INTRODUCTION
Chronic urticaria (CU) is a common skin disorder, defined by recurrent wheals and pruritus, with or without angioedema for at least 6 weeks [
1]. CU accounts for 25% of urticaria, and the prevalence of CU is 0.1% to 0.6% [
2]. In acute urticaria (AU) possible eliciting factors, such as drug or food, are often identified by detailed patient history taking. The course of AU is self-limited, by avoiding the offending factors, and is characterized by rapid responses to symptomatic treatment. However, about 80% patients with CU, the underlying cause is not identified and its pathogenesis is not clearly established [
1,
3]. These patients whose urticaria occurs without obvious extrinsic stimuli are diagnosed with chronic spontaneous urticaria (CSU).
Urticaria is a mast cell-driven disease and histamine is considered to be the key mediator. Degranulation of skin mast cells by heterogeneous activating signals results in sensory nerve activation, vasodilation, plasma extravasation, and cell recruitment to urticarial lesions [
3]. Many studies have shown that about 50% of CU is related to autoimmunity [
2,
3]. Elevated histamine releasability from skin mast cells and skin infiltration of blood basophils have also been noted in the pathogenesis of CU [
3].
Health-related quality of life (HR-QOL) in CU can be impaired substantially due to its unpredictable symptoms and long-term nature. In a previous study, the impairment of QOL in CU was comparable with that of patients with coronary artery disease awaiting coronary artery bypass grafting [
4]. Additionally, recent studies have shown psychological stress and emotional disturbances to be common in patients with CU [
5-
7]. Ozkan et al. [
8] showed that 60% of CU patients had a psychiatric diagnosis and their QOL was reduced.
In most cases, the evaluation of CU has focused on clinical symptoms; however, understanding and dealing with QOL in CU patients is also necessary to improve patient management, address economic costs, and further clinical research. Recent guidelines recommended the use of the urticaria activity score (UAS) as a tool for assessing urticaria disease activity, and the CU-QOL questionnaire [
9], which was the first measurement device developed in Italy to assess disease-specific QOL in patients with CU [
2]. However, HR-QOL generally depends on the disease entity and social environment in which patients live. We developed a CU-specific QOL (CU-QOL) questionnaire as a validated tool, which considers Korean culture and language [
10].
This study was aimed at evaluating QOL impairment in CSU patients using our own CU-QOL questionnaire and the correlation between UAS and CU-QOL. Additionally, we sought to investigate possible predictors of impaired CU-QOL.
DISCUSSION
Recently, concerns about the deterioration of QOL of patients with CU have been heightened. In addition to clinical symptoms, like pruritus, wheals, and angioedema, many other factors, including the unexpectedness of symptom occurrence, sleep disturbances, and changes in appearance, are major problems for patients with CU. Thus, we need a more integrated approach to understand the whole influence of CU on patients. In the same context, recent guidelines and many health authorities have recommended using CU-QOL measurements and other patient-reported outcomes, like the urticaria control test [
12], as major parameters in clinical practice and research [
2,
13].
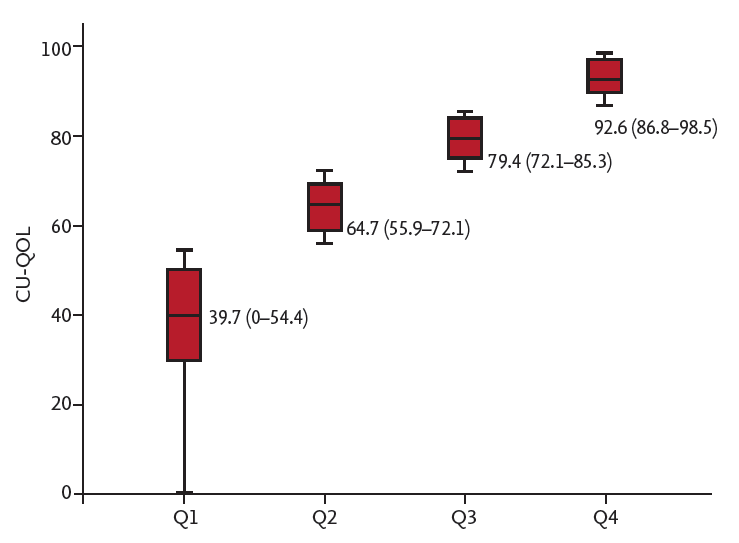
The present study demonstrated substantial impairment in disease-specific QOL among patients with CSU. When patients were stratified into four quartiles, based on total CU-QOL scores, each of the four different dimensions also varied significantly. The US domain, which reflects on physical symptoms, was the most impacted domain. However, significant reductions in the ED and FE domains also suggest that CSU patients do not suffer only from physical symptoms but also from impairment in their emotional well-being and daily or social activities. Thus, understanding QOL in CSU patients is important to improve patient management.
