 |
 |
|
|
|
Abstract
Background/Aims
The prevalence and effects of airway diseases, including asthma, eosinophilic bronchitis (EB), chronic obstructive pulmonary disease (COPD), and asthma-COPD overlap (ACO) have not been thoroughly studied in patients with idiopathic pulmonary fibrosis (IPF). This study aimed to evaluate the prevalence of airway diseases in patients with IPF and to identify the differences in symptoms based on the presence of airway diseases.
Methods
This single-institution prospective cohort study was conducted from June 2017 to September 2018, at the Seoul National University Hospital. Spirometry with bronchodilator, methacholine bronchial provocation test, induced sputum with eosinophil stain, and exhaled nitric oxide were performed to confirm the presence of airway disease. The modified Medical Research Council (mMRC) dyspnea scale, COPD assessment test (CAT), St. George’s Respiratory Questionnaire (SGRQ), EuroQol-5 dimension (EQ-5D) index, and cough-specific quality of life questionnaire (CQLQ) data were collected to assess symptom severity.
Results
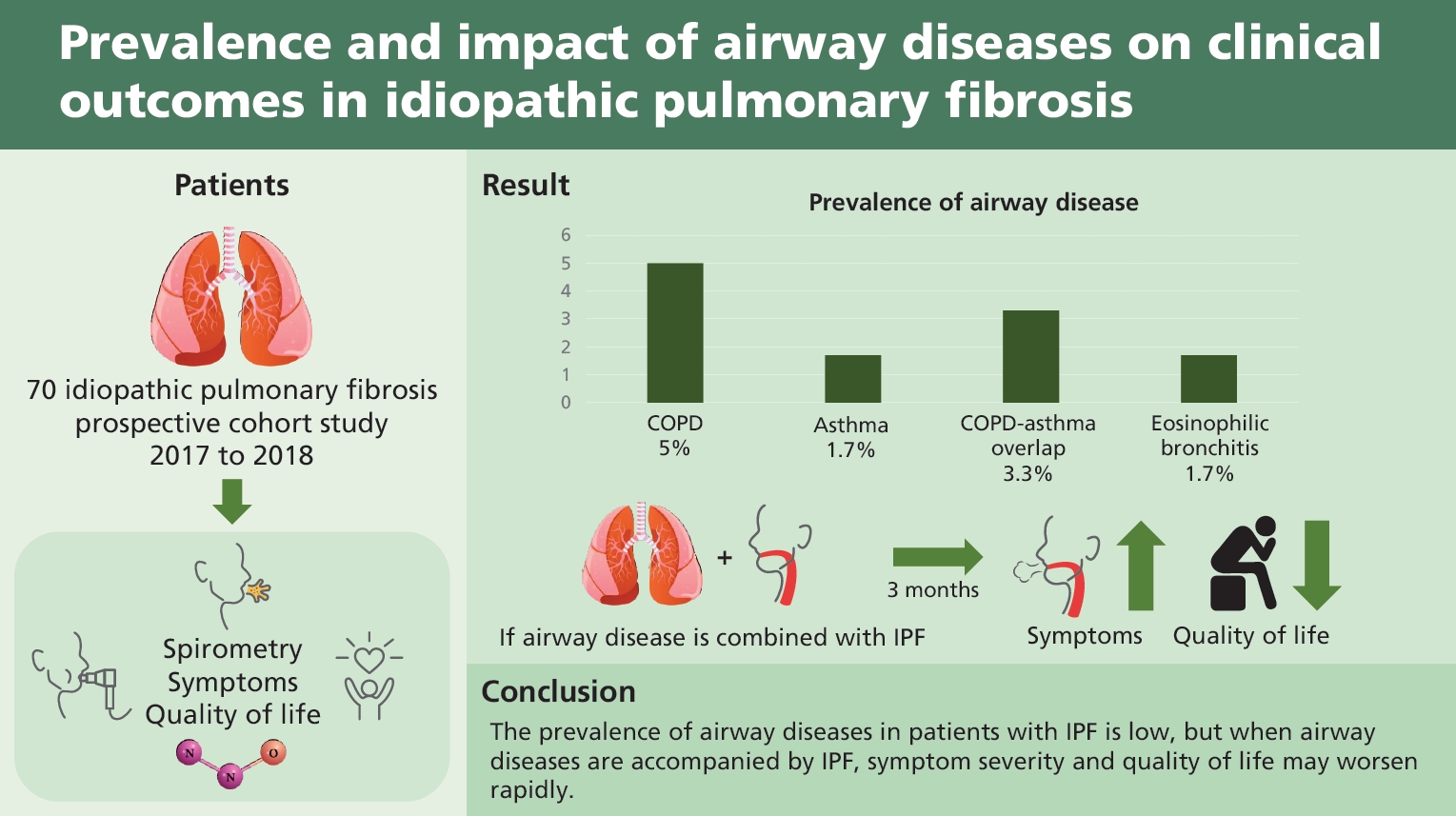
Total 147 patients with IPF were screened, and 70 patients were analyzed. The prevalence of airway diseases in the participants was as follows: 5.0% had COPD, 1.7% had asthma, 3.3% had ACO, and 1.7% had EB. The mMRC, CAT, SGRQ, EQ-5D, and CQLQ scores did not differ regardless of combined airway disease. After 3 months, the SGRQ (p = 0.028) and CQLQ (p = 0.030) scores were significantly higher in patients with airway disease than in those without.
Idiopathic pulmonary fibrosis (IPF) is a specific form of chronic, progressive fibrosing interstitial pneumonia of unknown etiology. It primarily occurs in elderly and is limited to the lungs. It is characterized by progressive worsening of dyspnea and lung function, which is associated with poor prognosis [1]. A recent analysis, based on healthcare claims data from a large health plan in the United States, estimated the prevalence of IPF to be between 14.0 and 42.7 per 100,000 persons [2].
The main symptoms of IPF are dyspnea on exertion and persistent dry or mildly productive cough, affecting over 70% to 85% of patients with IPF [3–5]. Additionally, airway diseases such as chronic obstructive pulmonary disease (COPD; prevalence rate 8.4% to 15.0%) [6], asthma (4.2% to 4.4%) [7], eosinophilic bronchitis (EB; 1.5% to 9.6%) [8], and asthma-COPD overlap (ACO) induce dyspnea and cough.
To date, the prevalence and effects of airway diseases have not been thoroughly studied in patients with IPF. Unlike IPF, airway diseases are relatively well treated using inhaled bronchodilators (BDs) or inhaled corticosteroids (ICSs). Therefore, if patients with IPF have concurrent airway diseases, treatment of these diseases may improve their quality of life (QOL).
The purpose of this study was to evaluate the prevalence of airway diseases in patients with IPF and to identify the differences in symptoms based on the presence of airway diseases.
This single-institution prospective cohort study was conducted from June 2017 to September 2018, at the Seoul National University Hospital. The study was conducted in accordance with the amended Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB no.: 1703-111-840). The participants were explained the purpose of this study, and written informed consent was obtained from each participant. The study was registered with ClinicalTrials.gov (NCT03215147).
