INTRODUCTION
The prevalence of heart failure (HF) is increasing worldwide, particularly among the elderly. Moreover, numerous studies have shown that high maximal oxygen consumption (VO
2max) can lower the risk of cardiovascular disease (CVD), specifically HF, and all-cause mortality. HF is caused by either systolic or diastolic dysfunction, both of which reduce the cardiac output response to exercise and thus reduce exercise tolerance. Reduced cardiac output is correlated with poor exercise capacity [
1].
Cardiorespiratory fitness (CRF) is an important independent predictor of CVD outcomes in patients with HF and is defined as the cardiovascular and pulmonary capacity that provides oxygen to skeletal muscles during exercise [
2]. CRF is measured by VO
2max and is expressed in terms of the metabolic equivalents (METs) of this metabolic demand at a given work level. One MET is defined as the amount of oxygen consumed while at rest and is equal to 3.5 mL oxygen per kilogram of body weight per minute (*3.5 mL O
2/kg/min) [
3]. CRF is based on the peak activity rate on a treadmill or cycle ergometer, with measurements of oxygen and carbon dioxide. A gas analyzer is one of the requirements for measuring VO
2max. The American Heart Association states that gas analysis with traditional testing can provide the most accurate and noninvasive quantification of VO
2max [
4]. For CRF measurements to be feasible under various conditions, such as without a gas analyzer, estimation using an equation is required.
The commonly used regression equation is the American College of Sports Medicine (ACSM) equation [
5,
6] (
Table 1,
2). Many scientists have compared the estimated VO
2max by the ACSM equation to measured values and found it to be inaccurate, especially when used in populations other than those for which it was developed [
2,
3,
7,
8]. An important limitation of the ACSM equation is that it is based on a cohort of < 200 young adults who underwent submaximal treadmill exercise testing to achieve a steady-state aerobic requirement [
5ŌĆō
7]. Although VO
2max was extrapolated from the value at steady state, it is unrealistic for peak exercises and is limited by the fact that it provides a higher estimate of VO
2max of 1ŌĆō1.5 METs than the actual value [
7]. Therefore, a better regression equation must be developed to predict VO
2max.
Recently, the Fitness Registry and the Importance of Exercise National Database (FRIEND) equation was developed for healthy individuals [
8] (
Table 2). The FRIEND equation is more accurate than the ACSM equation in estimating VO
2max in healthy individuals [
2,
8].
Kokkinos et al. [
9] recently reported that the newly developed FRIEND equation for patients with HF (FRIEND-HF) was superior to the ACSM and FRIEND equations in predicting directly measured VO
2max in patients with HF (
Table 2). They validated the results in the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) [
10] cohort, in which participants were medically stable HF patients with left ventricular ejection fraction (EF) Ōēż 35%.
However, the FRIEND-HF equation has not been validated in patients with HF with preserved ejection fraction (HFpEF). Considering that the characteristics of HFpEF are different from those of HF with reduced ejection fraction (HFrEF), it is not clear whether the FRIEND-HF equation can be applied to patients with HFpEF. In this study, three conventional equations for estimating VO
2max were compared with the directly measured VO
2max in patients with HFpEF. In addition, we developed and internally validated the Kor-HFpEF equation to estimate the VO
2max in patients with HFpEF. In
Table 1 [
2,
9,
11] we summarize the comparison of VO
2max between directly measured VO
2max and estimated VO
2max using the three conventional equations.
METHODS
Of the 1,848 patients who underwent a cardiopulmonary exercise test using a treadmill at the Korea University Guro Hospital between May 2015 and December 2021, we analyzed 521 patients with medically stable HFpEF (EF Ōēź 50%). Patients were excluded from the study based on the following criteria: 1) N-terminal pro-B-type natriuretic peptide (NTpro-BNP) level <125 pg/mL; 2) EF <50%; and 3) EF Ōēź50%, but with stable or unstable angina, acute myocardial infarction, peripheral arterial disease, and arrhythmia other than HFpEF (full exclusion criteria are provided in the
Supplementary Fig. 1). Consequently, 1,327 patients were excluded from our cohort (239 patients with EF < 50%, 215 patients without two-dimensional (2D) echocardiography, 593 patients with NTpro-BNP level < 125 pg/mL, and 280 patients with other CVDs without HFpEF, such as stable or unstable angina, acute myocardial infarction, and peripheral arterial disease).
