See Article on Page 191-202
Arthritis, particularly osteoarthritis (OA), is the leading cause of disability and is characterized by degenerative joint changes [1]. OA has become more prevalent with the aging of the population and increasing prevalence of obesity. The Fourth Korea National Health and Nutrition Examination Survey in 2008 showed that the proportion of the population over 80 years of age is estimated to be about 34.5% [2]. OA is thought to be caused by the aging process and long-standing mechanical stress, which lead to articular cartilage degradation and chondrocyte activation [1,3].
Although the major risk factor for the development of OA is aging, the overall purpose of treatment is to improve pain control and prevent, reduce, or reverse cartilage damage [4]. The average number of comorbid conditions is higher in aged than in young individuals, and other chronic diseases that limit activity and reduce the efficacy of drug therapy may frequently develop. Management of multiple chronic conditions is an important part of the care of patients with OA [5,6]. To enhance the specificity of the treatment recommendations for individuals with varying health profiles and OA burdens, the OA Research Society International (OARSI) defined clinical subphenotypes without/with comorbidities such as diabetes, hypertension, cardiovascular (CV) disease, renal failure, gastrointestinal (GI) bleeding, depression, and physically limiting conditions such as obesity [7]. Consideration of these comorbidities, especially renal failure and GI bleeding, is very important to determine the most appropriate pharmacological treatments.
The most frequently used pharmacological interventions for reducing joint pain are acetaminophen (paracetamol), nonsteroidal anti-inflammatory drugs (NSAIDs), (both nonselective and selective cyclooxygenase [COX]-2 inhibitors), and opioids [4,6]. NSAIDs are widely used because they are highly effective in controlling OA pain and are appropriate for individuals without comorbidities [6,7]. NSAIDs act through their ability to block COX, completely or partially inhibiting prostaglandin production; this is the main pathogenesis of NSAID-induced gastric damage [8]. A 2011 comparative effectiveness review reconfirmed that NSAIDs are associated with higher risks of serious GI, CV, and renal failure than is placebo [9]. In the search for another option for pain control and protection of cartilage damage, many dietary supplements (DS) have been developed for patients with OA, especially for aged patients with comorbidities. These DS have been considered as alternative treatments for OA by many patients and even medical supervisors. A meta-analysis of clinical trials indicated that some OA supplements are termed structure/disease-modifying OA drugs [7,10,11].
D-002 is a new natural active ingredient, comprised of a mixture of aliphatic saturated fatty alcohols, with antioxidant and antiulcer effects. It is isolated and purified from beeswax [12]. D-002 has been shown to be effective in experimental OA models of acute and chronic inflammation [13]. Puente et al. [14] investigated the effect of D-002 on OA symptoms. Their randomized, double-blind, placebo-controlled study showed significant improvements in the pain, stiffness, physical function, and total WOMAC scores after treatment with D-002 (50 to 100 mg/day for 6 weeks). The authors emphasized the finding that D-002, which is devoid of gastrotoxic effects, may be useful in managing OA pain and discomfort. Moreover, 6 weeks of treatment with D-002 was well tolerated and had a good safety profile. Considering the fact that the adverse effects of NSAIDs frequently include GI discomfort, such as that induced by peptic ulcers and gastritis, it is surprising that only one case of diarrhea has been reported in association with D-002 [14]. This result is in line with the fact that some OA supplements with good pain modulation, good anti-inflammatory effects, and safe GI profiles have shown benefits in both experimental models and patients with OA. If a further large clinical study with uniformly characterized inclusion criteria and assessment tools could be performed, D-002 may be shown to be an important part of the treatment regimen for patients with OA and comorbidities.
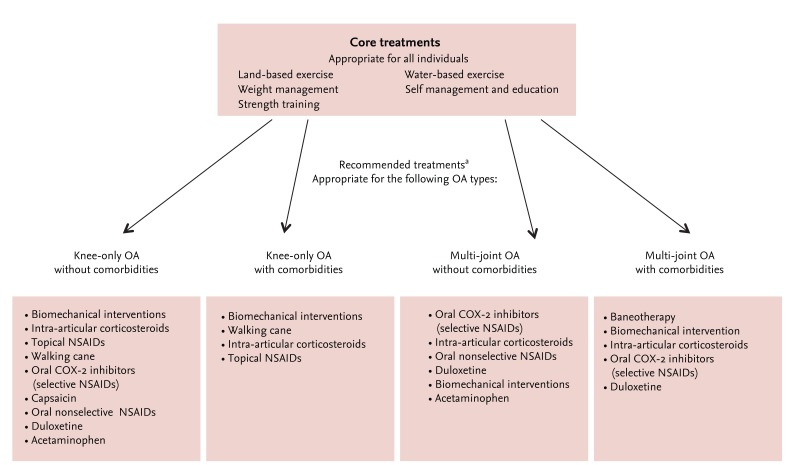
A number of OA management guidelines exist, and some recommendations are conflicting. The 2013 OARSI guidelines contain many treatment modalities not addressed by the American College of Rheumatology guidelines, such as avocado, soybean unsaponifiables, risedronate, diacerein, and rosehip [7]. According to the quality assessment results of a meta-analysis of randomized controlled trials, these OA supplements have shown evidence of reducing moderate to severe pain in certain groups of patients with OA [7,11]. DS are natural biological compounds that are traditionally known as harmless and slightly beneficial for many types of discomfort. Numerous DS preparations for OA are currently available. The most popular OA supplements in the United States are fish oil and glucosamine. In most parts of Asia, Africa, and Latin America, DS are also available in varying potencies and qualities. Most results from published randomized controlled trials and meta-analyses of OA supplements have mixed with pools from retail-based, online, and direct-sell companies [5,11]. To obtain the best available evidence, the most appropriate publications should be selected by experts. All of these variations have impacted the assessment of DS for OA, as reflected in the current 2013 OARSI recommendations for knee OA (Fig. 1) [7,11]. Although the long-term use of OA supplements is generally safe, routine use is not recommended for the treatment of OA. To obtain appropriate and reliable evidence for the establishment of OA recommendations, OA supplements should be evaluated based on uniformity and general agreement among outcome measures in experimental and clinical trials.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





