 |
 |
| Korean J Intern Med > Volume 37(1); 2022 > Article |
|
Abstract
Background/Aims
Methods
Results
Acknowledgments
Figure 1

Figure 2

Figure 3

Figure 4
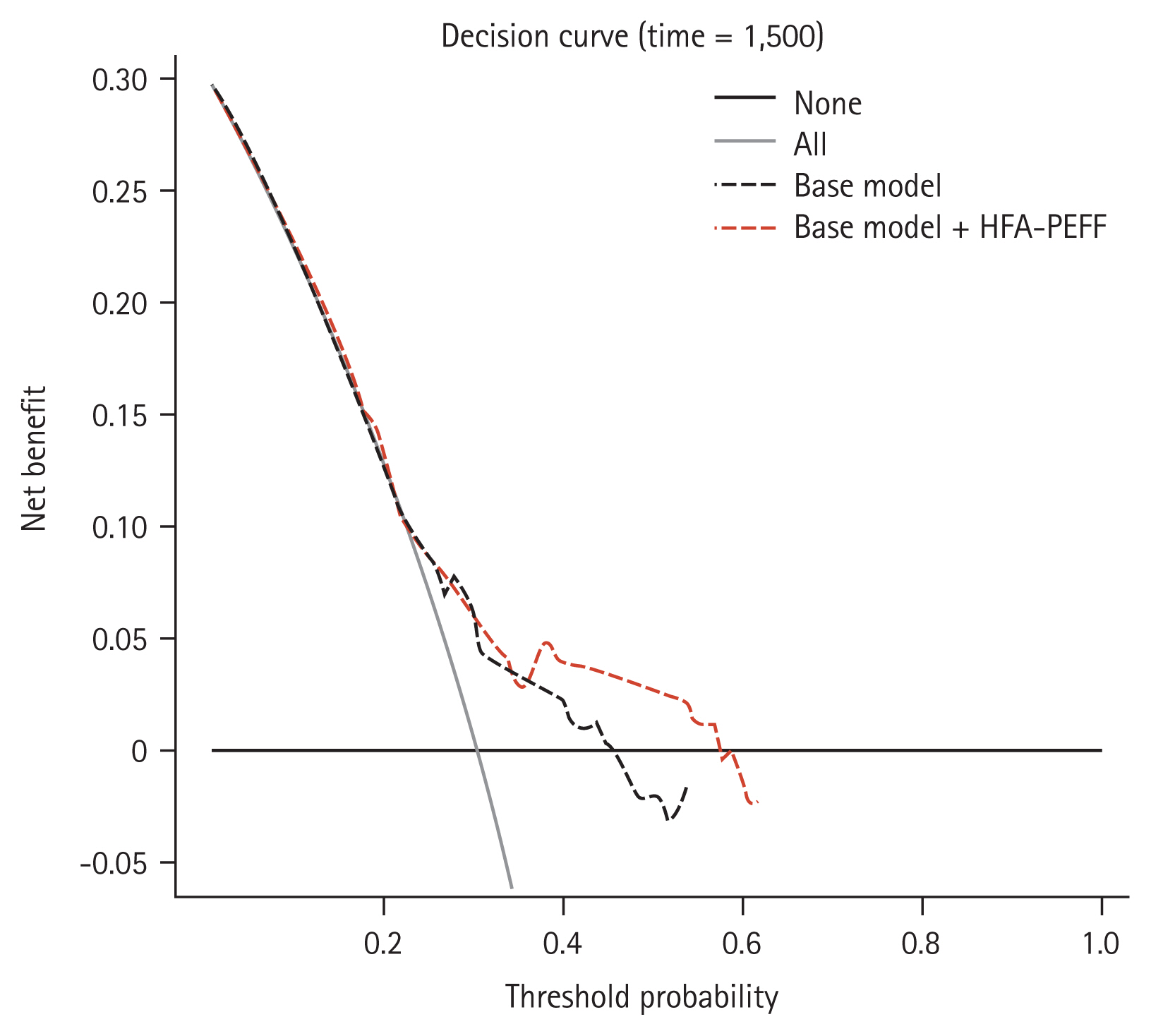
Table 1
HFpEF, heart failure with preserved ejection fraction; BMI, body mass index; HF, heart failure; NYHA, New York Heart Association; IHD, ischemic heart disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; hs-CRP, high-sensitivity C-reactive protein; eGFR, estimated glomerular filtration rate; BNP, brain natriuretic peptide; LVEF, left ventricular ejection fraction; SVI, stroke volume index; LAD, left atrium diameter; LVMI, left ventricular mass index; E/e′, the ratio of early transmitral flow velocity to early diastolic mitral annular velocity; TR-PG, tricuspid regurgitation pressure gradient; PAP, pulmonary artery systolic pressure; ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker; HFA-PEFF, Heart Failure Association-PEFF.
Table 2
Table 3
| Variable | Univariate regression | Multivariate regression | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Model 1 (I-PRESERVE) | Model 2 | ||||||||
|
|
|
|
|||||||
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |
| Age, yr | 1.01 | 0.99–1.03 | 0.381 | 1.00 | 0.99–1.02 | 0.658 | |||
|
|
|||||||||
| Male sex (yes) | 0.78 | 0.53–1.13 | 0.190 | ||||||
|
|
|||||||||
| BMI, kg/m2 | 0.97 | 0.92–1.02 | 0.242 | ||||||
|
|
|||||||||
| Previous hospitalization for HF (yes) | 2.30 | 1.51–3.50 | < 0.001 | 2.02 | 1.32–3.10 | 0.001 | 1.43 | 0.89–2.30 | 0.143 |
|
|
|||||||||
| NYHA III or IV (yes) | 2.83 | 1.89–4.23 | < 0.001 | 1.70 | 1.07–2.70 | 0.026 | |||
|
|
|||||||||
| Diabetes mellitus (yes) | 0.83 | 0.55–1.28 | 0.403 | 0.92 | 0.60–1.41 | 0.696 | |||
|
|
|||||||||
| Hypertension (yes) | 0.57 | 0.38–0.85 | 0.007 | 0.65 | 0.42–1.01 | 0.054 | |||
|
|
|||||||||
| Dyslipidemia (yes) | 0.82 | 0.53–1.28 | 0.379 | ||||||
|
|
|||||||||
| IHD (yes) | 0.56 | 0.38–0.82 | 0.003 | 0.74 | 0.48–1.13 | 0.158 | |||
|
|
|||||||||
| Atrial fibrillation (yes) | 1.76 | 1.20–2.59 | 0.004 | 1.21 | 0.78–1.87 | 0.390 | |||
|
|
|||||||||
| SBP, mmHg | 1.00 | 0.99–1.01 | 0.473 | ||||||
|
|
|||||||||
| DBP, mmHg | 1.00 | 0.98–1.01 | 0.570 | ||||||
|
|
|||||||||
| Hemoglobin, g/dL | 0.85 | 0.76–0.94 | 0.001 | 0.87 | 0.79–0.97 | 0.011 | |||