Participants were divided into two randomly assigned groups: group A (n = 253) and group B (n = 268). Data from group A were used to create the Kor-HFpEF equation, whereas data from group B were used to validate the estimated VO2max using the Kor-HFpEF. The Kor-HFpEF equation was internally validated based on directly measured VO2max during a maximal exercise test using a treadmill in patients with HFpEF.
All patients underwent a treadmill test based on the modified Bruce Ramp protocol for the CRF test, a 6-minute walk test, and 2D and Doppler transthoracic echocardiography. Brachial blood pressure, oxygen saturation, and heart rate were measured during the pre-exercise test. Demographic characteristics, including age, sex, body mass index (BMI), and medical history, including hypertension (HTN), diabetes mellitus (DM), and dyslipidemia, were reviewed. HFpEF was identified by reviewing the medical records of patients with symptoms and/or signs of HF, NTpro-BNP levels > 125 pg/mL, EF Ōēź 50%, and other echocardiographic findings associated with HFpEF, such as left atrial enlargement, left ventricular hypertrophy, and diastolic dysfunction.
The VO
2max measured by cardiopulmonary exercise test is the gold standard for assessing CRF [
4,
12ŌĆō
14]. The modified Bruce ramp protocol was designed to achieve each individualŌĆÖs estimated VO
2max over a specific period with personalized speed and fractional grade settings [
12,
14]. In 2013, the ACC/AHA supported the use of the modified Bruce ramp protocol in special populations, such as patients with HF, by defining a standard for treadmill exercise testing [
4].
Statistical analysis
Statistical analysis was performed using IBM SPSS (version 22.0; IBM Corp., Armonk, NY, USA) and MedCalc Statistical Software version 19.2.1 (MedCalc Software Ltd., Ostend, Belgium). Statistical values are presented as mean ┬▒ standard deviation for numerical variables and their percentages for categorical variables. The difference between the directly measured VO2max and estimated VO2max was compared using PearsonŌĆÖs correlation and StudentŌĆÖs t-tests for paired data. Independent sample t-tests and chi-square analyses were used to compare baseline characteristics between the control and validation groups.
In our study, we performed random sampling and allocation using SPSS software. We aimed to control the baseline characteristics of both groups and achieve a balance in their characteristics. Our dataset, designated by simple random sampling, included age, HTN, DM, dyslipidemia, atrial fibrillation, smoking, alcohol, total cholesterol, triglyceride, low-density lipoprotein, high-density lipoprotein, and HbA1c levels.
Development of new Kor-HFpEF equation
We applied multivariate linear regression analysis to identify the most relevant variables associated with VO2max to construct the best prediction model for VO2max estimation using data from group A. The stepwise linear regression selection was adopted, and the selection entry and removal stepping method criteria were 0.05 and 0.10, respectively. The variables considered were treadmill speed (m/min), treadmill fractional grade (%), interaction between treadmill speed and fractional grade, heart rate at rest, blood pressure at rest, BMI, height, age, and sex. The excluded variables were heart rate at rest, blood pressure at rest, BMI, height, age, and sex; these variables were not significantly correlated with the dependent variable. In the final model, stepwise regression analysis was performed with the variables treadmill speed and the interaction between treadmill speed and grade in group A. The coefficients in the prediction equation for directly measured VO2max included treadmill speed and fractional grade, and we checked whether these variables contributed significantly to the VO2max prediction model (mean p < 0.05). A new equation was developed using the values of the unstandardized regression coefficients from the linear regression analysis. In the final model, stepwise regression analysis was performed with the variables treadmill speed and the interaction between treadmill speed and grade in group A.
