 |
 |
|
|
|
See the letter "The causal association between sarcopenia and colorectal cancer: a Mendelian Randomization analysis" on page 266.
Abstract
Background/Aims
Although several studies have shown that sarcopenia is associated with poor outcomes in colorectal cancer patients, the impact of sarcopenia on the development of colorectal neoplasia remains unclear. We aimed to evaluate the prevalence and association of colorectal neoplasia, especially advanced colorectal neoplasia, in adults with sarcopenia.
Methods
We retrospectively analyzed the data for 10,676 adults who underwent first-time colonoscopy and bioelectrical impedance analysis (BIA) on the same day in a health screening program at a single center. Sarcopenia was diagnosed using established BIA-based criteria as adjusted appendicular skeletal muscle mass (ASM) divided by body mass index (BMI) (ASM/BMI), height (ASM/height2), or weight (ASM/weight). Prevalence of overall and advanced colorectal neoplasia and their association with sarcopenia, as established by the aforementioned diagnostic criteria, were evaluated.
Results
Among 10,676 subjects, 583 were diagnosed with sarcopenia using ASM/BMI. Subjects with sarcopenia had a higher prevalence of colorectal neoplasia than those without. In the multivariate analysis after adjusting for confounding factors, sarcopenia was an independent risk factor for any colorectal neoplasia (odds ratio [OR], 1.31; 95% confidence interval [CI], 1.09 to 1.56) and advanced colorectal neoplasia (OR, 1.97; 95% CI, 1.27 to 3.06). The association between sarcopenia and advanced colorectal neoplasia remained significant for all sarcopenia measures including ASM/height2 (OR, 2.19; 95% CI, 1.24 to 3.85) and ASM/weight (OR, 2.41; 95% CI, 1.54 to 3.77).
Colorectal cancer (CRC) is the fourth most commonly diagnosed malignancy and the second leading cause of cancer-related death worldwide [1]. The global burden of CRC has been increasing rapidly due to population growth, changes in demographics, and transition to more westernized lifestyles [2,3]. Since the majority of CRC cases develop from colorectal adenoma, the early detection and removal of colorectal adenoma has been considered the most effective strategy for reducing CRC incidence and related mortality [4]. In addition to genetic factors, the development of colorectal neoplasia is reportedly associated with environmental factors including physical activity, dietary habits, smoking, and alcoholic consumption [5,6]. Moreover, factors associated with the development of metabolic syndrome including obesity, dyslipidemia, impaired glucose tolerance, and insulin resistance have been reported as potential risk factors for colorectal neoplasia [7ŌĆō10].
Recent studies have reported that sarcopenia, which is the loss of skeletal muscle mass and strength, may be one of the risk factors for poor outcomes in several chronic disorders [11ŌĆō16]. This could be related to a prolonged catabolic state associated with immune dysfunction and systemic inflammatory response in the host [11,17,18]. Several studies have also reported that sarcopenia is associated with metabolic syndrome, diabetes mellitus (DM), and non-alcoholic fatty liver disease, which are risk factors for colorectal neoplasia [14ŌĆō16]. Although this suggests that colorectal neoplasia and sarcopenia may have similar pathophysiological mechanisms, studies on the association between the risk of colorectal neoplasia and sarcopenia are still lacking.
Various tools, with different criteria even for the same tool, are utilized for measuring muscle mass in studies. Among these, the bioelectrical impedance analysis (BIA), using a non-invasive body composition analyzer, is a convenient and cost-effective tool for the evaluation of lean body mass. Recent studies have also reported its use for the assessment of skeletal muscle mass and a conclusive diagnosis of sarcopenia [19,20]. We aimed to investigate the association between sarcopenia, as indicated by three currently accepted diagnostic criteria, and the risk of colorectal neoplasia, especially advanced neoplasia, in a large sample of asymptomatic, average-risk population.
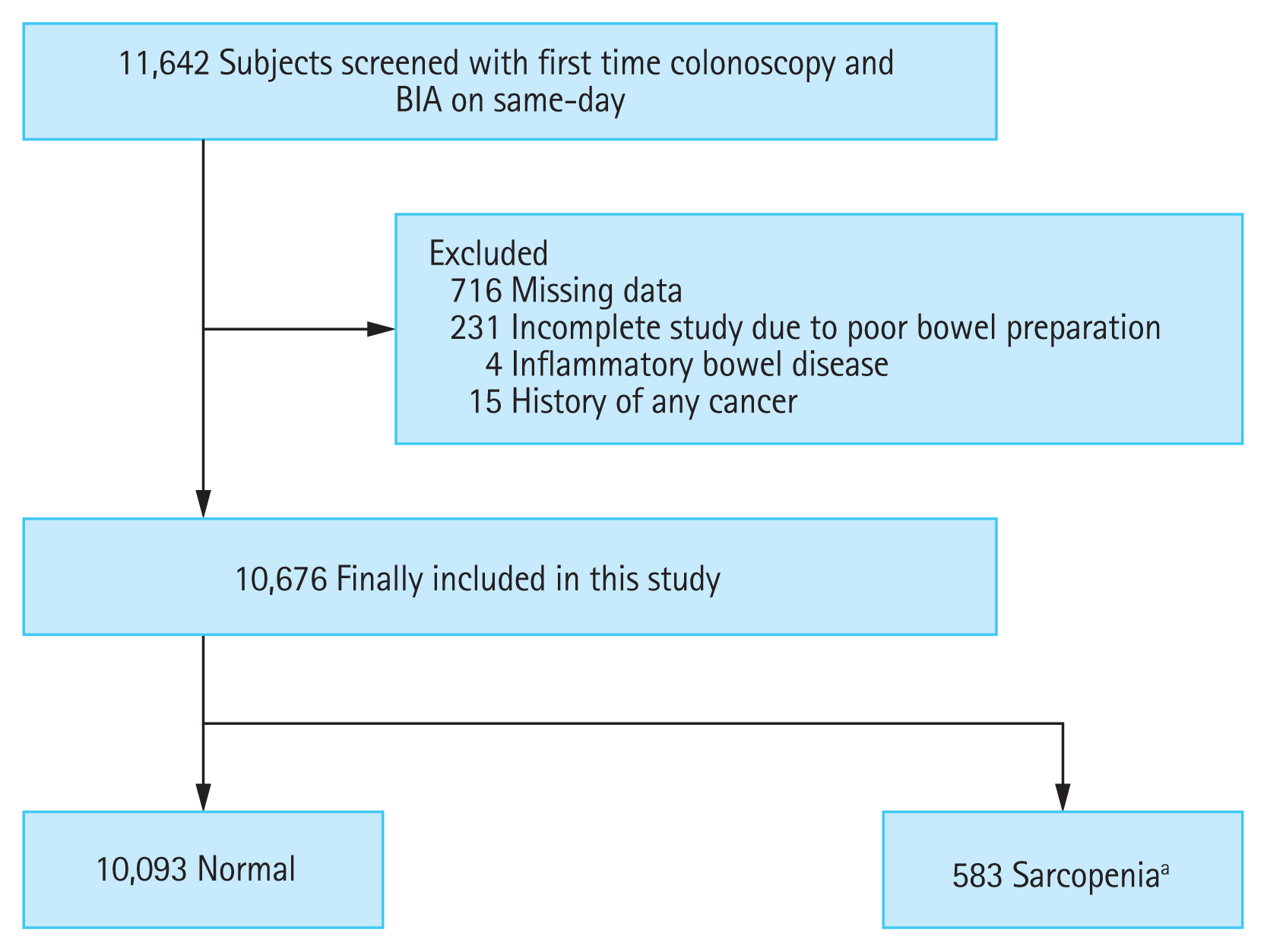