Patients who already had IPF or were newly diagnosed with IPF were included in the study. Subjects were excluded if they had contraindications for spirometry with bronchodilator response (BDR) or methacholine bronchial provocation test (MBPT), or if any of the following conditions were met: forced expiratory volume in 1 second (FEV1) < 1.5 L or < 60%, resting peripheral oxygen saturation (SpO2) < 90%, home oxygen use, systemic steroid use, or acute exacerbation of IPF within the last 6 months. In case of patients who were using an inhaler at the time of registration, the study was conducted after 1 day off medication when patients were using short-acting beta 2 agonist/short-acting muscarinic antagonist, 2 days off medication when patients were using long-acting beta 2 agonist (LABA), 1 week off medication when patients were using long-acting muscarinic antagonist (LAMA), and 4 weeks off medication when patients were using an ICS.
Demographic findings such as age, sex, body mass index, smoking history, and medications were collected for baseline evaluation of the enrolled patients. Pulmonary function test (PFT) and 6-minute walk test (6MWT) data which includes nadir SpO2 and 6-minute walk distance (6MWD) were collected to assess physiological status. In this study, the clinical outcomes were limited to assessment of symptom severity and QOL. The modified Medical Research Council (mMRC) dyspnea scale, COPD assessment test (CAT), St. George’s Respiratory Questionnaire (SGRQ), EuroQol-5 dimension (EQ-5D) index, and cough-specific quality of life questionnaire (CQLQ) data were collected to assess symptom severity. PFT with BDR, MBPT, induced sputum (IS) examination with eosinophil stain, multiple antigen simultaneous test (MAST), fractional exhaled nitric oxide (FENO), serum immunoglobulin E (IgE), and blood eosinophil count were performed to confirm the presence of airway diseases.
If the patient was diagnosed with COPD, they received treatment according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD, 2017) guidelines [9] for 3 months. If asthma, ACO, or EB was confirmed, the patient was treated according to the Global Initiative for Asthma (GINA, 2017) guidelines [10]. After 3 months of treatment, the patients were evaluated to assess symptom improvement using mMRC, CAT, SGRQ, 6MWT, EQ-5D, and CQLQ.
IPF was diagnosed according to the American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association (ATS/ERS/JRS/ALAT) guidelines [1]. First, we excluded patients with interstitial lung disease caused by other known etiologies, such as drug toxicity, environmental exposure, and connective tissue disease. A definite usual interstitial pneumonia pattern on high-resolution computed tomography (HRCT) and/or surgical lung biopsy is required to diagnose IPF.
COPD, asthma, and ACO were defined according to the GOLD 2017 [9] and the GINA 2017 guidelines [10]. COPD is defined as a post-BD FEV1/forced vital capacity (FVC) ratio of < 0.7. Asthma is defined by a positive BD reversibility test showing an increase in FEV1 of > 12% and > 200 mL from baseline 10–15 minutes after 200–400 μg albuterol or equivalent, or by positive MBPT showing a fall in FEV1 from baseline of ≥ 20% with standard doses of methacholine. ACO is defined when the following two conditions are satisfied: post-BD FEV1/FVC < 0.7, and post-BD FEV1 > 12% and > 200 mL from baseline.
The primary outcome of this study was the prevalence of airway disease in patients with IPF. Prevalence was analyzed using descriptive statistics expressed as mean and standard error. Tests to confirm airway disease and allergic predisposition were partially completed in some subjects. For prevalence analysis, the total number was defined as the number of patients who completed all the tests.
The secondary outcome of this study was the differences in symptom severity according to the presence of combined airway diseases and the improvement of symptoms after treatment for 3 months.
For categorical variables, data were analyzed using Fisher’s exact test or Pearson’s chi-squared test. For numerical variables, Mann-Whitney test or Student’s t test was undertaken. We used propensity score-matched (1:3) analysis to match participants with or without airway disease. We analyzed repeated-measured variables using the Wilcoxon signed-rank test or repeated measures analysis of variance (RM-ANOVA).