Multivariate linear regression analysis using the enter method yielded the following Kor-HFpEF equation:
Microsoft Excel 2016 was used to calculate the mean absolute percentage error (MAPE) for equivalent analysis in both groups. MAPE measures accuracy as a percentage and can be calculated as shown in the following equation:
Box and Bland-Altman plots were used for the differential average of the regression equations. LinŌĆÖs concordance correlation coefficient (CCC) was calculated to test the concordance between two variables. The statistical significance level was set at 5% (p < 0.05). This study was approved by the Institutional Review Board (IRB) of the Korea University Guro Hospital (IRB number 2020GR0060). The requirement for written informed consent was waived due to the retrospective design of the study.
RESULTS
We analyzed 521 patients with HFpEF and divided them into two randomly assigned groups: group A (n = 253) and group B (n = 268). Patients in both groups had similar base line characteristics. The percentages of men in Groups A and B were 73.1% and 78.7%, respectively. Baseline characteristics of the study population, medical history, laboratory values, and echocardiography records are presented in
Table 3.
In the total HFpEF population, VO
2max values predicted using the FRIEND-HF equation were significantly lower than the directly measured VO
2max (Direct 21.2 ┬▒ 5.9 vs. FRIEND-HF 14.2 ┬▒ 4.9 mL/kg/min,
p < 0.001), whereas those predicted by the ACSM (Direct 21.2 ┬▒ 5.9 vs. ACSM 32.5 ┬▒ 13.4 mL/kg/min,