We reviewed the clinical data of 11,642 adults (Ōēź 18 years) who underwent their first colonoscopy screening and BIA on the same day during a health screening program at Yeungnam University Medical Center from June 2009 to June 2017. Subjects were excluded if they met any of the following criteria: missing data (n = 716); incomplete colonoscopy due to poor bowel preparation (n = 231); inflammatory bowel disease such as CrohnŌĆÖs disease and ulcerative colitis (n = 4); and previous history of any type of cancer (n = 15). Finally, 10,676 subjects were included in this analysis and divided into two groups according to the presence of sarcopenia, based on three diagnostic criteria (Fig. 1). We first investigated the difference in the prevalence of colorectal neoplasia stratified according to the presence of sarcopenia. Next, we evaluated the association between sarcopenia and the risk of colorectal neoplasia after adjusting for potential confounding factors. Furthermore, our analysis included the risk of advanced colorectal neoplasia and multiple colorectal neoplasia. This study was approved by the Institutional Review Board of Yeungnam University Medical Center, and the requirement of consent was waived due to its retrospective study design (YUMC-2020-08-021).
During the health screening program, the height, weight, waist circumference, and blood pressure (BP) of the subjects were measured by trained nurses. Data on smoking status, alcohol consumption, personal medical history, and medication use were collected using a self-administered questionnaire. The subjectŌĆÖs smoking status was categorized as never, former, or current smoker. Alcohol consumption was calculated using the number of drinks consumed multiplied by the frequency, and Ōēź 210 g/week for men and Ōēź 140 g/week for women were defined as excessive drinking.
A blood sample was collected in the morning after at least 10 hours of fasting. This was analyzed by trained staff in the hospitalŌĆÖs clinical laboratory for parameters including complete blood count, blood chemistry, glucose level, and lipid profile. Body mass index (BMI) was calculated by dividing the weight (kg) by the height squared (m2), and obesity was defined as a BMI Ōēź 25 kg/m2, according to the criteria for the Asia-Pacific region. Visceral obesity was defined based on waist circumference (Ōēź 90 cm in men and Ōēź 80 cm in women based on ethnicity-specific values). Hypertension was defined as systolic BP Ōēź 140 mmHg or diastolic BP Ōēź 90 mmHg or a history of disease/medication use. DM was defined as a fasting glucose level Ōēź 126 mg/dL or glycated hemoglobin level Ōēź 6.5% or a history of disease/medication use. Hyperlipidemia was defined as a serum triglyceride level Ōēź 150 mg/dL or serum cholesterol level Ōēź 200 mg/dL or a history of disease/medication use. Metabolic syndrome was defined in accordance with the International Diabetes Federation criteria as central (visceral) obesity plus any two of the following four factors: increased triglyceride level (Ōēź 150 mg/dL) or specific treatment for this lipid abnormality; reduced high-density lipoprotein cholesterol level (< 40 mg/dL in men and < 50 mg/dL in women) or receiving specific treatment for this lipid abnormality; systolic BP Ōēź 130 mmHg or diastolic BP Ōēź 85 mmHg or treatment of previously diagnosed hypertension; and an increased fasting plasma glucose level (Ōēź 100 mg/dL) or a previous diagnosis of type 2 DM.
Colonoscopies were performed by five faculties from the gastroenterology department in Yeungnam University Medical Center, each with at least 5 years of experience after board certification for gastrointestinal endoscopy. This procedure was done using a conventional colonoscope (CV-160 Evis EXTRA and CV-180 Evis EXTRA II, Olympus, Tokyo, Japan) after bowel preparation. All specimens of colorectal neoplasia were histologically assessed by two experienced board-certified pathologists. We investigated the following in the medical record: (1) complete colonoscopy examination; (2) status of bowel preparation; (3) size, location, and number of colorectal neoplasia; and (4) histopathology of colorectal neoplasia. Overall colorectal neoplasia was defined as cancer or any adenoma (tubular, tubulovillous, villous, and serrated adenoma), and advanced colorectal neoplasia was defined as cancer or advanced adenoma. Advanced adenoma was defined as an adenoma with a diameter Ōēź 10 mm, high grade dysplasia, or > 25% villous component. Multiple colorectal neoplasia was defined as the presence of more than three colorectal neoplasia.
