori infection and telomerase were assessed. After H. pylori eradication, 15 patients were re-evaluated and compared after 4 months.
Results:
Thirty-four (79.1%) patients were infected with H. pylori. The incidence of H. pylori infection was borderline correlated to the severity of IM (p=0.076). Telomerase was elevated in eight (18.6%) patients. Telomerase tends to be high in subtype III and endoscopic grade III of IM. After H. pylori eradication, endoscopic extent (p=0.039) and histologic severity (p=0.074) showed improvements, and telomerase decreased significantly (p=0.0001).
Conclusion:
Our data suggest that telomerase is associated with the severity and extent of IM and that H. pylori eradication improves the endoscopic and histologic features in IM, and decreases telomerase activity. H. pylori eradication can be considered one of the methods to prevent gastric cancer in patients with H. pylori-infected IM. Further long-term and large-scaled study will be needed.
INTRODUCTION
Gastric cancer is one of the most common types of cancer worldwide1). Despite some improvement in its treatment, the 5-year survival rate of gastric cancer is still low. The prevalence of intestinal metaplasia (IM) is closely related to H. pylori infection2). Furthermore, chronic gastritis due to H. pylori infection may progress to IM and even gastric cancer3–5). H. pylori-induced inflammation facilitates gastric carcinogenesis by increasing the rate of cell replication, decreasing the concentration of ascorbic acid and inducing DNA damage via reactive oxygen species6, 7). Both environmental and genetic factors are crucial in the multistage model of gastric tumorigenesis3). A preliminary observation has suggested that both IM and H. pylori infection are important targets in the prevention of gastric cancer8).
Telomerase, located at the distal end of human chromosomes, comprises simple, repetitive and G-rich hexameric sequences (TTAGGG), and is vital for chromosomal stability and replication. Telomerase is a ribonucleoprotein polymerase that adds telomeric sequences onto the ends of the chromosome to compensate for the DNA end-replication problem. Thus, this activity is strongly associated with cell immortalization and carcinogenesis, and its presence is elevated in 85% of human cancers, including those associated with the stomach, colon, breast, bladder, prostate, liver, lung and ovary. The measurement of telomerase activity may become a diagnostic tool for cancer9). However, telomerase activity can also be found in precancerous lesions, such as IM10).
In this study, we evaluated the relationships of both H. pylori infection and telomerase activity with endoscopic and histologic features in IM. The effects of H. pylori eradication on endoscopic, histologic and biochemical changes were evaluated.
PATIENTS AND METHODS
This study involved 43 patients who, between January 1999 and June 2000, were diagnosed with IM without any gastroenterologic diseases by an upper gastrointestinal endoscopic examination at a gastrointestinal endoscopy unit of Soonchunhyang University Chonan hospital. Thirty-four of these patients were diagnosed as having H. pylori infection. Eight patients exhibited elevated telomerase activity. Twenty-three of the patients with H. pylori infection were treated with an H. pylori eradication regimen and fifteen of them were able to be followed up for changes in endoscopic, pathologic and telomerase results after 4 months of complete therapy.
1) Gastric tissue sampling by upper gastrointestinal endoscopy
Six gastric specimens were obtained by endoscopic biopsy of the IM lesion. Two of these specimens were fixed in 10% buffered formalin, embedded in paraffin and sectioned, and then one was stained with hematoxylineosin (H & E) and/or WarthinStarry silver stain for detecting H. pylori. The other specimens were stained using the high-iron diamine (HID)/alcian blue (AB) technique to differentiate acidic mucin into sialomucins (blue) and sulfomucins (brown-black)11). The other two specimens were used to detect telomerase activity. One set of gastric tissue from the antrum and body was obtained and used for the Campylobacter Like Organism (CLO) test and monitored for color changes for up to 24 h at room temperature.