p < 0.001) and FRIEND (Direct 21.2 ┬▒ 5.9 vs. FRIEND 29.1 ┬▒ 11.8 mL/kg/min,
p = 0.001) equations were significantly higher than the directly measured VO
2max (
Table 4,
Fig. 1A).
The MAPE for the FRIEND and FRIEND-HF equations was 35.5 ┬▒ 13.4% and ŌłÆ33.2 ┬▒ 16.4%, respectively, whereas the ACSM equation showed the highest prediction error (% Error = 50.7 ┬▒ 46.2).
We developed a new equation, the Kor-HFpEF equation, to estimate directly measured VO
2max in patients with HFpEF. The Kor-HFpEF equation was created using the interaction between treadmill speed and grade from stepwise regression analysis using group A data (
Table 2). In group B, similar to the total HFpEF population, the FRIEND-HF equation underestimated VO
2max (
p < 0.001) and showed high prediction error (% Error = ŌłÆ33.2 ┬▒ 16.4); the estimated VO
2max by the ACSM and FRIEND equations significantly overestimated the directly measured VO
2max. The newly developed Kor-HFpEF equation from group A was more accurate than the other three equations in predicting directly measured VO
2max in patients with HFpEF in group B (Kor-HFpEF 21.3 ┬▒ 4.6 vs. Direct 21.7 ┬▒ 5.9 mL/kg/min,
p = 0.124) (
Table 5,
Fig. 1B). As shown in
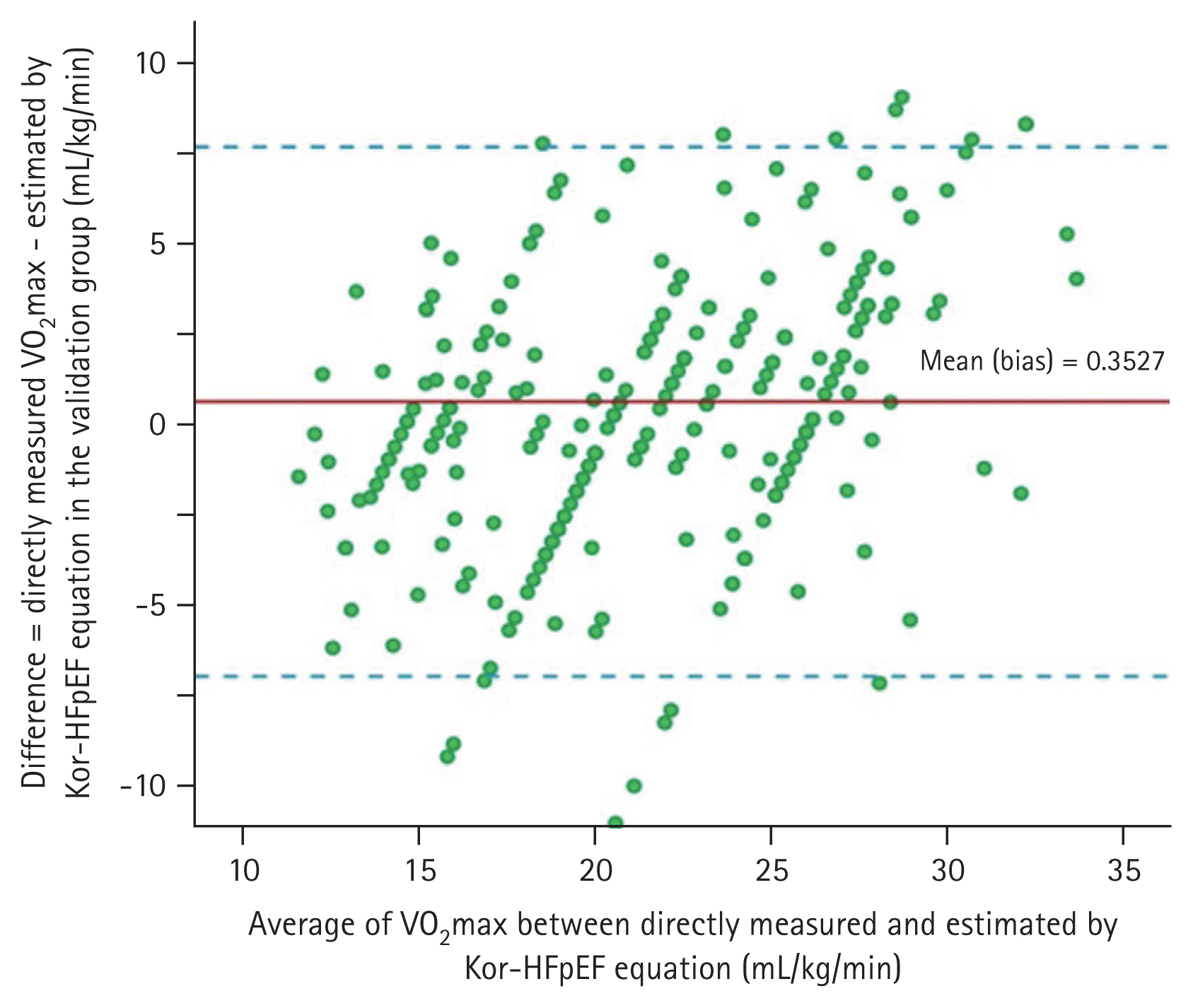
Fig. 2, there was no significant difference in VO
2max between the directly measured values and estimated values using the Kor-HFpEF equation with minimal bias (0.353 mL/kg/min; 95% CI: ŌłÆ6.97 to 7.68;
p = 0.124) in group B (LinŌĆÖs CCC = 0.999,
p < 0.001).