BIA was performed to measure the appendicular skeletal muscle mass (ASM) using Inbody 720 (Biospace, Seoul, Korea), a non-invasive body composition analyzer, by well-trained staff on the same day as the colonoscopy procedure. ASM was calculated as the sum of lean muscle mass in the bilateral upper and lower extremities. In this study, sarcopenia was defined using the following three formulas, all of which have been previously reported as validated measures of sarcopenia. (1) BMI-adjusted ASM was defined as ASM divided by BMI (ASM/BMI); as suggested by the Foundation for the National Institutes of Health, sarcopenia was defined in terms of ASM/BMI using cutoffs of < 0.789 for men and < 0.512 for women [21]. (2) Height-adjusted ASM was defined as ASM divided by height in meters squared (ASM/height2, kg/m2); as suggested by the Consensus Report of the Asian Working Group for Sarcopenia, sarcopenia was defined in terms of ASM/height2 using cutoffs of < 6.75 kg/m2 for men and < 5.07 kg/m2 for women, which are more than two standard deviations for sex-specific values in young healthy adults [22]. (3) Weight-adjusted ASM was defined as ASM divided by body weight (ASM/weight, %); sarcopenia was defined in terms of ASM/weight with cutoffs of < 29.1% for men and < 23.0% for women, which are more than two standard deviations for sex-specific values in young healthy adults, according to the national health examination in Korea [23].
Continuous and categorical variables were expressed as mean ┬▒ standard deviation and number (%), respectively. The characteristics of the study subjects were analyzed based on sarcopenia status using the StudentŌĆÖs t test for continuous variables and the chi-square test for categorical variables. The association between sarcopenia and colorectal neoplasia was assessed using logistic regression analysis. Three models with increasing levels of adjustment to account for potential confounding factors were used to determine risk factors for any colorectal neoplasm, advanced neoplasm, and multiple polyps. Model 1 was adjusted for age and sex. Model 2 was further adjusted for current smoking status, excessive alcohol intake, obesity, and visceral obesity. Model 3 was additionally adjusted for hypertension, DM, dyslipidemia, and metabolic syndrome. The odds ratio was considered to be statistically significant if the 95% confidence interval did not include 1.0. Statistical analysis was performed using SPSS version 25.0 (IBM Co., Armonk, NY, USA), and the level of statistical significance was set at p < 0.05.
Of the 10,676 subjects enrolled in this study, 583 (5.5%), 297 (2.8%) and 685 (6.4%) subjects had sarcopenia defined by the BMI-adjusted, height-adjusted, and weight-adjusted ASM models, respectively. Of these subjects with sarcopenia, 35 subjects met all three BIA-based criteria, 358 subjects met two of three BIA-based criteria, and 744 subjects met only one BIA-based criterion. The baseline characteristics of the subjects according to sarcopenia defined using the three BIA-based diagnostic criteria are shown in Table 1. Subjects with sarcopenia defined using the BMI-adjusted ASM model were older (56.3 ┬▒ 10.8 vs. 48.7 ┬▒ 10.3, p < 0.001), predominantly male (79.2% vs. 62.0%, p < 0.001), and had a higher BMI (26.9 ┬▒ 3.6 vs. 24.0 ┬▒ 2.9, p < 0.001) and waist circumference (92.1 ┬▒ 9.9 vs. 85.6 ┬▒ 8.4, p < 0.001) than those without sarcopenia. The prevalence of hypertension (46.7% vs. 23.9%, p < 0.001), DM (18.4% vs. 4.7%, p < 0.001), dyslipidemia (68.3% vs. 60.4%, p < 0.001), and metabolic syndrome (35.7% vs. 18.5%, p < 0.001) was higher in subjects with sarcopenia defined using the BMI-adjusted ASM model than in those without sarcopenia. Subjects with sarcopenia defined using weight adjusted ASM model had a similar tendency. However, BMI and waist circumference values and the prevalence of metabolic syndrome were lower in subjects with sarcopenia defined using the height-adjusted ASM model than in those without sarcopenia.