2) Grading of intestinal metaplasia
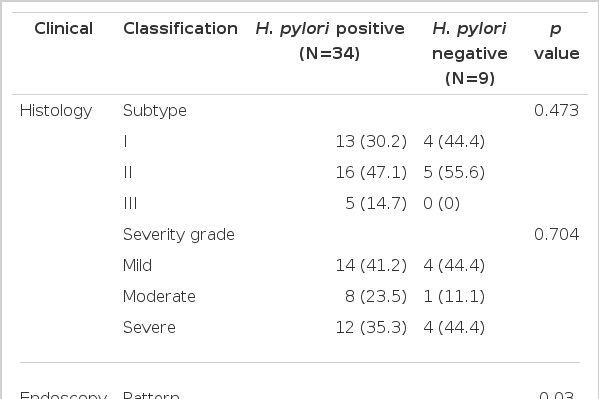
Endoscopic grades were classified according to the degree and pattern of mucosal elevation: I, granular; II, nodular; III, irregular or confluent. The extent of IM, as assessed endoscopically, was graded as follows: I, confined to antrum; II, extending to lower body; III, extending to upper body and fundus. Histologic grading is classified by HID/AB and H&E staining. Metaplastic lesions were classified into three subtypes: type I, complete IM characterized as resembling the normal intestinal epithelium; type II, incomplete IM expressing sialomucins but not sulfomucin; and type III, incomplete IM expressing sulfomucins. If IM of more than one subtype was present in a given sample, the case was assigned to the least mature subtype, as proposed previously2).
3) Telomerase assay
Protein extracts were obtained and a telomerase activity assay was performed as described previously12). One to three milliliters of tissue extract (1–50 μg of total protein) was subjected to a modified, semiquantitative telomeric repeat amplification protocol, which included an internal telomerase assay standard13). A polymerase chain reaction (PCR) was performed using a thermal cycler (DNA engine: Peltier thermal cycler, MJ Research, Waltham, Mass., USA) using primer elongation at 25°C for 10 min and inactivation at 94°C for 5 min. Thirty-three PCR cycles were performed which consisted of 94°C for 30 s, 50°C for 30 s and 72°C for 90 s. After hybridization using digoxin and a telomeric repeat sequence, the absorbancy was measured at 450 nm by a microtiter plate (ELISA) reader. Negative and positive controls were read using a PCR ELISA kit (TeloTAGGG Telomerase PCR ELISAPLUS, Roche, Nutley, N.J., USA). The final value was obtained by subtraction of the mean of the absorbance readings of the negative controls from the absorbance readings of the samples (i.e., AsAs.o). Samples are considered as telomerase-positive if the difference in absorbance (A) is higher than two times the background activity. Quantification of telomerase activity was determined as the relative telomerase activity using a formula provided in the PCR ELISA kit.
4) H. pylori eradication and follow-up
The H. pylori eradication regimen was administrated to 15 patients with H. pylori infection for 2 weeks as 20 mg omeprazole, 1000 mg amoxicillin and clarithromycin 500 mg twice per day. Four months later we performed endoscopic, histologic, and biochemical evaluations.
5) Statistical analysis
Statistical analysis was performed using the Stata program (Release 5.0; Stata, College Station, Texas, USA). The chi-square or Fishers exact test was utilized to analyze differences in the proportions of H. pylori infection status or telomerase activity in the endoscopic and histologic findings. The Wilcoxon signed rank test was utilized when comparing paired samples, p values less than 0.05 were considered to be statistically significant.
RESULTS
1) General profiles
The mean (SD) age of the subjects was 52.2 (11.1) years and 29 (67.4%) of them were male. The nodular pattern and grade-III extent was prevalent (24, 55.8%; 20, 46.5%; respectively) in the endoscopic findings. The prevalence of subtype II (21, 44.8%) and mild grade (18, 41.9%) was highest in histologic findings. Thirty-four (79.1%) patients showed H. pylori infection and eight (18.6%) had elevated telomerase activity (Table 1).