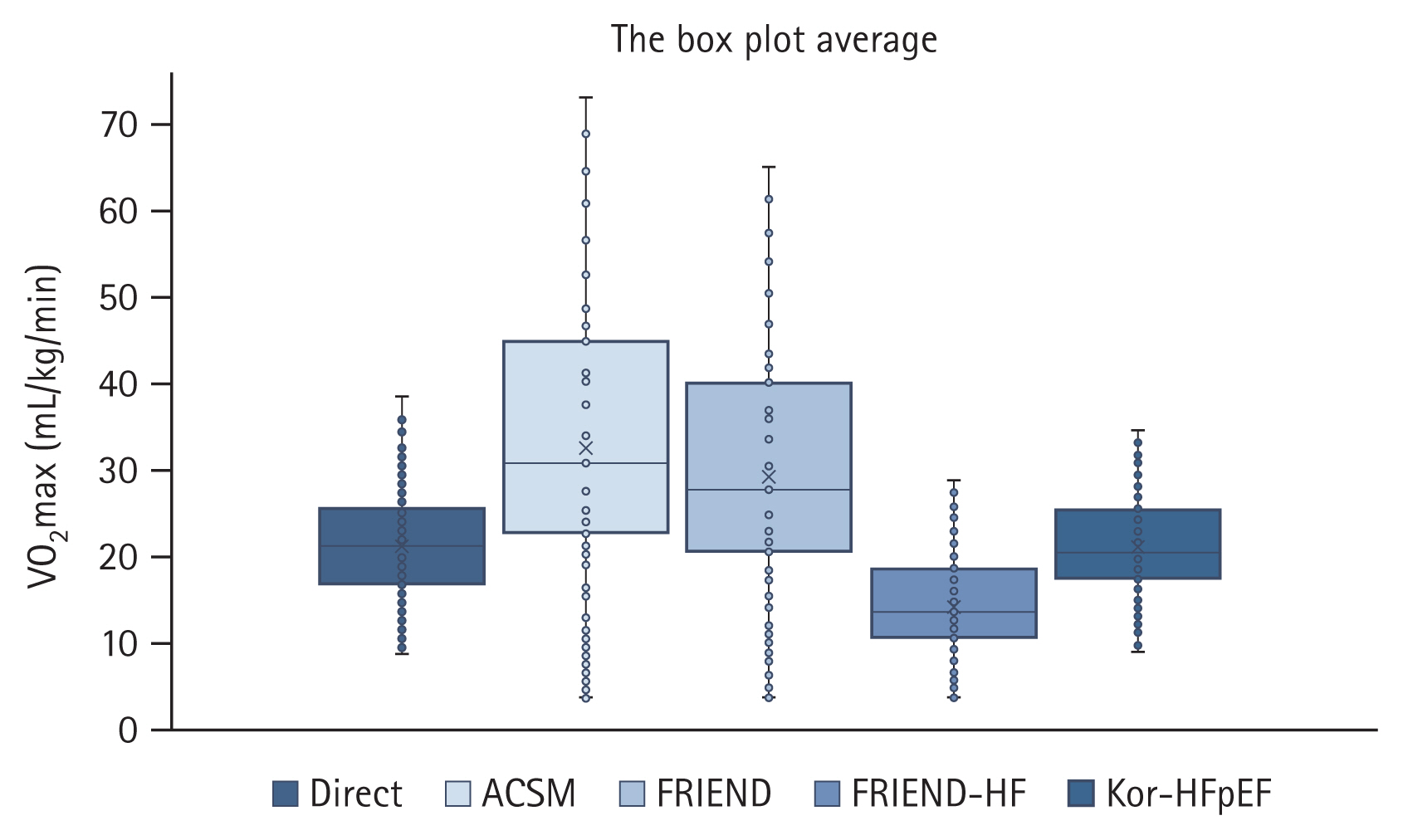
Fig. 3 shows the box plot average of the directly measured VO
2max and the estimated VO
2max using the ACSM, FRIEND, FRIEND-HF, and Kor-HFpEF equations for group B. The Kor-HFpEF equation predicted VO
2max values closer to the directly measured values in group B.
DISCUSSION
Lifelong healthy behaviors are key to preventing chronic diseases [
15,
16]. HFpEF is a chronic disease associated with a high disease burden on patients, their families, and the health care system [
17]. Numerous studies have shown that a high VO
2max can lower the risk of CVD, particularly HF, and all-cause mortality. Interestingly, one MET improvement in CRF resulted in a 10ŌĆō25% reduction in mortality [
18,
19]; therefore, CRF measurement is important, not only for healthy people but also for patients with HFpEF.
The gold standard method for assessing CRF is to measure VO
2max using a cardiopulmonary exercise test [
12,
13,
19ŌĆō
22]. This method is difficult to implement in all institutions because it requires maximum exercise on a treadmill or cycle ergometer with expiratory gas analysis [
23]. In the absence of a gas analyzer, the CRF must be estimated using an equation. The most commonly used equation is ACSM (
Table 2). One of the important limitations of the ACSM equation is that it considers an unrealistic steady state for peak exercises, which has resulted in the overestimation of VO
2max in this and previous studies [
3,
9].