During the study period, 3,054 (28.6%) subjects were found to have colorectal neoplasia during their first-time colonoscopy. The prevalence of colorectal neoplasia according to sarcopenia based on different diagnostic criteria are shown in Table 2. Subjects with sarcopenia, irrespective of diagnostic criteria, had a significantly higher prevalence of any colorectal neoplasia than the subjects without sarcopenia. The prevalence of advanced colorectal neoplasia and multiple colorectal neoplasia was also significantly higher in subjects with sarcopenia, irrespective of diagnostic criteria, than in those without sarcopenia. However, among subjects with advanced colorectal neoplasia, there was a significant difference in the prevalence of advanced adenoma when subjects were stratified according to the presence of sarcopenia. However, no such difference could be seen for adenocarcinoma.
Table 3 shows the association between sarcopenia, as defined by each of the three formulas, and colorectal neoplasia including advanced colorectal neoplasia and multiple colorectal neoplasia after stepwise adjustment for potential confounders and metabolic parameters. Sarcopenia defined using BMI or weight adjusted ASM was associated with a significantly higher risk of any colorectal neoplasia. However, sarcopenia defined using height adjusted ASM was not significantly associated with the risk of overall colorectal neoplasia. For advanced colorectal neoplasia, sarcopenia defined using any of the ASM measures was associated with a higher risk of advanced colorectal neoplasia, and the magnitude of the association was stronger than that with any colorectal neoplasia. These results were consistent even after stepwise adjustment for several confounding factors, and a similar trend was seen for multiple colorectal neoplasia.
In this large-scale, cross-sectional retrospective study, we found a significant association between the presence of sarcopenia and the risk of colorectal neoplasia in asymptomatic adults who had undergone first-time colonoscopy. Even after adjusting for demographic characteristics and currently reported metabolic risk factors for colorectal neoplasia, our study showed that sarcopenia is a significant independent risk factor for colorectal neoplasia. Furthermore, sarcopenia was more strongly associated with advanced colorectal neoplasia and multiple colorectal neoplasia than with overall colorectal neoplasia.
Previous studies have found that sarcopenia is associated with an increased risk of several chronic disorders such as cardiovascular disease, obstructive pulmonary disease, metabolic syndrome, DM, and non-alcoholic fatty liver disease [13ŌĆō16,24]. Additionally, the relationship between sarcopenia and CRC has been previously reported. According to these studies, sarcopenia is associated with poor clinical outcomes and surgical advanced events in CRC patients, which may be related to a prolonged catabolic state associated with immune dysfunction and systemic inflammatory responses [11,17,18]. However, there have been limited studies on the relationship between sarcopenia and the development of colorectal neoplasia. Only three studies have reported that sarcopenia is a risk factor for colorectal neoplasia; all studies used the established BIA-based diagnostic criteria for sarcopenia. Among these, two studies defined sarcopenia using ASM adjusting by body weight [25,26], whereas one defined it as a value below the lower limit or < 90% of the standard [27]. Although dual-energy X-ray absorptiometry (DEXA) and computed tomography (CT) are currently considered as gold standard methods for measuring of the skeletal muscle mass, radiation exposure may be a drawback in actual clinical settings.
BIA, a non-invasive body composition analyzer, is a convenient, safe, and cost-effective tool for measuring skeletal muscle mass, and several studies have validated its clinical usefulness and accuracy when compared with either DEXA or CT [28ŌĆō30]. However, the cutoff value of the skeletal muscle mass index for a conclusive diagnosis of sarcopenia using BIA varies from study to study. Therefore, to compensate for this shortcoming, various formulas for defining sarcopenia using BIA, such as BMI-adjusted ASM model, height-adjusted ASM model, and body weight-adjusted ASM model, were used in the analysis.