In a previous study validating the CU-QOL in Korean patients, patients with CU as well as with physically inducible urticaria were enrolled. The main result from that study was that UAS, dermographism, and emotional stress were important predictors of CU-QOL in Korean CU patients [
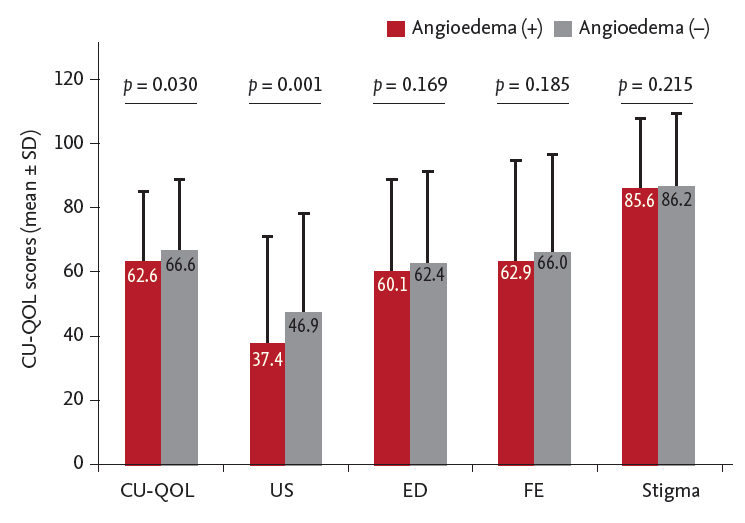
10]. The present study focused on measuring CU-QOL and exploring predictors of QOL impairment in patients with CSU. In addition to UAS-15, the presence of angioedema and log (total IgE) were also identified as significant predictors of impaired CU-QOL.
Most studies have reported that about 30% to 50% of CU patients have angioedema [
10,
14,
15]. Toubi et al. [
15] reported that more patients with accompanying angioedema were still suffering from urticaria at up to 5 years of follow-up compared with patients who exhibited only wheals (45% vs. 12%). We found a significant association between co-existing angioedema and poor scores in the US domain of CU-QOL and both UAS-15 and UAS-6. Although neither UAS-15 nor UAS-6, nor CU-QOL, consider angioedema sufficiently, more prominent disease activity and a significant difference in a specific QOL domain were noted in patients having angioedema, indicating that CU-QOL was a more reliable measurement for Korean patients with CU. However, additional impairment in QOL in patients with CSU presenting with angioedema was not evident in a prior study using a different QOL measurement [
16]. Thus, specific instruments to measure angioedema activity and angioedema-related QOL impairment in CU patients with recurrent angioedema with or without wheals have been proposed [
17,
18].