The results were considered significant if p values were < 0.05. All analyses were conducted using the SPSS version 22.0 (IBM Co., Armonk, NY, USA).
A total of 147 patients with IPF were screened, and 73 patients were excluded. The remaining 74 patients provided written informed consent and participated in the study. Amongst these, four patients were lost to follow-up, and thus 70 patients were included in the analysis (Fig. 1). Baseline characteristics of the subjects are presented in Table 1. The median age was 70 years, and 74.3% of the subjects were men. PFT results were 77.25% ± 1.78% for FEV1, 89.97% ± 2.09% for FVC and 66.18% ± 2.05% for diffusing capacity for carbon monoxide (DLco). The average 6MWD was 474.81 ± 10.95 m, and 12 patients (17.2%) had mMRC dyspnea scale of 2 or greater (Table 1). The prevalence of airway diseases, which was the primary outcome of this study, was 5.0% for COPD, 1.7% for asthma, 3.3% for ACO, and 1.7% for EB. One patient had both COPD and EB. Because some subjects did not undertake all the tests, the number of patients who completed all tests was taken as the total number of subjects (n = 60). We also analyzed each test to evaluate the allergic predisposition in patients with IPF. The results are shown in Table 2. Among the tests, IS eosinophilia and increased IgE (total IgE > 100 IU/mL) were observed in 23.3% and 31.7% of participants, respectively (Table 2).
Baseline characteristics and clinical outcomes, such as symptom severity and QOL, were compared between the two groups according to the presence of airway diseases, including COPD, asthma, ACO, and EB. There was a significant difference in FEV1 (91.53 ± 2.16 vs. 73.83 ± 3.88, p = 0.006), FEV1/FVC (81.66 ± 0.74 vs. 67.67 ± 2.84, p < 0.001), 6MWD (484.30 ± 10.76 vs. 388.50 ± 43.92, p = 0.036), but the clinical outcomes were not different between the two groups (Supplementary Table 1). Eleven patients without 3-month follow-up data were excluded when comparing the longitudinal differences in clinical outcomes between the two groups with and without airway diseases. Finally, clinical outcomes after 3 months were compared in 59 patients: CAT (14.68 ± 1.16 vs. 17.10 ± 1.24, p = 0.011), SGRQ (30.09 ± 2.72 vs. 33.50 ± 2.75, p = 0.010), and mMRC (0.81 ± 0.107 vs. 1.07 ± 0.130, p = 0.037) scores were significantly worse after 3 months (Table 3). The results of PFT after 3 months showed that, FEV1 was not significantly different between the groups (91.16 ± 2.36 vs. 78.83 ± 4.57, p = 0.086) and FEV1/FVC was significantly lower in the airway disease group (81.54 ± 0.87 vs. 70.33 ± 3.31, p = 0.003) (Supplementary Table 1).
Next, we compared the changes in clinical outcomes between the two groups according to the presence of airway disease. The CAT (p = 0.052), SGRQ (p = 0.040), and CQLQ (p = 0.027) scores were significantly higher in patients with airway disease than in those without the disease (Supplementary Table 2). Propensity score-matched analysis was performed with FVC and FEV1 due to the low baseline lung function and exercise capacity of the group with airway disease. Matching was performed at a ratio of 1:3. Six patients with airway disease and 18 patients without airway disease were included in the analysis. In the baseline characteristics after correction, only FEV1/FVC (78.33 ± 1.17 vs. 67.67 ± 2.84, p < 0.001) was significantly lower in the group with airway disease. FEV1 (77.33 ± 2.29 vs. 73.83 ± 3.88, p = 0.450), FVC (69.67 ± 2.74 vs. 75.00 ± 5.03, p = 0.348), DLco (62.17 ± 3.05 vs. 52.60 ± 5.95, p = 0.160), and 6MWD (469.35 ± 20.78 vs. 388.50 ± 43.92, p = 0.076) were not significantly different between the two groups (Supplementary Table 3). The SGRQ (p = 0.028) and CQLQ (p = 0.030) scores were significantly higher in patients with airway disease than in those without (Fig. 2).