In our study, sarcopenia defined by the BMI or weight-adjusted ASM model showed a significant association with the risk of colorectal neoplasia, but sarcopenia defined by the height-adjusted ASM model did not. However, sarcopenia defined using any of the ASM models showed a significant association with the risk of advanced colorectal neoplasia and multiple colorectal neoplasia, and the magnitude of the association was stronger than that with any colorectal neoplasia. In other words, sarcopenia may be associated with the risk of any colorectal neoplasia but is more strongly associated with the risk of advanced colorectal neoplasia and multiple colorectal neoplasia. The insignificant association between sarcopenia defined by the height-adjusted ASM model and any colorectal neoplasia might be due to the patient sample. In our study, the height-adjusted ASM model was used for a relatively small number of sarcopenia subjects (297 subjects) compared to the other models (583 subjects in the BMI-adjusted ASM model and 685 subjects in the weight-adjusted ASM model). In addition, contrary to previous reports, patients for whom sarcopenia was defined by the height-adjusted ASM model had lower obesity, lower visceral obesity, and lower incidence of metabolic syndrome than normal subjects. This may have contributed to the difference in the strength of association mentioned earlier. Further studies with a larger number of patients for the height-adjusted ASM model are required.
Although the role of sarcopenia in the development of colorectal neoplasia has not been fully elucidated yet, several observational and experimental studies have suggested a putative mechanism to explain such an association. These studies also indicate that sarcopenia and colorectal neoplasia may have common risk factors. Several studies have reported that sarcopenia is associated with decreased physical inactivity and the accumulation of visceral fat [31,32], both of which are associated with the development of colorectal neoplasia [33,34]. Decreased physical activity leads to an abnormal accumulation of visceral fat, which activates inflammatory pathways. Metabolically active visceral adipose tissue produces proinflammatory cytokines including tumor necrosis factor-╬▒ (TNF-╬▒), interleukin (IL)-6, and IL-8, which play a key role in angiogenesis, promotion of cellular proliferation, and inhibition of apoptosis during the development of colorectal neoplasia [35ŌĆō37]. These conditions are also observed when there are changes in the body composition associated with sarcopenia, including decreased skeletal muscle mass and increased visceral fat [38]. Beyer et al. [39] reported that age-related chronic low-grade inflammation is an important causative factor for sarcopenia, and sarcopenia associated inflammation is mediated by adipocytokines such as TNF-╬▒ and IL-6. In addition, a reduction in skeletal muscle mass lowers the insulin-mediated glucose uptake in myocytes, and in turn, induces insulin resistance [16,40]. Furthermore, insulin resistance elevates the insulin-like growth factor, which may promote the development of colorectal neoplasia through proliferative and anti-apoptotic effects [41]. Recently, metabolic syndrome has been reported to be an important risk factor for the development of colorectal neoplasia. Additionally, high BP, hyperglycemia, and dyslipidemia, which contribute to metabolic syndrome, are also associated with sarcopenia [16,40,42]. The interaction between these factors may consist of a complex vicious cycle, and multiple mechanisms have been implicated in the association between sarcopenia and colorectal neoplasia.
