INTRODUCTION
The incidence and mortality of colorectal cancer has gradually increased in Korea. According to recent cancer statistics in Korea, colorectal cancer is the second most common and fourth leading cause of cancer-related deaths and places a significant burden on the public health system [1,2]. In nonmetastatic colon cancer, curative surgical resection is the mainstay of treatment, and adjuvant chemotherapy may be considered according to the pathologic stage after surgery [3]. Recent guidelines on surveillance colonoscopy after colorectal cancer resection recommend that (1) patients with colorectal cancer should undergo high-quality perioperative clearing colonoscopy preoperatively (or within 6 months after colectomy if preoperative colonoscopy was not possible); (2) the first surveillance colonoscopy should be performed 1 year after surgery; and (3) if there was no significant lesion, the surveillance interval can be increased to 3 and 5 years (i.e., 1, 4, and 9 years after colectomy) [4].
In general, adenomatous polyps are precancerous lesions and should be removed. Post-polypectomy surveillance interval should be determined based on the patientŌĆÖs risk of metachronous neoplasm, and a shorter surveillance interval is recommended in high-risk group [5]. According to post-polypectomy surveillance guidelines, the following characteristics are associated with an increased risk of advanced metachronous neoplasms (i.e., high-risk neoplasms): (1) number of adenomas Ōēź 3; (2) size of adenomas Ōēź 10 mm; (3) adenomas with high-grade dysplasia; (4) adenomas with villous histology; or (5) serrated adenomas Ōēź 10 mm [6,7]. However, risk factors for advanced metachronous neoplasms during postoperative surveillance colonoscopy have not been fully evaluated. Thus, we aimed to evaluate risk factors for advanced metachronous neoplasms during postoperative surveillance colonoscopy.
METHODS
Study patients
Patients newly diagnosed with nonmetastatic colon cancer who underwent curative intent surgery between January 2002 and December 2012 in a single tertiary hospital were included in this study. We retrospectively reviewed the patientsŌĆÖ clinical data. Inclusion criteria were as follows: (1) patients Ōēź 18 years; (2) patients with biopsy-proven colon adenocarcinoma; and (3) patients who underwent curative colon resection. Exclusion criteria were as follows: (1) patients diagnosed with initially metastatic (stage 4) colon cancer; (2) patients diagnosed with rectal cancer (cancer below the rectosigmoid junction); (3) patients who underwent total colectomy; (4) patients who underwent R2 resection (gross remnant disease after colectomy); (5) patients who underwent neoadjuvant chemotherapy or radiotherapy; and (6) patients with missing or incomplete endoscopic surveillance data. This study was approved by Dankook University Hosiptal Institutional Review Board (IRB no. 2018-07-015). The requirement for written informed consent was waived due to the retrospective nature of this study.
Data collection
At diagnosis, patients underwent baseline workup, including clinical evaluation, laboratory tests, chest radiography, colonoscopy, and abdominopelvic and chest computed tomography (CT). Positron emission tomography-CT was selectively performed for suspected distant metastasis. PatientŌĆÖs age, sex, body mass index (BMI), presence of underlying diseases, smoking, and alcohol consumption were analyzed. The location of tumor was classified as proximal or distal (compared to the splenic flexure). Tumor differentiation was classified as differentiated (well-to-moderately differentiated adenocarcinoma) or undifferentiated (poorly differentiated adenocarcinoma).
Perioperative clearing colonoscopy was performed at the time of diagnosis (or within 6 months after colectomy in case of failed preoperative total colonoscopy due to significant obstruction). Endoscopists adhered to colonoscopy quality indicators, such as cecal intubation, withdrawal time Ōēź 6 minutes, and adequate bowel preparation. Synchronous neoplasms were removed by proper endoscopic resection methods. Patients were then classified into low-risk or high-risk group according to the characteristics of the synchronous neoplasms at diagnosis. The high-risk group had at least 1 or more features of high-risk neoplasms at baseline, based on previously mentioned characteristics (number of adenomas Ōēź 3, size of adenomas Ōēź 10 mm, adenomas with high-grade dysplasia, adenomas with villous histology, or serrated adenomas Ōēź 10 mm) [6,7].