2) Clinical features and H.pylori infection
The granular type was significantly higher in H. pylori infection (11, 32.4% vs 0, 0%, p=0.03) and the increase in grade-I extent was borderline significant (9, 26.5% vs 0, 0% p=0.076) in endoscopic findings. However, H. pylori infection rates were not significantly different according to histologic subtype (p=0.473) or severity (p=0.704) (Table 2).
3) Clinical features and telomerase activity
Telomerase activity was detected in eight (18.6%) patients. Telomerase activity showed a high tendency in subtype III of IM, but without statistical significance (p=0.179). There was no relation between histologic severity of IM and telomerase activity (p=0.731). There were no significant relationships between endoscopic pattern and telomerase activity, but telomerase activity showed a high tendency in a wide extent of IM (Table 3).
4) Follow-up after H. pylori eradication
We performed repeat endoscopic and histologic examinations and evaluated telomerase activity in 15 patients to compare the quantitative changes in the activities (relative telomerase activity). Eight and ten out of 15 patients were downgraded or improved according to histologic subtype (p=0.671) and severity (p=0.074), respectively. In endoscopy, five and six patients showed improvement of pattern (p=0.151) and extent (p=0.039), respectively. There was a significant difference in extent grading between initial and follow-up after H. pylori eradication according to endoscopic findings (p=0.039) (Table 4). Telomerase activity showed a significant decrease in this period (1321.5±870.8 vs 373.2±560.68, p=0.0001) (Figure 1).

Comparisons between initial and follow-up conditions after H. pylori eradication in 15 patients with IM
DISCUSSION
Telomerase activity in humans has been detected in germ lines and tumor tissues as well as in established cultured-cell lines14). In normal somatic cells, the absence or low expression of telomerase is thought to result in progressive telomeric shortening with each cell division. Therefore, it has been proposed that reactivation of telomerase is a critical step in tumorigenesis. The expression of telomerase may occur at different stages of cancer progression, depending on the type of malignancy15–19).
Telomerase activity has been detected in approximately 35% of noncancerous lesions, such as gastric IM,10) although the level is generally lower than in cancerous tissue. In addition, genetic alterations, such as p5320) and APC gene mutations21), are found in IM and gastric cancer. These genetic changes and the activation of telomerase in IM and so-called precancerous lesions may contribute to the carcinogenic process in intestinal gastric cancers20). Furthermore, detection of telomerase activity in both cancer and IM suggests that telomerase activation is an early event in stomach carcinogenesis. In our study, telomerase activity was detected in 18.6% of patients.
IM is the common endoscopic feature that occurs at an average age of 14 years after superficial and/or atrophic gastritis. The occurrence of chronic atrophic gastritis has a complex relationship to H. pylori infection, genetics, diet, drugs, smoking, alcohol, high salt intake, vitamin-C deficiency and environmental factors. IM is the replacement of gastric mucosal cells with intestinal cells (including goblet cells). Regression of IM after H. pylori eradication is debatable in many histologic studies22–31), and studies on endoscopic changes are also limited.
We randomly classified the endoscopic grade and extent of IM according to the nature of gross severity. Through the classification, we assessed the relationship between endoscopic features and H. pylori infection or telomerase activities and we evaluated the significance of these endoscopic findings on the basis of histology and molecular biology. We also observed the changes of these endoscopic classifications and histologic and molecular biologic changes as detailed markers of precancerous lesions after H. pylori eradication. In our study, changes in the grade of the pattern of IM were not statistically significant and the extent of IM tended to decrease. These changes represent the possibility of regression of endoscopic findings. The H. pylori infection rate was high in the endoscopic granular type which was suspected as being a relatively low-grade lesion. The H. pylori infection rate had a tendency to be higher in the grade-I extent of IM than grade-II or III extent. These results suggest indirectly that the H. pylori infection rate is high in the early stages of IM, and suggest that H. pylori cannot survive for long enough to allow migration from the antrum to the upper body by the progression of IM. Among the eight patients with telomerase activity, there was no significant relationship between the endoscopic pattern of IM and telomerase activity, but telomerase activity tended to be detected in grade-III extent. These findings suggest that the endoscopic extent of IM represents the duration of H. pylori infection and premalignant tendency of IM.