Our study has certain limitations. First, because of the cross-sectional design of this study, any causality between sarcopenia and colorectal neoplasia could not be inferred. Second, the study subjects that participated in the health screening program at a single center could not be representative of the general population. Third, there was a lack of data on physical activity, a factor associated with both sarcopenia and colorectal neoplasia in this study. Fourth, although BIA is a convenient and cost-effective tool for assessing the skeletal muscle mass index, it is not the gold standard for sarcopenia diagnosis. Another limitation is that sarcopenia includes not only lowered skeletal muscle mass but also reduced muscle functions such as gait speed and grip strength. However, BIA does not provide information about the latter. Therefore, further study is needed to analyze the association between sarcopenia and colorectal neoplasia, wherein muscle function is included as a parameter. Nevertheless, this study is meaningful as it confirms the association between sarcopenia, irrespective of diagnostic criteria, and colorectal neoplasia with a relatively large number of subjects who underwent first-time colonoscopies. In addition, the temporal bias was minimized by stepwise adjustment for potential confounding factors for colorectal neoplasia.
In conclusion, sarcopenia was a significant risk factor for any colorectal neoplasia independent of traditional risk factors. Furthermore, the presence of sarcopenia was more significantly associated with the risk of advanced colorectal neoplasia and multiple colorectal neoplasia than with the risk of any colorectal neoplasia. However, longitudinal studies are needed to elucidate the exact causal relationship between sarcopenia and colorectal neoplasia.
1. In this large-scale, cross-sectional retrospective study showed that sarcopenia was a significant risk factor for colorectal neoplasia independent of traditional risk factors.
2. Sarcopenia was more strongly associated with advanced colorectal neoplasia and multiple colorectal neoplasia than with overall colorectal neoplasia.
Figure┬Ā1
Flow diagram for the selection of study subjects. BIA, bioelectrical impedance analysis. aWhen sarcopenia was defined using body mass index-adjusted appendicular skeletal muscular mass model, one of the three established BIA-based diagnostic criteria in defining sarcopenia.
Table┬Ā1
Baseline characteristics according to sarcopenia defined using the three bioelectrical impedance analysis-based diagnostic criteria
Table┬Ā2
The prevalence of colorectal neoplasia according to sarcopenia
Table┬Ā3
Risk of colorectal neoplasia, advanced colorectal neoplasia, and multiple colorectal neoplasia according to sarcopenia
| Any colorectal neoplasia | Advanced colorectal neoplasia | Multiple colorectal neoplasia | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| BMI-adjusted ASMa | ||||||
|
|
||||||
| ŌĆāUnadjusted | 1.89 (1.60ŌĆō2.25) | < 0.001 | 2.71 (1.77ŌĆō4.17) | < 0.001 | 2.37 (1.78ŌĆō3.15) | < 0.001 |
|
|
||||||
| ŌĆāModel 1 | 1.38 (1.15ŌĆō1.64) | < 0.001 | 2.02 (1.30ŌĆō3.12) | 0.002 | 1.58 (1.18ŌĆō2.11) | 0.002 |
|
|
||||||
| ŌĆāModel 2 | 1.34 (1.12ŌĆō1.60) | 0.001 | 2.04 (1.32ŌĆō3.16) | 0.001 | 1.52 (1.13ŌĆō2.03) | 0.005 |
|
|
||||||
| ŌĆāModel 3 | 1.31 (1.09ŌĆō1.56) | 0.003 | 1.97 (1.27ŌĆō3.06) | 0.002 | 1.49 (1.11ŌĆō1.99) | 0.008 |
|
|
||||||
| Height-adjusted ASMa | ||||||
|
|
||||||
| ŌĆāUnadjusted | 1.38 (1.08ŌĆō1.76) | 0.009 | 2.89 (1.65ŌĆō5.04) | < 0.001 | 2.20 (1.48ŌĆō3.25) | < 0.001 |
|
|
||||||
| ŌĆāModel 1 | 1.08 (0.84ŌĆō1.38) | 0.570 | 2.28 (1.30ŌĆō4.00) | 0.004 | 1.57 (1.05ŌĆō2.35) | 0.028 |
|
|
||||||
| ŌĆāModel 2 | 1.17 (0.91ŌĆō1.51) | 0.218 | 2.22 (1.26ŌĆō3.90) | 0.006 | 1.80 (1.19ŌĆō2.71) | 0.005 |
|
|
||||||
| ŌĆāModel 3 | 1.16 (0.90ŌĆō1.50) | 0.254 | 2.19 (1.24ŌĆō3.85) | 0.007 | 1.77 (1.17ŌĆō2.67) | 0.007 |
|
|
||||||
| Weight-adjusted ASMa | ||||||
|
|
||||||
| ŌĆāUnadjusted | 1.60 (1.36ŌĆō1.87) | < 0.001 | 2.49 (1.64ŌĆō3.77) | < 0.001 | 2.17 (1.65ŌĆō2.85) | < 0.001 |
|
|
||||||
| ŌĆāModel 1 | 1.34 (1.14ŌĆō1.58) | 0.001 | 2.12 (1.40ŌĆō3.22) | < 0.001 | 1.77 (1.34ŌĆō2.34) | < 0.001 |
|
|
||||||
| ŌĆāModel 2 | 1.25 (1.06ŌĆō1.49) | 0.009 | 2.48 (1.59ŌĆō3.89) | < 0.001 | 1.60 (1.19ŌĆō2.14) | 0.002 |
|
|
||||||
| ŌĆāModel 3 | 1.21 (1.02ŌĆō1.43) | 0.029 | 2.41 (1.54ŌĆō3.77) | < 0.001 | 1.58 (1.18ŌĆō2.11) | 0.002 |
Model 1: adjusted for age and sex; Model 2: further adjusted for current smoking, excessive alcohol intake, obesity (BMI Ōēź 25 kg/m2), and visceral obesity; Model 3: further adjusted for hypertension, diabetes mellitus, dyslipidemia, and metabolic syndrome.
REFERENCES
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394ŌĆō424.