Subjects who needed inhaler were defined as those with airway disease, positive results for IS, or high FENO. Compliance was assessed by confirming whether the patient was using the inhaler correctly and by evaluating whether the inhaler was used continuously for 3 months.
One patient with both COPD and EB used ICS, one patient with COPD used ICS/LABA, and one patient with COPD was not prescribed an inhaler. One ACO patient used ICS/LABA, another patient used ICS/LABA + LAMA, and one patient with asthma used ICS/LABA. Seven IS-positive patients used ICS, one of the two high FENO patients used ICS/LABA, and the other patient used ICS. ICS was not indicated in one patient; however, the patient used it, and was therefore excluded from further analysis.
To evaluate the effect of appropriate inhaler use on clinical outcomes, we compared the changes in symptom severity and QOL scores between patients who used inhalers appropriately according to their airway disease and those who did not. The following 11 participants were excluded from this analysis: seven patients who had airway disease or allergic disposition but did not use an inhaler, one patient who was not indicated for inhaler use but used an inhaler, and three patients with poor inhaler compliance. The remaining 48 participants who were divided into two groups according to their airway disease, use of an inhaler, and the changes in clinical outcomes were analyzed. There was no difference in the baseline characteristics between the two groups (Supplementary Table 4). The CAT (p = 0.012) and mMRC (p = 0.013) scores significantly increased in patients using inhalers than in those not using them (Fig. 3).
Finally, among the total patients who needed to use inhalers, 11 with good inhaler compliance and 10 who were not using an inhaler appropriately were compared. There was no difference in the baseline characteristics between these two groups (Supplementary Table 5). Only the CAT (p = 0.012) scores were significantly increased in patients with good inhaler compliance (Fig. 4). When the same analysis was performed with only three patients with COPD and two patients with ACO, there were no significant differences in the symptom severity scores, which may have been due to the small sample size (Supplementary Fig. 1).
In the general population, the prevalence of COPD is 8.0% to 15.0% [6], asthma prevalence is 4.1% to 44.4% [7], and the prevalence of EB is 1.8% to 4.6% [8]. However, the prevalence of airway diseases such as COPD, asthma, ACO, and EB in patients with IPF has not been well researched. In this study, the prevalence of airway diseases in patients with IPF was 5.0%, 1.7%, 3.3%, and 1.7% for COPD, asthma, ACO, and EB, respectively; this is lower than that observed in the general population. A recent review showed that the resistance of the conducting airway was reduced in patients with IPF [14]. The mean value of FEV1/FVC obtained from the data set of 55 patients with IPF was 0.83, which was higher than 0.74 for men and 0.75 for females according to the ERS reference equations [15]. The ratio of the forced expiratory flow at 25% to 75% of FVC (FEF 25%–75%) to FVC (FEF 25%–75%/FVC) was positively correlated with the degree of IPF progression confirmed through HRCT [16]. When volumetric capnography was used to measure the volume of the conducting airways, patients with IPF had higher conducting airway volumes than those without [17]. The dilation of the conducting airway is thought to be associated with a lower prevalence of airway disease in patients with IPF than in the general population.