The FRIEND equation was devised recently. The FRIEND cohort study established in 2014 included healthy adults (n = 7,983) to discover normative CRF values in the United States [
8,
13,
14,
23ŌĆō
25]. The FRIEND equation predicts VO
2max more accurately than the ACSM equation in healthy adults [
7]. When the FRIEND equation was applied to patients with HF from the FRIEND dataset, the prediction error was relatively high [
9]. Therefore, Kokkinos et al. [
9] developed a new formula specifically for patients with HF (FRIEND-HF) using the FRIEND HF cohort dataset and found it to be superior to the ACSM and FRIEND equations in predicting directly measured VO
2max in an external cohort of patients with HFrEF (HF-ACTION cohort) [
10]. Although the FRIEND-HF equation was developed based on the data of patients with HF irrespective of left ventricular EF and validated in the HFrEF cohort [
6], it was unclear whether it was also applicable to patients with HFpEF. Indeed, the FRIEND-HF equation systematically underestimated the directly measured VO
2max in the HFpEF cohort (
Table 4,
5,
Fig. 1).
The accuracy of estimating VO
2max is important, and the need for a more accurate regression equation is emphasized, considering its prognostic value in patients with HFpEF [
26,
27]. As conventional equations estimating VO
2max cannot accurately predict the true VO
2max in patients with HFpEF, we developed the Kor-HFpEF equation from the HFpEF cohort. The estimated VO
2max using the Kor-HFpEF equation closely matched the directly measured VO
2max in the internal validation (
Table 5,
Fig. 1B,
2,
3). The MAPE is often used in practice as a statistical method to determine the accuracy of a prediction method [
9]. In the results of the regression quality measurement, the MAPE value of the Kor-HFpEF equation was the lowest when compared with that of the ACSM, FRIEND, and FRIEND-HF equations (1.7% vs. 52.0%, 36.6%, and ŌłÆ33.0%, respectively). In other words, unlike the other three equations, the Kor-HFpEF equation estimated VO
2max comparable to the direct measurement with the lowest prediction error. This may be useful in environments where CRF cannot be directly measured in patients with HFpEF. LinŌĆÖs CCC analysis for VO
2max showed almost perfect concordance between direct measurement and estimation using the Kor-HFpEF equation (CCC = 0.999,
p < 0.001) with minimal bias in the Bland-Altman plot. These findings support the accuracy of estimated VO
2max using the Kor-HFpEF equation in patients with HFpEF (
Fig. 3). To our knowledge, this is the first study to devise and validate a high-quality regression equation for CRF using a treadmill in a Korean population with HFpEF.
The phenotypic differences between HFpEF and HFrEF have been investigated in several studies related to decreased cardiac output and oxygen transport capacity [
13,
28,
29]. There is clear evidence that exercise intolerance in HFrEF is associated with vascular dysfunction, capillary scarcity, the absence of red cell flux in most capillaries at rest, decreased microvascular oxygen pressure, and increased muscle deoxygenation [
30,
31]. Unlike in HFrEF, our understanding of the pathophysiology of exercise intolerance in HFpEF is limited. Little is known about the skeletal muscle function in HFpEF, which may contribute to exercise intolerance in HFpEF [
31].
In addition, interracial differences are substantial when the prediction equations for Western populations are applied to Asian populations. Standardized reference values for CRF test indices, including directly measured VO
2max, have been published in Western and Asian countries [
3,
20,
32,
33]. However, the steady state of CRF is influenced by several factors, including the study population, sample size, equipment such as a treadmill or cycle ergometer, testing protocols, and CRF measurements. Normal values for Asian populations have been shown to be lower than those for Western populations [
20,
25,
29,
30,
32,
33]. In Korea, only a few population-based studies have investigated the potential of CRF as a predictor of all-cause and CVD mortality. Yun et al. [
34] found that physical inactivity was independently associated with an increased risk of mortality. Park et al. [
35] reported an association between physical fitness and mortality in Korean men, which was significant among participants who did not exercise regularly, but not among those who did. In a previous population-based prospective cohort study, all-cause and CVD mortality were inversely correlated with estimated CRF in Korean adults [
36].