2. Wong MC, Ding H, Wang J, Chan PS, Huang J. Prevalence and risk factors of colorectal cancer in Asia. Intest Res 2019;17:317ŌĆō329.



3. Jackman RJ, Mayo CW. The adenoma-carcinoma sequence in cancer of the colon. Surg Gynecol Obstet 1951;93:327ŌĆō330.

4. Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993;329:1977ŌĆō1981.


5. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology 2010;138:2044ŌĆō2058.


6. Tao S, Hoffmeister M, Brenner H. Development and validation of a scoring system to identify individuals at high risk for advanced colorectal neoplasms who should undergo colonoscopy screening. Clin Gastroenterol Hepatol 2014;12:478ŌĆō485.


7. Wei EK, Ma J, Pollak MN, et al. A prospective study of C-peptide, insulin-like growth factor-I, insulin-like growth factor binding protein-1, and the risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev 2005;14:850ŌĆō855.


8. Frezza EE, Wachtel MS, Chiriva-Internati M. Influence of obesity on the risk of developing colon cancer. Gut 2006;55:285ŌĆō291.



9. Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology 2007;132:2208ŌĆō2225.


10. Edwards BK, Noone AM, Mariotto AB, et al. Annual report to the nation on the status of cancer, 1975ŌĆō2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 2014;120:1290ŌĆō1314.


11. Reisinger KW, van Vugt JL, Tegels JJ, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg 2015;261:345ŌĆō352.


12. Pedersen M, Cromwell J, Nau P. Sarcopenia is a predictor of surgical morbidity in inflammatory bowel disease. Inflamm Bowel Dis 2017;23:1867ŌĆō1872.