|
|
|||||||||
| hs-CRP, mg/L | 1.03 | 0.97–1.10 | 0.317 | ||||||
|
|
|||||||||
| eGFR, mL/min/1.73 m2 | 1.00 | 0.99–1.01 | 0.863 | ||||||
|
|
|||||||||
| LVEF, % | 0.98 | 0.95–1.01 | 0.150 | ||||||
|
|
|||||||||
| SVI, mL/min | 0.99 | 0.97–1.01 | 0.169 | ||||||
|
|
|||||||||
| ACE-I or ARB (yes) | 1.24 | 0.83–1.85 | 0.290 | ||||||
|
|
|||||||||
| CCB (yes) | 0.72 | 0.50–1.05 | 0.084 | ||||||
|
|
|||||||||
| Beta-blocker (yes) | 1.03 | 0.71–1.50 | 0.889 | ||||||
|
|
|||||||||
| Statin (yes) | 0.73 | 0.50–1.07 | 0.108 | ||||||
|
|
|||||||||
| High HFA-PEFF score (yes) | 2.18 | 1.49–3.19 | < 0.001 | 1.98 | 1.35–2.92 | 0.001 | 1.66 | 1.11–2.50 | 0.014 |
|
|
|||||||||
| TRVmax, m/sec (> 2.8) | 1.93 | 1.24–3.02 | 0.004 | ||||||
|
|
|||||||||
| Averaged E/e′ (≥ 15) | 0.84 | 0.50–1.43 | 0.526 | ||||||
|
|
|||||||||
| High LVMIa (yes) | 1.40 | 0.96–2.04 | 0.080 | ||||||
|
|
|||||||||
| LV wall thickness, mm (≥ 12) | 1.40 | 0.95–2.07 | 0.092 | ||||||
|
|
|||||||||
| RWT (> 0.42) | 1.02 | 0.66–1.59 | 0.915 | ||||||
|
|
|||||||||
| High BNPb (yes) | 1.94 | 1.33–2.82 | 0.001 | ||||||
Model 1: age, previous hospitalization for HF, diabetes mellitus and HFA-PEFF score; Model 2: variables of statistical significance in the univariate analyses (p < 0.05).
HF, heart failure; I-PRESERVE, Irbesartan in Patients with Heart Failure and Preserved Ejection Fraction; HR, hazard ratio; CI, confidence interval; BMI, body mass index; NYHA, New York Heart Association; IHD, ischemic heart disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; hs-CRP, high-sensitivity C-reactive protein; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; SVI, stroke volume index; ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker; HFA-PEFF, Heart Failure Association-PEFF; TRVmax, maximum velocity of tricuspid regurgitation; E/e′, the ratio of early transmitral flow velocity to early diastolic mitral annular velocity; LVMI, left ventricular mass index; LV, left ventricular; RWT, relative wall thickness; BNP, brain natriuretic peptide.
Table 4
REFERENCES
- TOOLS
-
METRICS

- Related articles
-
Breakthrough in heart failure with preserved ejection fraction: are we there yet?2016 January;31(1)




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print