IS analysis is a safe, non-invasive method and a suitable tool for evaluating respiratory tract inflammation in patients with IPF [18]. It has previously been reported that IS eosinophilia is observed in 40% to 60% of patients with IPF [19]. A study by Peterson et al. [20] showed that patients with IPF who failed to respond to treatment with corticosteroids had increased eosinophil counts in bronchoalveolar lavage. However, because steroids are not currently a treatment option for IPF, their clinical significance is unknown. According to the official ATS clinical practice guidelines, FENO ≥ 50 ppb is used to indicate bronchial eosinophilic inflammation and can, therefore, be an indicator of responsiveness to corticosteroids in symptomatic patients [11]. Conversely, in one study, the median FENO value of patients with IPF was 22 ppb, and in an another one, the value was 8.6 ppb [12,13]. Therefore, in this study, we defined EB in patients with IPF according to the following two conditions: eosinophil count in IS above 3% without airflow limitation, and FENO value above 50 ppb.
The effect of airway diseases on the clinical outcomes of patients with IPF is also unknown. It is well known that in the general population, the clinical outcomes of patients with airway diseases are worse; however, they improve with the use of an inhaler. In this study, the clinical outcomes at the 3-month follow-up of patients with airway disease were worse than those of patients without airway diseases.
The use of inhalers was thought to improve the clinical outcome; however, analysis of total subjects who required an inhaler (with airway disease or positive result of IS or high FENO) showed no significant differences in outcomes except CAT scores between patients with good inhaler compliance and patients not appropriately using an inhaler. Rather, the CAT score significantly increased in the group using inhalers. The baseline symptom severity and QOL scores of patients who used inhalers were worse than those of patients who did not use inhalers (Fig. 4). It is appropriate to infer that patients with severe symptoms probably showed higher compliance than those with light symptoms, but the inhaler was of no use to patients with severe symptoms and resulted in worse results on their second visit. Therefore, this may have acted as a bias in the distribution of patients with worse CAT scores as those who used inhalers well. In addition, the use of an inhaler may cause complications, such as cough, which may have also affected the deterioration of the CAT score.
The inflammation or hyperresponsiveness of airway diseases may have a minor impact on the patient’s symptoms and QOL, since the main cause of symptoms and QOL deterioration in patients with IPF is pulmonary parenchymal destruction due to pulmonary fibrosis.
This study had several limitations. The prevalence of IPF was low; thus, few patients were enrolled, and the examined period of inhaler use and follow-up was short too. The results after a 3-month follow-up showed that the clinical outcomes were worse in patients with airway disease, but the initial clinical outcomes did not show a significant difference between the two groups. This study likely needs to be conducted with a longer follow-up period and a larger sample size. In addition, to determine whether the use of inhalers improves actual clinical outcomes, more specific grouping should be used to examine patients who may see great benefit from inhaler use. Thereafter, the group should have been divided into groups using inhalers, not inhalers, and placebo groups. However, another limitation of this study was that no such randomization with a control group was done.
In conclusion, the prevalence of airway diseases in patients with IPF is low, but when airway diseases are accompanied by IPF, clinical outcomes may worsen rapidly. Patients with IPF and allergic predisposition are unlikely to benefit from inhaler therapy. Inhalers themselves may have side effects (cough, infection, etc.), and it is therefore advisable that its use be limited to patients in need of inhaler therapy.