Omalizumab (anti-IgE) is currently recommended for CU patients resistant to antihistamines, based on demonstrated efficacy and safety in recent clinical trials [
19-
21]. The importance of serum IgE levels in CU patients is increasingly recognized due to the clinical benefit of omalizumab, although it was effective in patients with CU regardless of IgE levels. Kessel et al. [
22] reported that total serum IgE levels are frequently elevated in CU patients and these are significantly related to disease severity and duration. Increasing evidence suggests that autoreactivity including not only IgG autoantibodies against IgE and/or FcεRI but also IgE antibodies to endogenous antigens, in patients with CSU plays an important role [
23]. The IgE-occupied mast cells become more sensitive due to a decreased threshold for degranulation as well as those cells can store more mediators as compared with mast cells without IgE engagement [
23]. A significant correlation between total IgE levels and urticaria disease activity was shown in the present study. However, log (total IgE) showed a significant correlation with UAS-15, but not with UAS-6. Log (total IgE) was associated significantly with the distribution range and mean diameter of wheals in CU patients, whereas we found no significant correlation between total IgE levels and the numbers of wheals and the intensity of pruritus. UAS-6 does not consider other factors of urticaria disease activity, except wheal numbers and pruritus intensity. A sequential assessment of wheal numbers and pruritus every day for a week (UAS-7) has been adopted in recent guidelines and clinical research [
2,
19]. Despite a wider range (0 to 42) of scores in UAS-7 compared with UAS-6 (0 to 6), both UAS-7 and UAS-6 are limited in the evaluation of wheal numbers and pruritus. However, our results suggest that a more comprehensive assessment of the disease activity is appropriate for understanding the clinical characteristics of CU and for identifying major drivers of QOL impairment in CSU patients. We also found that log-transformed serum total IgE levels in patients with CSU correlated significantly with total CU-QOL scores and the ED domain. This relationship may be explained by the effect of IgE antibodies on the extent of mast cell activation and degranulation [
19,
22]. Checking the serum total IgE levels can be useful to predict urticaria severity and CU-QOL in patients with CSU.
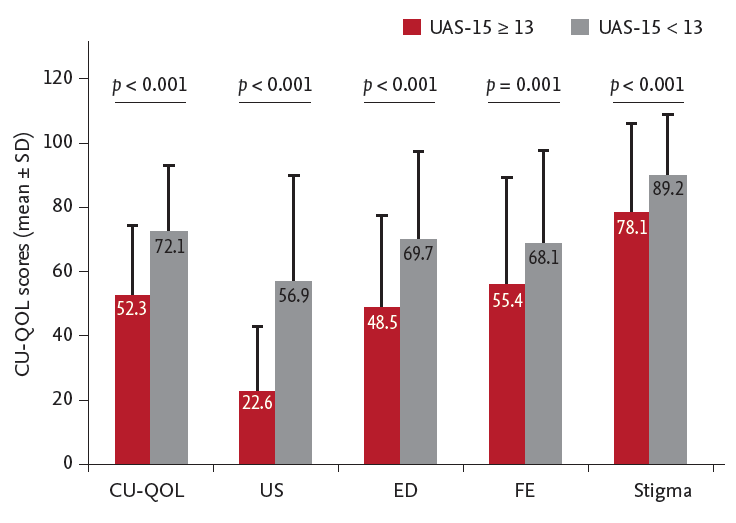
Based on previous studies, the UAS, which combines wheal numbers and pruritus intensity, correlated significantly with QOL impairment, as measured using the Dermatology Life Quality Index in CU patients [
24]. The present study showed the total CU-QOL scores and all individual domains correlated significantly with the UAS, and particularly well with the UAS-15, including duration, size, and distribution of wheals, when compared with the UAS-6. Of the five categories of UAS-15, UAS-2, which scores the distribution range of wheals, showed the strongest correlation with CU-QOL, but it is not included in UAS-6. Although the assessment of UAS-15 requires more time than UAS-6, UAS-15 is a more detailed and enhanced tool, and our results support that it is more suitable for the evaluation of impairment of CU-QOL.
We defined ‘severe’ CU as a UAS-15 score of 13 or more, as used previously [
11]. A multivariate logistic regression demonstrated that severe CU was a significant and independent predictor of impaired CU-QOL, and it also correlated significantly with each domain factor. Regarding more severe CU leading to more frequently uncontrolled CU [
11] and to a higher socio-economic burden [
25], careful consideration of how serious QOL impairment is caused by CU is needed, particularly in patients with severe CU. We attempted to identify predictors of impaired CU-QOL in CSU patients. There have been many studies investigating QOL impairment in patients with CSU, but clinical interpretation according to QOL score is rare. A multivariate analysis showed that severe CU, log (total IgE), and the presence of angioedema are significant predictors of impaired CU-QOL in Korean adults with CU.
In conclusion, physicians need to pay more attention to CU-QOL impairment in CSU patients for a better understanding of the disease burden in individual patients. Additionally, the assessment of patients in more comprehensive ways, including UAS-15, total IgE, and the presence of angioedema, to identify major determinants of CU-QOL is necessary.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