After baseline workup, curative colectomy was performed according to the tumor location. It was classified as left-sided colectomy (including left hemicolectomy and anterior resection) and right-sided colectomy (including right hemicolectomy and transverse colon resection). TNM staging was assessed according to the final pathologic result.
After colectomy, abdominopelvic CT and colonoscopy were performed as routine surveillance at 1 year after surgery. Colon neoplasms detected Ōēź 1 year after perioperative clearing colonoscopy were defined as metachronous lesions [8-10]. If there was any advanced metachronous adenomas (adenomas Ōēź 10 mm, adenomas with high-grade dysplasia, or adenomas with villous histology) or metachronous cancer during surveillance, patients underwent proper endoscopic or surgical resection, and follow-up colonoscopy was performed 1 year after treatment. If there were no advanced metachronous neoplasms during surveillance, the follow-up interval was increased according to the previously mentioned guideline [4]. Follow-up data were collected and reviewed through December 2017.
Statistical analysis
Statistical analyses were performed using SPSS version 25.0 (IBM Co., Armonk, NY, USA). Categorical variables were presented as numbers and percentages and compared using the chi-square test or FisherŌĆÖs exact test. Continuous variables were presented as mean and standard deviation and compared using StudentŌĆÖs paired t test. Risk factors were evaluated by univariate and multivariate analyses and presented using odds ratio (OR) and 95% confidence interval (CI). Kaplan-Meier curve analysis was performed to evaluate the cumulative incidence of advanced metachronous neoplasms. A p value of < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
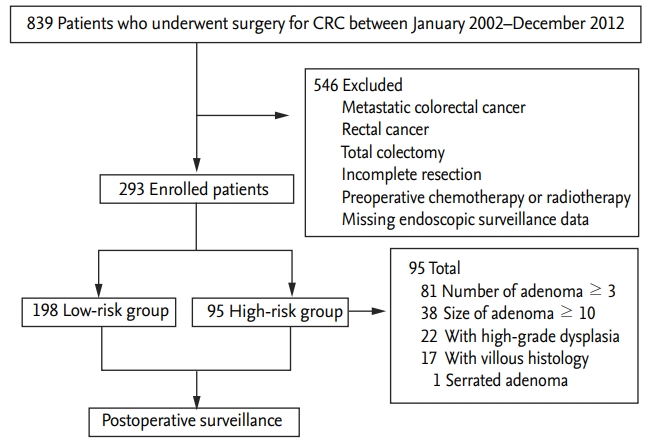
During the study period, 839 patients underwent surgery for newly diagnosed colorectal cancer. Among them, 546 were excluded and 293 were ultimately enrolled in this study (Fig. 1). There were 179 male patients (61.1%), and the male-to-female ratio was 1.6:1. The mean age was 63.2 ┬▒ 10.4 years (range, 34 to 89), and 155 patients (52.9%) were Ōēź 65 years old. The mean BMI was 24.2 ┬▒ 3.5 kg/m2 (range, 16.0 to 38.4), and 110 patients (37.5%) were obese (BMI Ōēź 25 kg/m2). Diabetes and hypertension were noted in 46 patients (15.7%) and 109 (37.2%), respectively. Current alcohol consumption and current smoking were noted in 82 patients (28.0%) and 71 (24.2%), respectively. According to tumor characteristics, distal colon cancer was more common (n = 184; 62.8%) than proximal colon cancer (n = 109; 37.2%), whereas advanced stage (stage 3, n = 108; 36.9%) and undifferentiated cancer (n = 38; 13.0%) were less common than stage 1ŌĆō2 (n = 185; 63.1%) and differentiated cancer (n = 255; 87.0%), respectively (Table 1).