1. The prevalence of airway diseases in patients with idiopathic pulmonary fibrosis (IPF) is low, but when airway diseases are accompanied by IPF, clinical outcomes may worsen rapidly.
2. Patients with IPF and allergic predisposition are unlikely to benefit from inhaler therapy.
3. Therefore, inhalers should not be routinely used in patients with IPF and concurrent airway disease.
Acknowledgments
This study was supported by a grant (No.04-2016-3030) from the Seoul National University Hospital research fund.
Figure 1
Flow chart of the study. IPF, idiopathic pulmonary fibrosis; ILD, interstitial lung disease; COPD, chronic obstructive pulmonary disease; ACO, asthma-COPD overlap; EB, eosinophilic bronchitis.
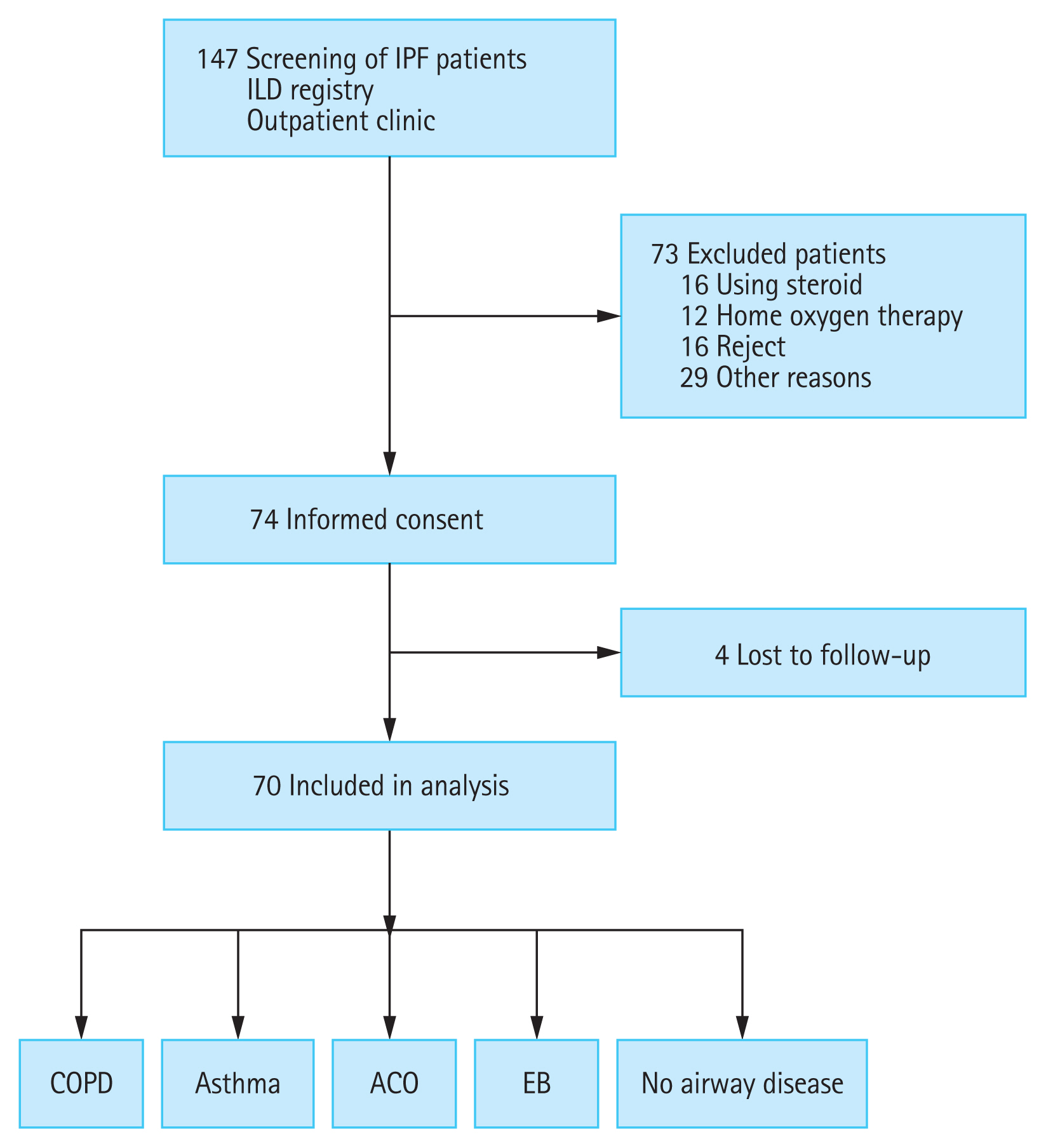
Figure 2
Changes in the clinical outcomes according to the presence of airway diseases in the groups after propensity score matching with forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1). (A) Chronic obstructive pulmonary disease (COPD) assessment test (CAT) score, (B) St. George’s Respiratory Questionnaire (SGRQ) score, (C) modified Medical Research Council (mMRC) dyspnea scale, (D) cough quality of life questionnaire (CQLQ) score, (E) EuroQol-5 dimension (EQ-5D) score, and (F) cough visual analogue scale (VAS) score. The analysis was carried out with 24 patients after excluding 11 patients who were lost to follow-up and matching by propensity score matched analysis with FVC and FEV1. Increasing scores indicate worsening outcomes in CAT, SGRQ, CQLQ, VAS, and mMRC. Decreasing scores indicate worsening outcomes in EQ-5D. Differences between the two groups were tested using repeated measures analysis of variance (RM-ANOVA). Visit 1, baseline; Visit 2, 3-month follow-up. ap < 0.05.
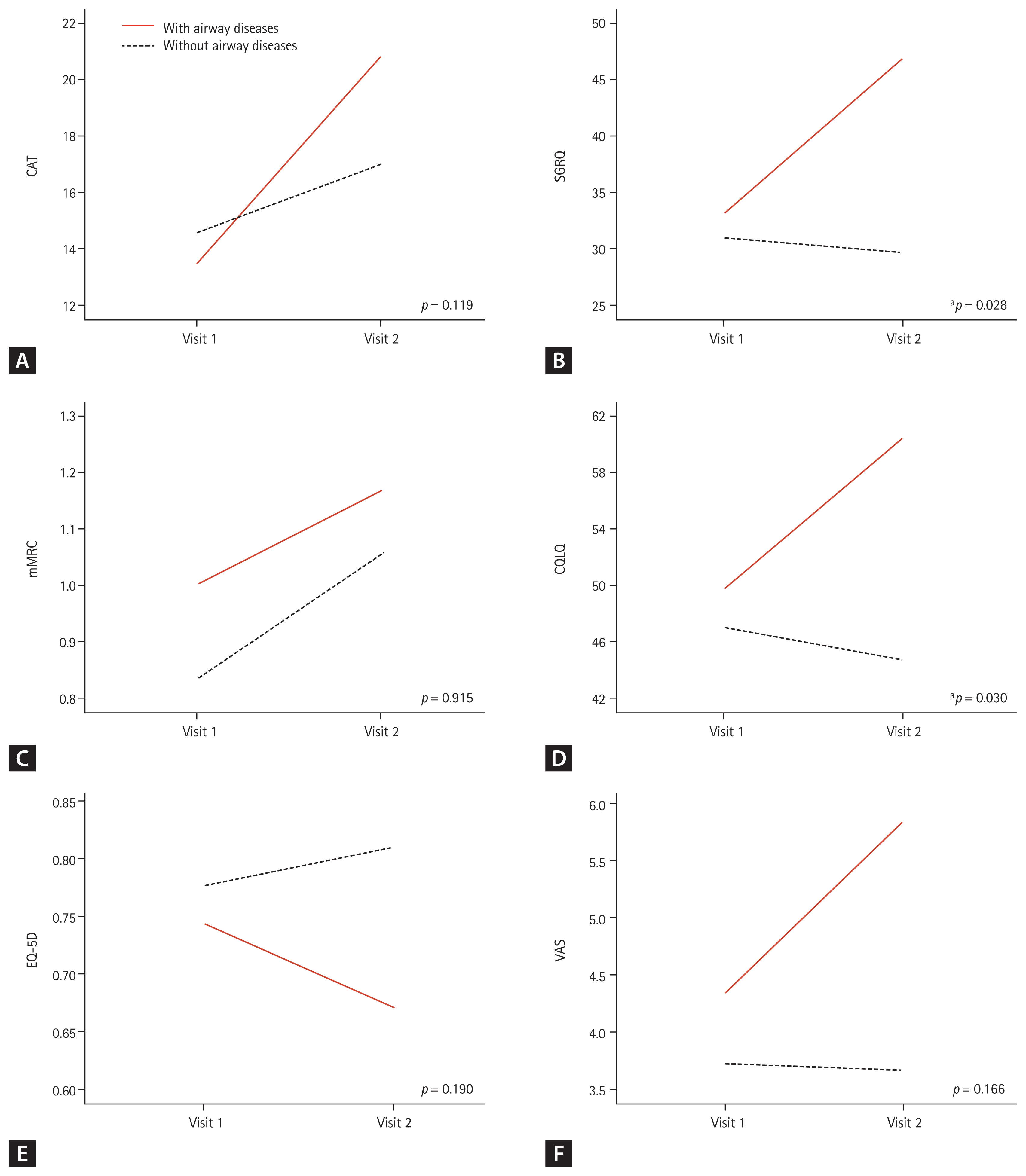
Figure 3
Comparison of changes in the clinical outcomes between patients who used inhalers for their airway diseases and those who did not use inhalers due to the absence of airway disease. (A) Chronic obstructive pulmonary disease (COPD) assessment test (CAT) score, (B) St. George’s Respiratory Questionnaire (SGRQ) score, (C) modified Medical Research Council (mMRC) dyspnea scale, (D) cough quality of life questionnaire (CQLQ) score, (E) EuroQol-5 dimension (EQ-5D) score, and (F) cough visual analogue scale (VAS) score. The analysis was carried out with 48 subjects excluding 11 patients who were lost to follow-up and 11 patients who used inhaler improperly. Increasing scores indicate worsening outcome in CAT, SGRQ, CQLQ, VAS, and mMRC. Decreasing scores indicate worsening outcome in EQ-5D. Differences between the two groups were tested using repeated measures analysis of variance (RM-ANOVA). Visit 1, baseline; Visit 2, 3-month follow-up. ap < 0.05.
Figure 4
Changes in clinical outcomes depending on the use of inhalers in patients who needed inhalers. (A) Chronic obstructive pulmonary disease (COPD) assessment test (CAT) score, (B) St. George’s Respiratory Questionnaire (SGRQ) score, (C) modified Medical Research Council (mMRC) dyspnea scale, (D) cough quality of life questionnaire (CQLQ) score, (E) EuroQol-5 dimension (EQ-5D) score, and (F) cough visual analogue scale (VAS) score. The analysis was carried out with 21 subjects who had airway diseases, positive result of induced sputum, or a high level of fractional exhaled nitric oxide. Increasing scores indicate worsening outcomes in CAT, SGRQ, CQLQ, VAS, and mMRC. Decreasing scores indicate worsening outcome in EQ-5D. Differences between the two groups were tested using repeated measures analysis of variance (RM-ANOVA). Visit 1, baseline; Visit 2, 3-month follow-up. ap < 0.05.