The relationships between the histologic characteristics of IM and H. pylori infection have been studied extensively. In the present study, the H. pylori infection rate did not differ significantly with histologic subtype and severity. Telomerase activity tends to be high in subtype III of IM; this subtype is known to be more precancerous in nature than other histologic types in pathologic studies.32) The number of subjects in our study is too small to allow us to say definitively that subtype III of IM has a precancerous nature at the molecular level. There was no relation between the histologic severity of IM and telomerase activity. The nature of the precancerous lesion did not depend on histologic severity, but subtype is more important in the prediction of cancerous changes in IM. The histologic subtype was changed in 73.3% of cases with IM and the severity of IM tended to decrease. Metaplastic changes disappeared in 20% of cases. Taken together, these observations suggest the possibility of reversibility of IM after H. pylori eradication.
Our study suffered from some limitations associated with the use of endoscopic biopsies, but these are unavoidable in a clinical study. However, we used special staining techniques to maximize our ability to assess the pattern and extent of IM, and we recorded the lesion site to allow re-examining of the same site by endoscopic biopsy. Another problem is that a relatively small number of subjects was included in this study to induce a statistically significant result for comparing the relationship among various factors, which needs subsequent more studies to confirm the clinical significance of relating factors in patients with IM.
The changes in quantitation of telomerase activities (relative telomerase activity) was significantly decreased in patients regardless of the positivity of the telomerase activity. These results suggest that H. pylori eradication is needed in H. pylori-infected patients with IM, especially those that have endoscopic findings of widespread and histologic subtype III IM.
To conclude, in IM a widespread endoscopic lesion and histologic subtype III produces a tendency for high telomerase activity, which is a predictable marker of malignant changes. H. pylori eradication improved the endoscopic and histologic features and decreased telomerase activity. We carefully suggest that H. pylori eradication can be considered one of the methods to prevent gastric cancer in patients with H. pylori infected IM. Further long-term and large scaled study will be needed.

The endoscopic extent of IM. IM in antrum is defined as grade I (A), extending to lower body as grade II (B) and extending to upper body and fundus as grade III (C).
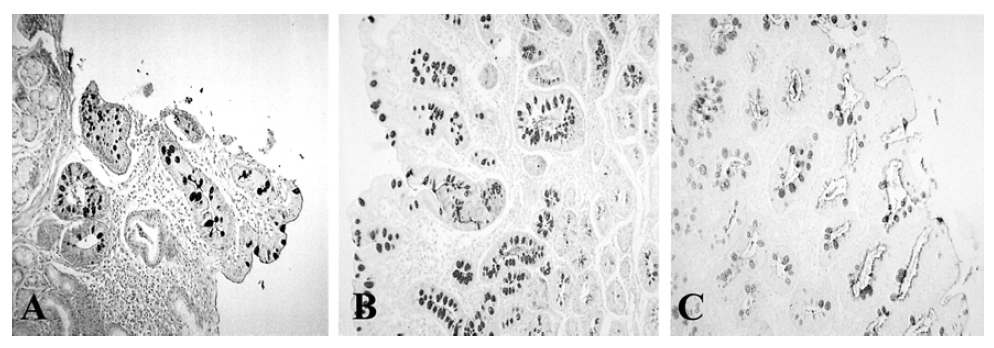
The histologic subtype of IM. Type I is incomplete IM characterized as resembling the normal intestinal epithelium (A), type II is complete IM expressing sialomucins but not sulfomucins (B) and type III is incomplete IM expressing sulfomucins (C).
Notes
Some of the results in this paper were presented at Digestive Disease Week (DDW), Atlanta, May 2001.