Risk factors for high-risk adenoma at perioperative clearing colonoscopy
Most patients underwent preoperative clearing colonoscopy before surgery (n = 216; 70.3%) than after surgery (within 6 months after surgery; n = 87; 29.7%). Based on synchronous neoplasms at perioperative clearing colonoscopy, the patients were classified into low-risk (n = 198; 67.6%) and high-risk (n = 95; 32.4%) groups. The proportion of male patients (p < 0.001), those aged Ōēź 65 years (p = 0.029), those with current alcohol consumption (p = 0.004), and who were currently smoking (p = 0.001) was significantly higher in the high-risk group than in the low-risk group, whereas the proportion of patients with higher T stage (T3ŌĆō4) was significantly lower in the high-risk group than in the low-risk group (Supplementary Table 1). The most common reason in the high-risk group was multiple adenomas (number Ōēź 3; 85.3%), following large adenoma (size Ōēź 10 mm; 40.0%), high-grade dysplasia (23.2%), villous histology (17.9%), and large serrated adenoma (size Ōēź 10 mm; 1.1%) (Fig. 1). The proportion of patients who were male (p = 0.009), who were obese (BMI Ōēź 25 kg/m2; p = 0.026), who had current alcohol consumption (p = 0.043), and who were currently smoking (p = 0.037) was significantly higher in the distal cancer group than in the proximal cancer group, whereas the proportion of patients with undifferentiated tumors was significantly lower in the distal cancer group than in the proximal cancer group (p < 0.001) (Supplementary Table 2).
Risk factors for advanced metachronous neoplasms during surveillance colonoscopy
During the follow-up period (mean 74.4 ┬▒ 36.4 months; range, 12.3 to 185.1), a median of 4 surveillance colonoscopies (interquartile range, 2 to 5) were performed. There was no significant difference in the number of surveillance colonoscopies between the high-risk and low-risk groups (p = 0.268). Metachronous adenomas were found in 187 patients (63.8%), including patients with adenomas Ōēź 3 adenomas (n = 29), size of adenomas Ōēź 10 mm (n = 32), adenomas with high-grade dysplasia (n = 9), and adenomas with villous histology (n = 10). Advanced metachronous neoplasms were found in 45 patients (15.4%), including metachronous cancer in four patients (1.4%). There was no significant difference in the number of surveillance colonoscopies between patients with and without advanced metachronous neoplasms (p = 0.170). Among four patients with metachronous cancer, three patients (75%) were associated with synchronous high-risk neoplasms, distal colon cancer, and lymph node metastasis (stage 3) at baseline. The median interval between initial curative surgery and diagnosis of metachronous cancer was 36.4 months (range, 13.5 to 53.0). All these patients underwent additional colectomy for metachronous cancer.
In univariate analysis to determine relevant risk factors, obesity (BMI Ōēź 25 kg/m2; OR, 1.935; 95% CI, 1.020 to 3.669; p = 0.043), hypertension (OR, 2.192; 95% CI, 1.154 to 4.163; p = 0.017), current alcohol consumption (OR, 2.400; 95% CI, 1.247 to 4.618; p = 0.009), distal colon cancer (OR, 4.617; 95% CI, 1.885 to 11.309; p = 0.001), and synchronous high-risk adenomas (OR, 3.550; 95% CI, 1.847 to 6.824; p < 0.001) were significant risk factors for advanced metachronous neoplasms. In multivariate analysis, distal colon cancer (OR, 4.402; 95% CI, 1.658 to 11.689; p = 0.003), synchronous high-risk adenoma (OR, 3.225; 95% CI, 1.503 to 6.918; p = 0.003), and hypertension (OR, 2.270; 95% CI, 1.058 to 4.874; p = 0.035), were significant risk factors for advanced metachronous neoplasms during surveillance colonoscopy (Table 2).