Many previous studies have shown that exercise-based cardiac rehabilitation (CR) has positive effects on patients with CVD, including reduced mortality [
37]. The prevalence of acute coronary syndrome in Korea is steadily increasing; however, the participation rate in CR is still insufficient [
38]. Choi et al. [
39] compared exercise capacity between a group that received CR training and a group that did not, targeting high-risk patients with myocardial infarction. CRF values, such as directly measured VO
2max and exercise time, showed a significant increase after 3 months in the CR participants. Recently, Jang et al. [
20] updated the age-related mean CRF reference values in an Asian population using a Korean cohort. In this study, sex- and age-predicted exercise capacity in Koreans was easily determined using a nomogram, and it was shown that there were clear interethnic differences in CRF. They also found that the CRF in patients with coronary heart disease was significantly lower than that in the healthy population.
Furthermore, data on CR in Korean patients with HF are limited. Although current guidelines recommend a CR program for all eligible patients with HF, CR costs reimbursed by KoreaŌĆÖs national healthcare system create substantial barriers to CR center operations owing to a lack of demand and profitability [
37]. Kim et al. [
40] reported that a higher level of physical activity was associated with a lower prevalence of chronotropic incompetence in Korean patients with HF, independent of potential risk factors. Previous studies have shown that physical inactivity and a higher BMI are significantly associated with an increased risk of de novo HF and hospitalization, particularly in patients with HFpEF [
40]. John et al. [
32] found no reliable predictive equation for VO
2max in the Indian population. It is essential to use prediction equations specific to different populations because lung function and maximal oxygen uptake can vary based on ethnicity. The VO
2max of healthy Koreans has been reported to differ from that of Westerners in terms of age-related reference values [
20]. In addition, Almakhaita et al. [
30] compared directly measured VO
2max with estimated VO
2max using equations for the Saudi female population. The directly measured VO
2max was lower than that obtained using the equations (Jones, Hansen, and Wasserman equations), and these equations were inadequate for the populations. Ultimately, it is hypothesized that the specific phenotype of HFpEF differs from that of HFrEF or healthy adults, and that different CRF based on race and/or ethnicity may have influenced the results of recent studies. Additionally, contrary to previous belief, the morbidity and mortality rates of patients with HFpEF are as ominous as those of patients with HFrEF [
16,
26,
27]. To date, there are no reliable regression equations for CRF applicable to a specific phenotype in the population with HF.
The strengths of the current study include the following: this is the first study in which conventional equations to estimate VO2max were compared with direct methods in a cohort of patients with HFpEF. The existing equations for patients with HF (FRIEND-HF) and healthy individuals (ACSM and FRIEND) were not applicable to patients with HFpEF. Further, this is the first external validation study of the FRIEND-HF equation used solely for HFpEF. Additionally, this is the first study to develop and internally validate a new equation (Kor-HFpEF) for estimating VO2max in patients with HFpEF. Additional studies are required to externally validate the Kor-HFpEF equation in other HFpEF cohorts.
This study has several limitations. First, it was conducted at a single center and included a relatively small number of patients and fewer women in the total population. Second, our VO2max prediction equation may have limited generalizability because this study included only Korean patients with HFpEF.
For the predicted VO2max, the accuracy of the newly developed Kor-HFpEF equation was higher than that of the other three traditional equations for patients with HFpEF. Therefore, the traditional equations were not applicable to Korean patients with HFpEF. Interestingly, the results suggest that the specific phenotype of HFpEF differs from that of HFrEF or healthy adults, and that different CRFs based on race and/or ethnicity may also have influenced the results. There is a need to improve our understanding of the pathophysiology of HFpEF, and we suggest that the Kor-HFpEF equation be validated externally in other HFpEF cohorts.








 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement figure 1
Supplement figure 1 Print
Print