13. Jones SE, Maddocks M, Kon SS, et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax 2015;70:213ŌĆō218.


14. Kim TN, Park MS, Yang SJ, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Care 2010;33:1497ŌĆō1499.



15. Lee YH, Jung KS, Kim SU, et al. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: nationwide surveys (KNHANES 2008ŌĆō2011). J Hepatol 2015;63:486ŌĆō493.


16. Lim S, Kim JH, Yoon JW, et al. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care 2010;33:1652ŌĆō1654.



17. Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer 2012;107:931ŌĆō936.



18. Malietzis G, Johns N, Al-Hassi HO, et al. Low muscularity and myosteatosis is related to the host systemic inflammatory response in patients undergoing surgery for colorectal cancer. Ann Surg 2016;263:320ŌĆō325.


19. Ling CH, de Craen AJ, Slagboom PE, et al. Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin Nutr 2011;30:610ŌĆō615.


20. Ida S, Watanabe M, Yoshida N, et al. Sarcopenia is a predictor of postoperative respiratory complications in patients with esophageal cancer. Ann Surg Oncol 2015;22:4432ŌĆō4437.


21. Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014;69:547ŌĆō558.



22. Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2006;61:1059ŌĆō1064.


23. Kim YS, Lee Y, Chung YS, et al. Prevalence of sarcopenia and sarcopenic obesity in the Korean population based on the Fourth Korean National Health and Nutritional Examination Surveys. J Gerontol A Biol Sci Med Sci 2012;67:1107ŌĆō1113.


24. Chin SO, Rhee SY, Chon S, et al. Sarcopenia is independently associated with cardiovascular disease in older Korean adults: the Korea National Health and Nutrition Examination Survey (KNHANES) from 2009. PLoS One 2013;8:e60119.



25. Jung YS, Kim NH, Ryu S, Park JH, Park DI, Sohn CI. Association between low relative muscle mass and the risk of colorectal neoplasms. J Clin Gastroenterol 2017;51:e83ŌĆōe89.


26. Hong JT, Kim TJ, Pyo JH, et al. Impact of sarcopenia on the risk of advanced colorectal neoplasia. J Gastroenterol Hepatol 2019;34:162ŌĆō168.


27. Park YS, Kim JW, Kim BG, et al. Sarcopenia is associated with an increased risk of advanced colorectal neoplasia. Int J Colorectal Dis 2017;32:557ŌĆō565.


28. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002;50:889ŌĆō896.


29. Bosaeus I, Wilcox G, Rothenberg E, Strauss BJ. Skeletal muscle mass in hospitalized elderly patients: comparison of measurements by single-frequency BIA and DXA. Clin Nutr 2014;33:426ŌĆō431.


30. Rangel Peniche DB, Raya Giorguli G, Aleman-Mateo H. Accuracy of a predictive bioelectrical impedance analysis equation for estimating appendicular skeletal muscle mass in a non-Caucasian sample of older people. Arch Gerontol Geriatr 2015;61:39ŌĆō43.


31. Ong ML, Holbrook JD. Novel region discovery method for Infinium 450K DNA methylation data reveals changes associated with aging in muscle and neuronal pathways. Aging Cell 2014;13:142ŌĆō155.


32. Yagi S, Kadota M, Aihara KI, et al. Association of lower limb muscle mass and energy expenditure with visceral fat mass in healthy men. Diabetol Metab Syndr 2014;6:27.



33. Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer 2009;100:611ŌĆō616.



34. Pedersen BK. Exercise-induced myokines and their role in chronic diseases. Brain Behav Immun 2011;25:811ŌĆō816.


35. Braunersreuther V, Viviani GL, Mach F, Montecucco F. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J Gastroenterol 2012;18:727ŌĆō735.



36. de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer 2006;6:24ŌĆō37.


37. Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005;7:211ŌĆō217.


38. Gomes MJ, Martinez PF, Pagan LU, et al. Skeletal muscle aging: influence of oxidative stress and physical exercise. Oncotarget 2017;8:20428ŌĆō20440.



39. Beyer I, Mets T, Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr Opin Clin Nutr Metab Care 2012;15:12ŌĆō22.


40. Lee SW, Youm Y, Lee WJ, et al. Appendicular skeletal muscle mass and insulin resistance in an elderly Korean population: the Korean social life, health and aging project-health examination cohort. Diabetes Metab J 2015;39:37ŌĆō45.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