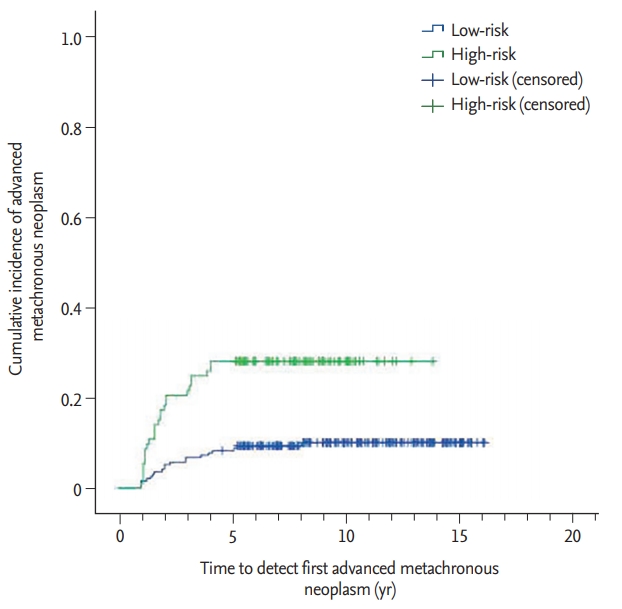
During the follow-up period, the incidence of advanced metachronous neoplasms was significantly higher in the high-risk group (those with synchronous high-risk adenomas at baseline) than the low-risk group (those without synchronous high-risk adenomas at baseline) (27.4% vs. 9.6%, p < 0.001). Fig. 2 shows the cumulative incidences of advanced metachronous neoplasms in the high-risk and low-risk groups.
DISCUSSION
In this present study, we analyzed risk factors for advanced metachronous neoplasms during surveillance colonoscopy after non-metastatic colon cancer resection. In multivariate analysis, distal colon cancer, synchronous high-risk adenomas, and hypertension were significant risk factors for advanced metachronous neoplasms. The cumulative incidence of advanced metachronous neoplasms was significantly higher in the high-risk group than in the low-risk group (27.4% vs. 9.6%, p < 0.001).
Patients who underwent colon cancer resection were at a high risk of metachronous neoplasms. In previous studies, the definition of metachronous neoplasms varies from 6 months to 3 years after colectomy. We defined metachronous neoplasms as any adenoma or carcinoma detected Ōēź 1 year after colectomy, as was done in previous studies [8-10]. In this present study, we performed high-quality perioperative clearing colonoscopy, and most patients underwent Ōēź 2 colonoscopies at 1 year after colectomy. Thus, the possibility of missing lesions was relatively low in our patients. In this present study, the incidence of metachronous adenomas (63.8%) is higher than that in previous reports (25% to 48%) [9-11]. One possible explanation is the relatively longer follow-up period (mean 74 months) and large number of surveillance colonoscopies (median, 4) than those in previous studies [11-13]. The incidence of metachronous cancer (1.3%) is comparable in previous reports (0.6% to 9.0%) [10,11]. At diagnosis, male sex, old age (Ōēź 65 years), current alcohol consumption, and current smoking were more common in the high-risk group (Supplementary Table 1). Some previous studies have suggested male sex, old age, and diabetes were risk factors of metachronous neoplasms after colectomy or polypectomy [11,13-15]. However, in this present study, there was no significant association between these factors and advanced metachronous neoplasms in multivariate analysis, although obesity and current alcohol consumption were significant factors of advanced metachronous neoplasms in univariate analysis. In addition, patients with advanced T stage (T3ŌĆō4) were more common in the low-risk group than in the high-risk group (Supplementary Table 1). However, previous studies reported no significant association between the stage of cancer and synchronous neoplasms [8,16]. Further studies including a large number of patients are needed to evaluate the relationship between the stage of cancer and synchronous neoplasms.