Table 1
Baseline characteristics of patients (n = 70)
BMI, body mass index; FEV1, forced expiratory volume in 1 second; % pred, % of predicted value; FVC, forced vital capacity; DLco, diffusing capacity for carbon monoxide; SpO2, resting peripheral oxygen saturation; QOL, quality of life; mMRC, modified Medical Research Council; CAT, COPD assessment test; SGRQ, St. George’s Respiratory Questionnaire; EQ-5D, EuroQol-5 dimension; CQLQ, cough quality of life questionnaire; VAS, visual analog scale.
Table 2
Prevalence of airway disease and allergic predisposition in patients with idiopathic pulmonary fibrosis
Table 3
Differences in the symptoms and quality of life between baseline and 3-month follow-up
| Variable | Initial score | Score at 3-month follow-up | p valuea |
|---|---|---|---|
| CAT | 14.68 ± 1.16 | 17.10 ± 1.24 | 0.011b |
| SGRQ | 30.09 ± 2.72 | 33.50 ± 2.75 | 0.010b |
| mMRC | 0.81 ± 0.107 | 1.07 ± 0.130 | 0.037b |
| CQLQ | 48.00 ± 1.81 | 48.36 ± 2.07 | 0.827 |
| EQ-5D | 0.777 ± 0.020 | 0.7807 ± 0.024 | 0.942 |
| Cough VAS | 3.66 ± 0.369 | 4.16 ± 0.386 | 0.158 |
CAT, COPD assessment test; SGRQ, St. George’s Respiratory Questionnaire; mMRC, modified Medical Research Council; CQLQ, cough quality of life questionnaire; EQ-5D, EuroQol-5 dimension; VAS, visual analogue scale.
REFERENCES
1. Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med 2015;192:e3–e19.


2. Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis. Evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788–824.


3. Crystal RG, Fulmer JD, Roberts WC, Moss ML, Line BR, Reynolds HY. Idiopathic pulmonary fibrosis. Clinical, histologic, radiographic, physiologic, scintigraphic, cytologic, and biochemical aspects. Ann Intern Med 1976;85:769–788.


4. Shorr AF, Wainright JL, Cors CS, Lettieri CJ, Nathan SD. Pulmonary hypertension in patients with pulmonary fibrosis awaiting lung transplant. Eur Respir J 2007;30:715–721.


5. Ryerson CJ, Hartman T, Elicker BM, et al. Clinical features and outcomes in combined pulmonary fibrosis and emphysema in idiopathic pulmonary fibrosis. Chest 2013;144:234–240.


6. Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health 2015;5:020415.



7. To T, Stanojevic S, Moores G, et al. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health 2012;12:204.



8. Gibson PG, Fujimura M, Niimi A. Eosinophilic bronchitis: clinical manifestations and implications for treatment. Thorax 2002;57:178–182.



9. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease. GOLD 2017.
10. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Fontana (WI): GINA, 2017.
11. Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011;184:602–615.



12. Chow S, Thomas PS, Malouf M, Yates DH. Exhaled breath condensate (EBC) biomarkers in pulmonary fibrosis. J Breath Res 2012;6:016004.


13. Guilleminault L, Saint-Hilaire A, Favelle O, et al. Can exhaled nitric oxide differentiate causes of pulmonary fibrosis? Respir Med 2013;107:1789–1796.


14. Plantier L, Cazes A, Dinh-Xuan AT, Bancal C, Marchand-Adam S, Crestani B. Physiology of the lung in idiopathic pulmonary fibrosis. Eur Respir Rev 2018;27:170062.


15. Pastre J, Plantier L, Planes C, et al. Different KCO and VA combinations exist for the same DLCO value in patients with diffuse parenchymal lung diseases. BMC Pulm Med 2015;15:100.



16. Lopes AJ, Capone D, Mogami R, Cunha DL, Melo PL, Jansen JM. Correlation of tomographic findings with pulmonary function parameters in nonsmoking patients with idiopathic pulmonary fibrosis. J Bras Pneumol 2007;33:671–678.


17. Plantier L, Debray MP, Estellat C, et al. Increased volume of conducting airways in idiopathic pulmonary fibrosis is independent of disease severity: a volumetric capnography study. J Breath Res 2016;10:016005.


18. Beeh KM, Beier J, Kornmann O, Buhl R. Neutrophilic inflammation in induced sputum of patients with idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 2003;20:138–143.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print