Tumor location is considered an important risk factor for metachronous neoplasms. In previous studies, distal colon cancer (left-sided colon cancer) was associated with a higher risk of metachronous neoplasm than proximal colon cancer (right-sided colon cancer) [12,13,17,18]. One possible explanation for this association is that interval colon neoplasm is more common in the proximal colon than in the distal colon. Adenomas in the proximal colon are generally difficult to detect and remove compared to those in the distal colon [5]. In addition, serrated adenomas are more common in the proximal colon than in the distal colon and show alternative carcinogenesis pathway (microsatellite instability) compared to the sporadic adenoma-to-carcinoma sequence. This result is not consistent with those of other studies where proximal colon cancer was reported as a risk factor for metachronous neoplasms [11,19,20]. However, most previous studies included rectal cancer, which has a different tumor nature and treatment option (e.g., preoperative chemotherapy or radiation therapy) compared to colon cancer [4]. Considering this, we excluded patients with rectal cancer and only patients with colon cancer above the rectosigmoid junction were included in the present study. Even after excluding patients with rectal cancer, distal colon cancer remains an independent risk factor for metachronous colon neoplasms, which was consistent with findings in previous studies [12,13,17,21].
In the present study, synchronous high-risk adenomas were identified as a significant risk factor for advanced metachronous neoplasms, which is consistent with those in previous studies. Choe et al [13]. reported that male sex, age > 65 years, distal colon cancer (left-sided index cancer), and synchronous high-risk adenomas (being in the high-risk group) were significant risk factors for advanced metachronous neoplasms. Lee et al. [11] reported that old age (Ōēź 60 years), synchronous adenoma, and diabetes were risk factors for metachronous neoplasms after colon cancer resection, but not in advanced metachronous neoplasms. In post-polypectomy surveillance, multiple high-risk findings might suggest a higher risk of advanced neoplasms [6,22]; however, these findings have not been fully evaluated in post-colectomy surveillance.
According to the present study, hypertension was a significant risk factor for advanced metachronous neoplasms. Yun et al. [12] reported that male sex, obesity, distal colon cancer, and hypertension were risk factors for metachronous adenomas after colectomy. Lin et al. [23] reported that hypertension combined with smoking, elevated liver function test, or multiple adenomas were risk factors of metachronous colorectal adenomas after polypectomy. Generally, metabolic syndrome, including hypertension, obesity, diabetes, and dyslipidemia, is associated with colon polyp and metachronous polyp recurrence after polypectomy [24,25]. However, obesity and diabetes were not identified as significant risk factors in this present study. Although hypertension was the only lifestyle risk factor in this present study, there was a significant association between hypertension and obesity (p = 0.006). We believe some unevaluated lifestyle factors associated with metabolic syndrome (e.g., fatty food, salty food, sedentary or westernized lifestyle) may elevate the risk of metachronous adenoma in this study. Further studies are needed to evaluate the relationship between hypertension and metachronous neoplasm.
The cumulative incidence of advanced metachronous neoplasm was significantly higher in the highrisk group (Fig. 2). During the follow-up period within 5 years, the incidence of advanced metachronous adenomas increased steeply in the high-risk group compared to that in the low-risk group. However, after 5 years, the difference between the groups was maintained. This result suggests the importance of postoperative surveillance colonoscopy, particularly in the early postoperative period. In addition, to improve the overall outcome, patients in the high-risk group should undergo more intensive postoperative surveillance colonoscopy than those to the low-risk group.
This study has some limitations. First, the retrospective nature of this study cannot eliminate selection bias. Second, the relatively long study period may have caused the difference in polyp detection and resection between the early and late study period. Third, although most patients underwent colonoscopy according to the post-colectomy surveillance guideline, the follow-up interval was irregular in some patients according to the patientsŌĆÖ medical condition. In addition, although all patients underwent at least two surveillance colonoscopies, only one-fourth of them underwent more than three colonoscopies.
In conclusion, during post-colectomy surveillance, distal colon cancer, synchronous high-risk adenomas, and hypertension are significant risk factors for advanced metachronous neoplasms. Patients with these risk factors may need more intensive surveillance colonoscopy, and a tailored surveillance strategy may improve the overall outcome in patients who undergo curative colon cancer resection.
KEY MESSAGE
1. Distal colon cancer, synchronous high-risk adenomas, and hypertension are significant risk factors of advanced metachronous neoplasm during post-colectomy surveillance.
2. Patients with these risk factors may need more intensive surveillance colonoscopy, and this tailored surveillance strategy may improve the overall outcome in patients who undergo curative colon cancer resection.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print





